Currently, gastric cancer accounts for 3% of all gastrointestinal tumors. The incidence of gastric signet ring cell carcinoma varies from 3.4% in Japan to 39% in Western countries, affecting yousnger subjects. At present, the intestinal strain has a higher incidence, but it has decreased in recent years. The diffuse subtypes, despite their lower incidence, have had a more marked increase over the last few decades. Calcifications in gastric adenocarcinoma are found in only 5% of cases. The tumors tend to be infiltrating, frequently compromising the lymph nodes, and having a very poor prognosis. In 1913, Gruber1 first reported a calcification in the context of gastric cancer. The number of reports of that entity is rising, given the advances in radiologic techniques. Nevertheless, it continues to be a relatively infrequent entity.
We present herein the case of a 56-year-old woman, with an unremarkable past medical history, that presented with dyspepsia and postprandial pain of 3-month progression.
Due to symptom persistence, upper gastrointestinal endoscopy was performed that identified conspicuous thickening of the gastric wall, especially in the greater curvature. A biopsy sample was taken from the antrum. The pathologic anatomy of the sample was consistent with diffuse gastric signet ring cell adenocarcinoma, and hematoxylin-eosin staining revealed extracellular calcifications.
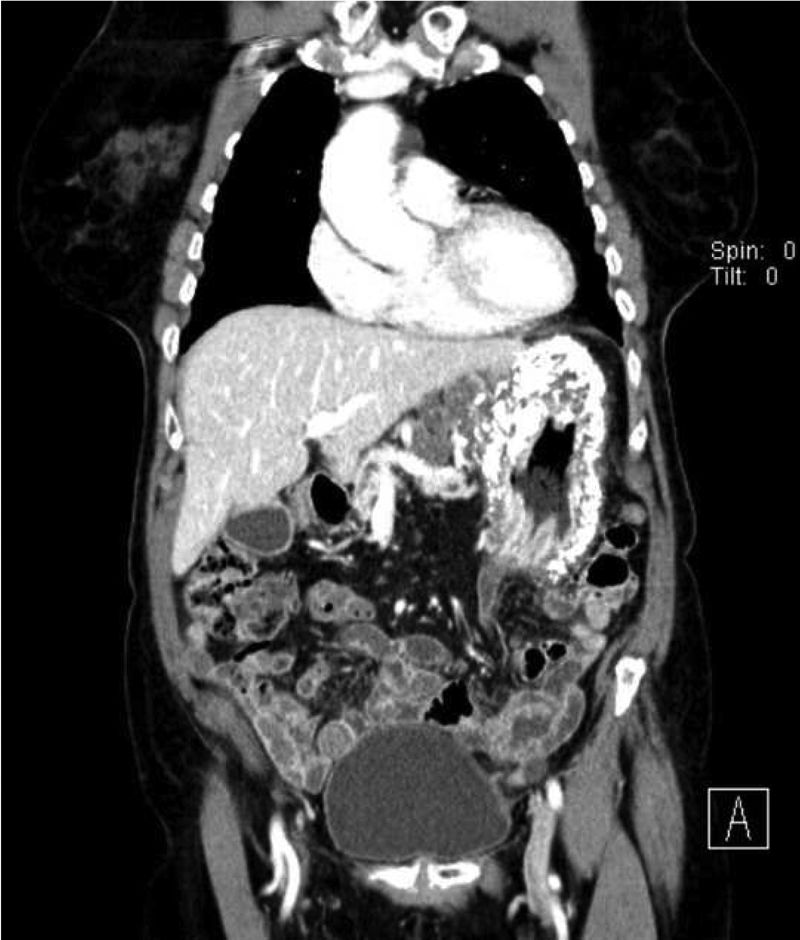
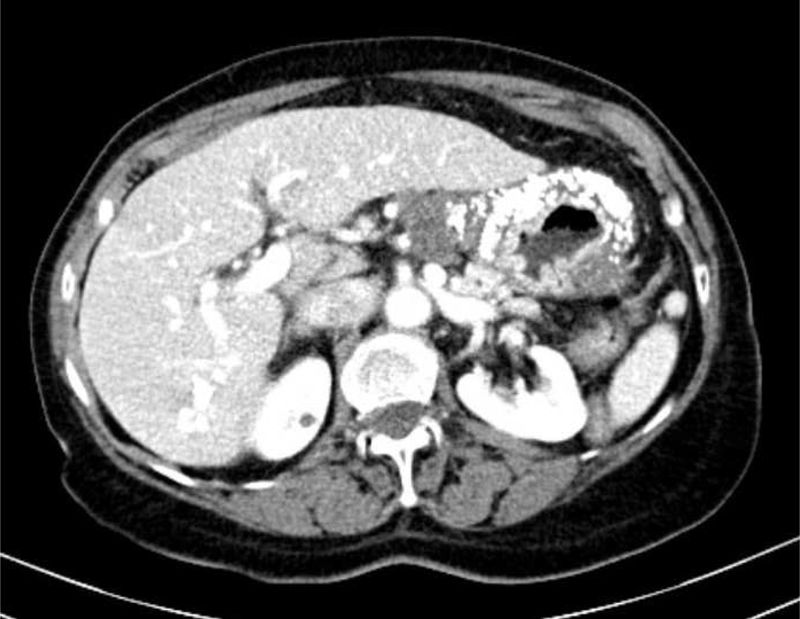
Computerized tomography (CT) showed multiple perigastric adenopathies and marked calcification of the entire gastric wall (Figs. 1 and 2). The patient’s laboratory test results reported elevated CEA, CA 125, and CA 19.9 levels. Calcium and phosphorus levels were not altered.
Initial neoadjuvant treatment was performed, given that the patient had compromised lymph nodes. After 4 cycles of chemotherapy, control CT showed important disease progression, with ovarian metastasis and peritoneal carcinomatosis, rejecting surgery as an option. The calcifications had no changes. Finally, the patient died after 14 months of treatment with palliative chemotherapy (FOLFOX).
There are different histologic classifications of gastric cancer that are not mutually exclusive:
- -
The WHO distinguishes 5 subtypes: papillary, tubular, mucinous, signet ring cell, and undifferentiated.2
- -
Lauren divides them into 2 large groups: intestinal or “well differentiated” and diffuse or “poorly differentiated”. Within the diffuse type, those that produce mucin are classified according to location: intracellular, corresponding to the signet ring cell subtype, and extracellular, corresponding to the mucinous subtype, as classified by the WHO.3 The second of those two subtypes needs a percentage of extracellular mucin above 50% to meet the WHO definition.
- -
Ming established 2 large groups: expanding and infiltrative. The mucinous type is included in the infiltrative subtype, given that it is proposed to be a variant of the signet ring cell subtype.
Calcification in gastric cancer belongs to a complex pathophysiology that is not completely understood. Said calcifications have been described as being induced by macrophage-produced osteopontin.4 In theory, 3 types of calcification are distinguished: coarse or heterogeneous; punctate or mucinous; and psammomatous.5
A priori, coarse calcification is larger and produced by dystrophic changes in a context of necrosis, bleeding, and inflammation that leads to the tissue degeneration inherent in hypoxia and tissue ischemia due to tumor size.6,7
On the other hand, punctate calcifications are posited to be produced in areas of ischemia and necrosis created by the tissue destruction due to rapid tumor growth or secondary to treatment with chemotherapy. In those cases, the environment or extracellular matrix has a high pH that perpetuates its alkalinity due to the presence of a carbon dioxide-rich hypoxic atmosphere. The denatured proteins from the alkaline environment preferably bind to free phosphate ions. At the same time, the glycoproteins and mucoproteins of that matrix act as ion exchangers in such a way that calcium is accumulated in the interior of the matrix, which when binding to phosphates, causes calcification of the matrix, giving rise to “mucin pools”.8 The levels of calcium and phosphate are normal in that type of calcification. Likewise, psammomatous calcification is associated with non-mucin producing intestinal tumor strains. In the abdomen, several types of tumors, such as the mucin-producing type and pseudopapillary neoplasias, frequently have intramural calcification.
The literature appears to describe a morphologic pattern in calcified gastric cancer that is not very constant in relation to the histologic type of tumor. The mucinous calcifications seem to form disperse calcifications that are punctate and small, albeit cases with large and isolated calcifications have been described. GIST tumors tend to calcify more coarsely.9 Based on that, authors, such as Zhao et al.,10 have suggested the complementary use of certain radiologic findings for making the diagnosis. In their case series of 166 patients with gastric tumors, they found that calcifications in CT predicted a mucinous gastric tumor over a non-mucin-producing tumor, with > 80% accuracy, high specificity (98.7%), and low sensitivity (33.3%). CT is a useful tool that can provide additional diagnostic information about the gastric lesions, when using water as an oral negative contrast medium, together with an intravenous contrast agent. We identified a signet ring cell histologic subtype, but the calcification pattern could also be considered more characteristic of a mucinous type. Isotopes (metastable technetium 99, 3-diphosphone-1, or 1-propanodicarboxylic) can sometimes be useful as bone markers, although their uptake in calcified gastric cancer is very infrequent. Despite that described above, we must not forget that the associations of the types of calcifications with histologic subtypes of gastric carcinoma are not reproducible and should not be used to make the differential diagnosis. Likewise, there are few autopsy case reports that describe the radiology-pathology correlation in calcified mucinous adenocarcinoma of the stomach.
In conclusion, our patient presented with diffuse signet ring cell gastric carcinoma, some of whose calcifications were characteristic of the mucinous subtype in the CT scan.5 Based solely on the present case and the variability of calcifications in the context of gastric tumor diseases, it is reasonable to think of the mucinous subtype and signet ring subtype as distinct entities at the practical level, but in reality, as the two extremes of the same entity, moving us closer to the position defended by Ming. Further studies are needed, not only at the anatomopathologic level, but also at the molecular level, to be able to determine the fine line that separates the tumor strains in gastric cancer.
Ethical considerationsNo intervention was performed on the patient for carrying out the present work, limiting us to describe the clinical process. The patient’s privacy has been protected at all times and none of the data or images contained herein can identify her.
Financial disclosureNo financial support from any private or public agency or company was received in relation to the present report.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Navarro-Martínez S, Payá-Llorente C, Martínez López E, Trullenque Juan R, Armañanzas Villena E. ¿Son los patrones radiológicos concordantes con la anatomía patológica en el cáncer gástrico calcificado? Revista de Gastroenterología de México. 2021;86:104–106.