The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. COVID-19 affected close to 2 million persons worldwide in fewer than 4 months, after the report of the first cases in China in December 2019. The relation of the disease caused by SARS-Cov-2 to immunosuppressive treatment used in different gastrointestinal disorders is uncertain, resulting in debate with regard to suspending immunosuppressive therapy to improve infection outcome. Said suspension implies the inherent risk for graft rejection or autoimmune disease exacerbation that can potentially worsen the course of the infection. Based on the presently available evidence, a treatment stance has been established for patients with gastrointestinal diseases that require immunosuppressive therapy.
La enfermedad por coronavirus 2019 (COVID-19) es causada por el virus de Síndrome Respiratorio Agudo Grave - Coronavirus 2 (SARS-CoV-2). COVID 19 afectó cerca de 2 millones de personas en todo el mundo en menos de 4 meses posterior al reporte de los primeros casos en China en diciembre 2019. La relación que guarda la enfermedad por SARS-Cov-2 con el tratamiento inmunosupresor utilizado en diversos trastornos gastrointestinales es incierta, esto genera el debate sobre suspender el tratamiento inmunosupresor para mejorar el pronóstico de la infección, lo cual incluye el riesgo inherente de rechazo de injerto o agudización de enfermedades autoinmunes que potencialmente pudieran agravar el curso de la infección. En base a la evidencia disponible se logra establecer una postura de tratamiento en pacientes con enfermedades gastrointestinales que requieren terapia inmunosupresora.
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. COVID-19 affected more than 6 million persons worldwide in fewer than 4 months after the report of the first cases in China in December 2019. In March 2020, the first cases in the United States were reported,1 and almost simultaneously the first imported case in Mexico City was reported. Since then, the number of cases on the continent have exponentially multiplied and are responsible for approximately 45% of all cases across the globe.
Seventy to eighty percent of cases have a mild disease course, but patients over 65 years of age and/or with comorbidities, such as diabetes, obesity, and high blood pressure, are considered risk groups for severe progression. Due to the speed at which the number of infections has increased and because we are dealing with a new pathogen, the effect COVID-19 may have on different chronic pathologies, including gastrointestinal ones, is unknown. In that regard, a preliminary multinational report on 103 patients with cirrhosis of the liver found that 23.3% of the cirrhotic patients needed to be admitted to intensive care, and invasive mechanical ventilation was required in 17.5%. In that same report, the mortality rate was 39.8%, greatly exceeding the rate in the general population with COVID-19 pneumonia.2
Equally important and uncertain is the relation of the disease caused by SARS-Cov-2 to immunosuppressive treatment utilized in different gastrointestinal disorders. That has resulted in a debate about suspending immunosuppressive treatment to improve infection outcome. Said suspension implies the inherent risk for graft rejection and autoimmune disease exacerbation that could potentially worsen the course of the infection.
Scientific activity is directed toward the search for a specific treatment and the development of vaccines, whereas clinical practice and health system priorities have been modified, directing a large part of the medical resources (human and technologic) toward the care of patients with COVID-19. Those actions, together with the physical distancing measures that have been implemented, have changed and limited the type of care and surveillance of patients with chronic illnesses, including those with gastrointestinal pathologies that require immunosuppressive treatment. Fortunately, there are few such pathologies and they are associated with low levels of incidence and prevalence (e.g., liver transplantation, autoimmune hepatopathy, autoimmune pancreatitis, inflammatory bowel disease). The risk for infection with COVID-19 does not appear to be greater in those patients than in the general population, but it is not known if its progression will be the same or if there will be an increased probability in the need for intrahospital care. Based on reports from China and other Asian countries, infected patients can present with elevated transaminases, diarrhea, or abdominal pain. In an acute case of COVID-19, it is difficult to differentiate whether those symptoms are directly caused by the viral infection, drug toxicity, systemic inflammatory response, or activity of the underlying disease.3
Other questions arise about the management of those diseases in the group of patients receiving some type of immunosuppression. Due to the scarcity and low quality of evidence available, the liver, pancreas, and liver transplantation clinics of the Instituto Nacional de ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ) decided to document and report their positioning and issue recommendations on the management of autoimmune liver diseases, liver transplantation, and autoimmune pancreatitis in patients infected with SARS-Cov-2.
In regard to potential therapeutic modifications, the lack of knowledge about the effect SARS-CoV-2 can have on those chronic diseases and how, together with immunosuppressive drug use, they can contribute to a severe disease course, must be considered.
Patients with autoimmune liver diseasesThe group of patients with autoimmune liver diseases includes those diagnosed with autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis. However, immunosuppressant use is only indicated in autoimmune hepatitis. Those patients may be at greater risk for infection or severe disease due to chronic immunosuppressant use and the presence of advanced liver disease. Therefore, maximizing general precautions and physical distancing is recommended in that group of patients.
Infection due to SARS-CoV-2 is associated with alterations in liver function tests and it is not known if that increases the risk for causing acute decompensation of the chronic liver disease.
The most commonly used medications in those pathologies include corticosteroids, antiproliferative drugs (e.g., azathioprine, mycophenolate mofetil), and calcineurin inhibitors (e.g., tacrolimus and cyclosporine) for both remission induction and maintenance therapy.4
Patients not infected with COVID-19By international consensus, the baseline immunosuppressive treatment regimen should not be modified during the COVID-19 pandemic in non-infected patients.5
- •
In patients recently diagnosed with autoimmune liver diseases, immunosuppressive treatment should be started in the same way as that established before the COVID-19 pandemic.4
- •
Do not routinely reduce the degree of immunosuppression.
- •
Verify that the patient has received the influenza and pneumococcal vaccines.
If the infection is acquired, treatment adjustment should be individualized, considering symptom severity,3 the current activity grade of the underlying liver disease, and the risk for its reactivation by modifying treatment, especially in the case of autoimmune hepatitis. It is very important to not assume that transaminasemia in a patient with COVID-19 infection is due to the underlying liver disease, because COVID-19 can alter liver function tests.5
At present, the following recommendations have been made for patients with COVID-19 infection that have an underlying autoimmune liver disease and take immunosuppressants:5–8
- •
Having liver function test alterations does not limit starting treatment for COVID-19.
- •
Consider reducing elevated doses of prednisone, maintaining at least 10 mg/day to prevent adrenal insufficiency.
- •
Consider reducing the dose of azathioprine, 6-mercaptopurine, or mycophenolate mofetil, especially in the context of lymphopenia, fever, or deterioration due to COVID-19 pneumonia.
- •
Consider reducing, but not suspending, the daily dose of calcineurin inhibitors, especially in the context of lymphopenia, fever, or deterioration due to COVID-19 pneumonia.
- •
Continuously monitor the interaction between immunosuppressants and the “off-label” drugs for COVID-19. Periodically check the website: https://www.covid19-druginteractions.org/
- •
Some of the most common interactions are between prednisone + hydroxychloroquine, causing a slight increase in the risk for convulsions, and between azathioprine + hydroxychloroquine, increasing the risk for cytopenia.9 Importantly, the FDA has issued warnings about the use of those medications in ambulatory patients due to the risk of developing arrhythmias.10
- •
In the case of cholestatic diseases, the same dose of ursodeoxycholic acid can be continued, as long as it is tolerated orally. That drug does not interact with the “off-label” medications currently used in COVID-19 management.9
- •
Consider an arbitrary delay in the surveillance of hepatocellular carcinoma of up to 2 months in patients that have had a normal ultrasound study within the previous 6 months. Alternatively, alpha-fetoprotein levels can be evaluated, with the limitations that implies.
- •
In patients with clinically significant portal hypertension that are under primary prophylaxis with band ligation, consider postponing follow-up endoscopies and changing the strategy to nonselective beta-blockers (NSBBs), titrating the dose according to heart rate (55-60 bpm), watching that systolic blood pressure is not below 90 mmHg.11
- •
In patients with a previous clinically significant diagnosis of portal hypertension that are programmed for screening endoscopy, consider postponing the study and starting NSBBs, at least temporarily, and titrating the dose (see above).
- •
In patients with no previous diagnosis of clinically significant portal hypertension that are programmed for screening endoscopy, consider postponing the study. Alternatively, the platelet count (< 150,000) can be evaluated and if it suggests clinically significant portal hypertension, start NSBBs, at least temporarily, titrating the dose (see above).
- •
In patients with suspicious liver nodules, the diagnostic approach should not be delayed, nor should treatment be delayed in the case of hepatocellular carcinoma.
- •
In patients that require imaging and/or laboratory studies, they should preferably be performed at peripheral diagnostic centers and not within hospital environments.
- •
In patients with recent commencement of primary prophylaxis with NSBBs, its titration can be carried out by telemedicine, phone call, or text message, if the patient can measure his/her heart rate and blood pressure.
- •
Adhere to the treatment protocols established by the international guidelines, but minimizing hospital stay as much as possible, and carry out inter-consultation with other services by telemedicine.12
- •
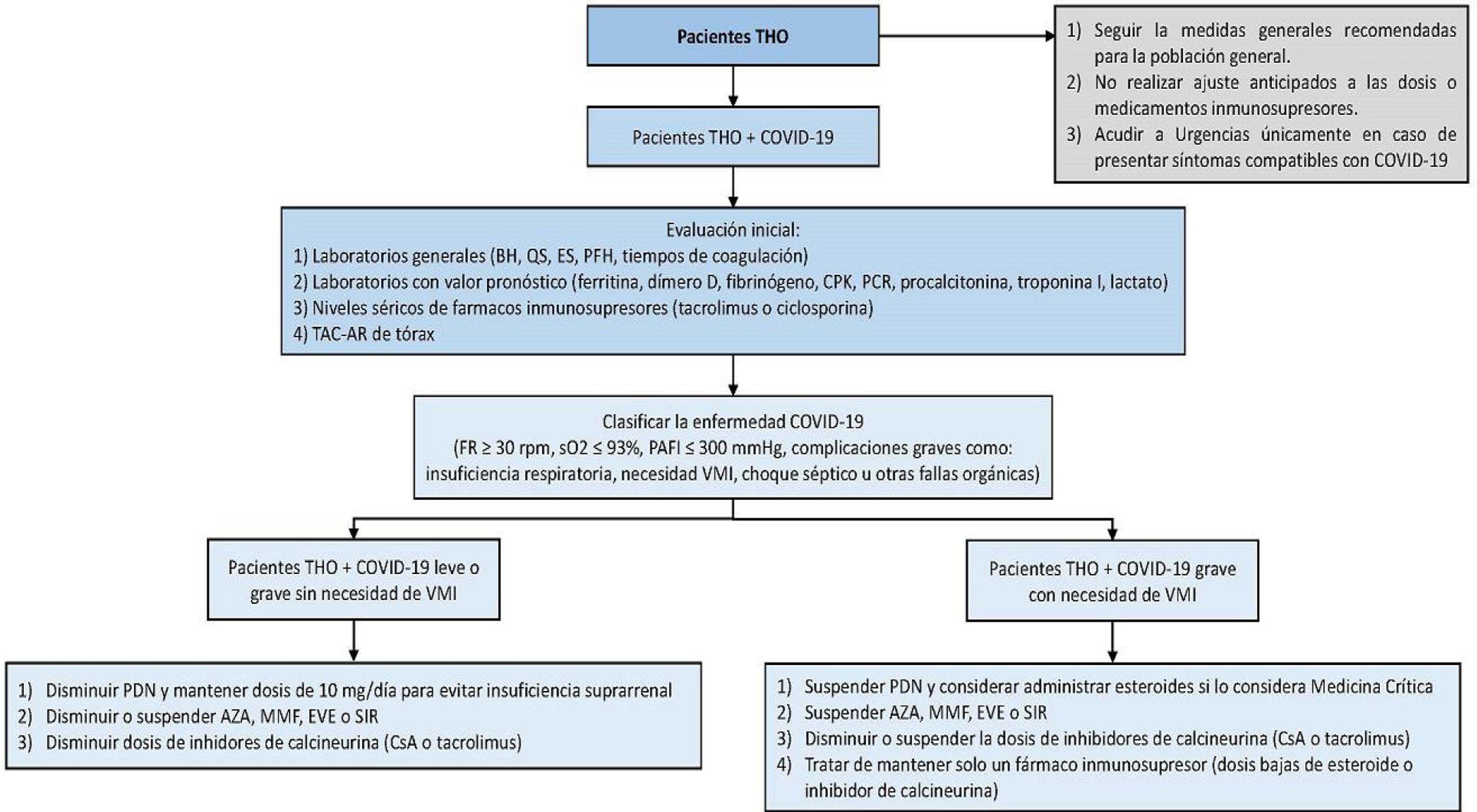
Place only those patients with poor short-term prognosis on the liver transplantation list, such as patients with a high MELD score (individualizing each case according to internal committee and liver transplantation protocols), hepatocellular carcinoma that is close to exceeding the Milan criteria, and patients with acute liver failure13 (see Fig. 1).
Figure 1.Management algorithm for patients presenting with liver transplantation and COVID-19.
AZA: azathioprine; BC: blood chemistry; CBC: complete blood count; CPK: creatine phosphokinase; CRP: C-reactive protein; CsA: cyclosporine; EVE: everolimus; HRCT: high resolution computed tomography; IMV: invasive mechanical ventilation; LFTs: liver function tests; MMF: mycophenolate mofetil; OLT: orthotopic liver transplantation; PAFI: ratio of partial pressure of oxygen in arterial blood to inspired oxygen fraction; PDN: prednisone; RR: respiratory rate; SaO2: oxygen saturation; SE: serum electrolytes; SIR: sirolimus.
(0.47MB). - •
Place special emphasis on optimizing prophylaxis for encephalopathy, variceal bleeding, and spontaneous bacterial peritonitis in the groups that merit it, because that will limit hospital visits.
- •
Preferably postpone that type of procedure.
- •
If seronegative primary biliary cholangitis is suspected, consider starting treatment, instead of performing liver biopsy.
If autoimmune hepatitis is suspected, disease severity should be taken into account to determine the need for urgent biopsy. Starting treatment without biopsy can also be considered in certain cases.5
Care of patients with liver transplantationImpact of COVID-19 on patients with transplantAt the time the present manuscript was drafted, there were no studies with a sufficient number of cases to enable the evaluation of SARS-CoV-2 (COVID-19) outcomes in the liver transplantation population. Nevertheless, some case reports indicate that the clinical, radiologic, and biochemical presentation is similar to that of immunocompetent patients, albeit disease progression, the rate of admissions to intensive care units, and frequency of bacterial superinfections can be higher.14 In lower respiratory tract viral infections caused by agents other than SARS-CoV-2, we know that the period of greater incidence is at early post-transplantation, with repercussions such as induced rejection and death, which in the case of syncytial respiratory virus, can reach 20%.15 In a correspondence article, the experience at a liver transplantation center in Northern Italy was reported. In that case series, 3 out of 111 patients (with a transplant period of more than 10 years) died from COVID-19. The 3 patients shared the following characteristics: they were men, over 65 years of age, overweight, and presented with high blood pressure and type 2 diabetes mellitus. In contrast, 3 out of 40 patients with liver post-transplantation status (with a transplant period under 2 years) that had COVID-19 pneumonia, had a benign disease course.16 It is not yet known whether immunosuppression in liver transplant patients makes them more susceptible to infection from SARS-CoV-2. However, at present, their mortality rate has not been higher than that of the general population and appears to be related more to the presence of metabolic complications after transplantation than to immunosuppression itself.
Liver function test alteration in patients with liver transplantationWe know that 14% to 53% of patients hospitalized with COVID-19 can present with liver function test alterations, mainly with elevated AST and ALT levels.17 The increase tends to be transitory and requires no specific treatment. The mechanisms of damage that have been suggested are:
- •
Direct damage. SARS-CoV-2 binds to the angiotensin-converting enzyme 2 receptor, enabling it to enter the target cells. That receptor is expressed in hepatocytes and cholangiocytes, making the liver a target organ of infection.17
- •
Pharmacologic toxicity. The therapeutic agents that form part of the treatment in Mexico, such as remdesivir and tocilizumab, can have hepatotoxic effects.
The American Association for the Study of Liver Diseases (AASLD) states that acute cell rejection should not be assumed in the setting of COVID-19 and liver transplantation. Nevertheless, there are reports that viral pneumonias can induce acute rejection. Therefore, suspicion should be maintained when there is moderate-to-severe transaminase elevation and/or lack of decrease in transaminases after COVID-19 infection has been resolved.
Recommendations for the diagnostic approach to a liver transplant patient include:
- •
Hepatotropic and non-hepatotropic viral hepatitis panel
- •
Imaging studies (USG or magnetic resonance cholangiography), only if there is a cholestatic pattern with suspected vascular problems and/or cholangitis (factor R < 2).
- •
Liver function tests should be monitored every 24-48 h.
- •
The presence of liver function test alterations does not limit starting treatment for COVID-19.
Given the lack of studies, there is no prevention strategy different from the general measures applied to the rest of the population. Current data suggest that the innate immune response may play an important role in pulmonary damage, and theoretically, immunosuppression could be a protective factor.18,19 We also know that immunosuppression was not a risk factor for death associated with previous outbreaks of SARS or MERS. At present, no association with greater risk for infection, severe disease forms, or mortality in patients with liver transplantation has been reported.19,20 Thus, at this point in time and in agreement with other associations, we conclude the following:
- •
Anticipatory adjustments to immunosuppressant doses should not be made.
- •
Changes in the immunosuppression regimen should not be recommended.
The general measures recommended for patients with liver transplant are the same as those for the general population.
- •
Frequent handwashing.
- •
The cleansing of surfaces that are frequently touched.
- •
Carrying out the measures of physical distancing and staying away from large crowds.
- •
Staying away from sick persons.
- •
Not travelling during the pandemic.
- •
Reducing face-to-face medical consultations to a minimum and utilizing telemedicine.
- •
Requesting a leave of absence from work or considering working from home.
The general recommendations for the management of patients with liver transplant and COVID-19 diagnosis are made according to the different possible scenarios faced in clinical practice. Basically, three possible scenarios are contemplated: 1) patients with liver transplantation and mild COVID-19, 2) patients with liver transplantation and severe COVID-19 with no need for invasive mechanical ventilation, and 3) patients with liver transplantation and severe COVID-19 and the need for invasive mechanical ventilation. When making decisions in relation to the transplanted patient with COVID-19, disease severity, immunosuppression status according to the time interval since transplantation, as well as possible drug interactions between the immunosuppressants and the “off-label” drugs utilized in those patients, should be taken into account. Doing so enables the risk-benefit of each of the interventions to be evaluated to make the best decision, always taking that which can put the patient’s life or graft survival at risk into consideration. On occasion it is necessary to individualize the decisions case by case. Fig. 1 shows the proposed treatment algorithm for patients with liver transplantation and COVID-19.
It is important to remember that all patients that present with any of the following, upon or after hospital admission, should be classified as severe cases: 1) tachypnea (≥ 30 breaths per min); 2) resting oxygen saturation ≤ 93%; 3) arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen ratio (PAFI) ≤ 300 mmHg; or 4) severe disease complications (respiratory failure, need for mechanical ventilation, septic shock, or other organ failure).
All patients with liver transplantation and demonstrated COVID-19 (RT-PCR) should be considered for hospital unit admission, because the effects of immunosuppression are currently not well-established. It is important to be able to monitor them for at least 72 h and thus determine the disease course (the need for supplementary oxygen or the development of hypoxemia). Severe cases have been described to take time (approximately 8 days from the beginning of the infection) before requiring greater support measures.
The initial evaluation of patients with liver transplantation and COVID-19 should include general laboratory tests (complete blood count, blood chemistry, serum electrolytes, liver function tests, and coagulation times), laboratory tests with prognostic value (ferritin, D dimer, fibrinogen, creatine phosphokinase [CPK], C-reactive protein, procalcitonin, troponin I, and lactate), and a high-resolution computed tomography scan of the chest.
Patients with liver transplantation and mild or severe COVID-19 with no need for invasive mechanical ventilation- •
Reduce high doses of prednisone but maintain a dose of 10 mg/day to prevent adrenal insufficiency.
- •
Reduce or suspend the dose of azathioprine, mycophenolate mofetil, everolimus, or sirolimus, especially if the patient has lymphopenia, fever, or worsening pneumonia, and if transplant progression allows it.
- •
Reduce the dose of calcineurin inhibitor, especially if the patient has lymphopenia, fever, or worsening pneumonia, and if transplant progression allows it.
- •
Measure calcineurin inhibitor levels upon admission and every 72 h.
- •
Perform baseline and serial electrocardiogram (ECG) on all patients taking chloroquine or hydroxychloroquine and azithromycin, due to the risk for developing arrhythmias secondary to QT segment prolongation. Importantly, the FDA has issued warnings about the use of those medications in ambulatory patients due to the risk of developing arrhythmias.10
- •
Remain in contact with the liver transplantation physicians.
- •
Suspend prednisone and consider administering steroids, according to the opinion of critical medicine physicians for preventing adrenal insufficiency or other indication, such as refractory shock.
- •
Suspend the dose of azathioprine, mycophenolate mofetil, everolimus, or sirolimus.
- •
Reduce or suspend the dose of calcineurin inhibitor, especially if the patient presents with fever or worsening pneumonia.
- •
Try to maintain only one immunosuppressant, whether low doses of a steroid or low calcineurin inhibitor levels (tacrolimus 2-5 ng/dl).
- •
Measure calcineurin inhibitor levels upon admission and every 72 h.
- •
Perform baseline and serial ECG on all patients taking chloroquine or hydroxychloroquine and azithromycin, due to the risk for developing arrhythmias secondary to QT segment prolongation. Importantly, the FDA has issued warnings about the use of those medications in ambulatory patients due to the risk of developing arrhythmias.10
- •
Remain in contact with the liver transplantation physicians.
In patients with liver transplantation and COVID-19 that are hospitalized, the implementation of certain measures is essential for their care because of the greater risk in the healthcare personnel for acquiring COVID-19 disease. The suggested measures for providing care to those patients are the following:
- •
Consider restricting providers with high-risk factors from direct patient care.
- •
Limit the number of personnel to the necessary minimum that can enter the patient’s room.
- •
Consider making telephone calls or utilize virtual conferencing to reduce direct personnel interactions.
- •
Limit the number of visitors to the patient.
- •
Request only the studies that are essential for patient care.
- •
Avoid the transport of patients to different areas of the hospital.
- •
Evaluate the needs of the patient before his/her release to determine his/her follow-up and the appropriate precautions for caregivers or family members.
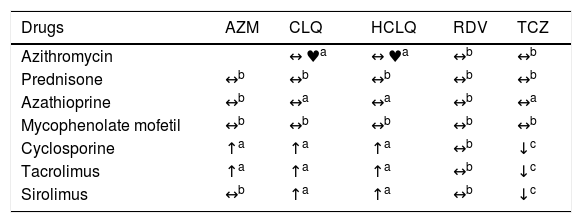
It is always important to investigate the pharmacologic interactions between the different “off-label” therapies utilized to treat patients with COVID-19 and the immunosuppressants used in patients with liver transplantation. Some of those combinations may require the performance of serial ECGs or immunosuppressant dose adjustments,9 as shown in Table 1.
Table of pharmacologic interactions between drugs used in the “off-label” treatment of COVID-19 and immunosuppressants.9
| Drugs | AZM | CLQ | HCLQ | RDV | TCZ |
|---|---|---|---|---|---|
| Azithromycin | ↔ ♥a | ↔ ♥a | ↔b | ↔b | |
| Prednisone | ↔b | ↔b | ↔b | ↔b | ↔b |
| Azathioprine | ↔b | ↔a | ↔a | ↔b | ↔a |
| Mycophenolate mofetil | ↔b | ↔b | ↔b | ↔b | ↔b |
| Cyclosporine | ↑a | ↑a | ↑a | ↔b | ↓c |
| Tacrolimus | ↑a | ↑a | ↑a | ↔b | ↓c |
| Sirolimus | ↔b | ↑a | ↑a | ↔b | ↓c |
AZM: azithromycin; CLQ: chloroquine; HCLQ: hydroxychloroquine; RDV: remdesivir; TCZ: tocilizumab.
♥ One or both medications could cause QT and/or PR prolongation. ECG monitoring is recommended if administered together.
↔ No significant effect.
↑ Possible increase in the levels of co-medication (not of the COVID drug).
↓ Possible decrease in the levels of co-medication (not of the COVID drug).
Source: modified from https://www.hep-druginteractions.org/.
In the INCMNSZ we have adhered to the previously mentioned recommendations. So far, the patients with autoimmune liver disease and COVID-19 infection that we have received at the institution have presented with mild disease, which we have been able to manage solely with support treatment and immunosuppressive treatment readjustment, as established in the recommendations. We have not found signs of reactivation or decompensation of the underlying disease.
At present, the liver transplantation program has been suspended at the national level, eliminating the need for evaluating or listing patients considered priorities. The majority of patient care has been carried out by telephone or telemedicine, reducing the risk of exposure for patients that results from hospital visits. Patients with hepatocellular carcinoma waiting for treatment have been referred to other healthcare institutions, to not postpone their treatment, or they have been treated with radiotherapy, which continues to be functioning at our hospital.
Autoimmune pancreatitisUp to the time the present article was written, there has been no information on the risk for infections in patients with immunosuppression and autoimmune pancreatitis (AIP), much less in the context of the new SARS-CoV-2 coronavirus. However, theoretically, that risk should not be higher than the risk seen with other types of infections. As in other pathologies, whether immunosuppression conditions a higher risk for severe cases of COVID-19 is not known.
Patients with AIP can present with acute pancreatitis (< 20%) but the majority have changes associated with chronic pancreatitis. In our experience, close to 50% present with bile duct strictures or increased pancreatic volume, which are the main indicators for immunosuppression. Similar to other immunologic disorders, initial treatment (induced remission) focuses on rapidly decreasing the inflammatory process and bringing about its remission, which is generally achieved through steroid use. Regarding AIP, the large majority of cases have treatment response and remain in remission after a 12-week course of steroids. A percentage of cases, especially those with extra-pancreatic manifestations, require maintenance treatment based on other immunosuppressants, such as azathioprine, 6-mercaptopurine, and in exceptional cases, rituximab.
It is well-known that the risk for infections with the use of systemic steroids is dependent on the time of administration and the intensity of the dosage.21,22 For inducing remission, adequate selection is required, along with the differentiation of patients that need emergency treatment (obstructive jaundice, abdominal pain) from patients with non-emergency indications (asymptomatic extra-pancreatic lesions, asymptomatic patients with a persistent pancreatic mass in imaging studies).23 Treatment delay, even in asymptomatic pancreatic lesions associated with the gradual development of chronic pancreatitis and exocrine and endocrine insufficiencies, should be considered and discussed with the patient.
Shortened induction regimens with 2 weeks of prednisone administration at a dose of 0.6 mg/kg/day, with later weekly dose reduction, should be preferable options.23 The risk-benefit should always be evaluated upon starting high doses. It is uncertain whether the use of steroids can be useful or harmful. A cytokine storm has been described in severe COVID-19 cases whose intensity could hypothetically be reduced with that type of drug. However, the effect in relation to viral load and replication is also uncertain.24
We believe patients that are in maintenance therapy should continue their treatment with no modifications, given that suspension could cause reactivation of the disease and add to comorbidity for the patient. Changes in therapy regarding the type of drug or dosage should be individualized and preferably postponed.
The severity of SARS-CoV-2 infection and the risk involved in maintaining immunosuppression should always be considered. Nevertheless, in the particular case of AIP, in which maintenance is usually carried out with azathioprine, drug depuration and its immunosuppressive effects tend to occur 3 to 6 months after its suspension and so its effects on immune response are not immediate. However, the dose should be reduced if there is lymphopenia, which is a datum associated with COVID-19 and confers a potentially poor outcome.25
Patients with mild disease on maintenance treatment with a low dose of prednisone (2.5-10 mg/day), azathioprine, or rituximab, could continue with no change to treatment, given that there is no evidence that their use increases the risk for severe infections.22,26–28 Immunoglobulin measurement in patients on maintenance therapy with rituximab is advisable and if levels are found to be reduced, mainly those of immunoglobulin G, intravenous immunoglobulin replacement could be beneficial in reducing mortality.29
Immunosuppressive therapy should be suspended in severe cases, critically ill patients, or if the physicians in charge of the patient consider it pertinent. Systemic steroids should be considered to prevent adrenal insufficiency.
Ethical considerationsThe articles cited herein meet the international regulations on bioethics research and have been authorized by the ethics committees where they were carried out. Likewise, the case series and case reports contain no personal information that can identify the patients.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Miranda-Zazueta G, González-Regueiro JA, García-Juárez I, Moctezuma-Velázquez C, López-Díaz FJ, Pérez-González B, et al. Manejo farmacológico de pacientes con enfermedades hepáticas y pancreáticas que involucran terapias inmunosupresoras. Posicionamiento en el marco de la pandemia de SARS-CoV-2 (COVID-19). Revista de Gastroenterología de México. 2020;85:312–320.