So far, available evidence suggests that patients with inflammatory bowel disease (IBD) are not at greater risk for developing COVID-19 infection. In regard to patients with IBD remission: 5-aminosalycylates (5-ASAs) do not increase the risk for infection and should be continued. There is no need to suspend them or lower the dose. Immunomodulating drugs, such as thiopurines and methotrexate, should be continued, without modifying doses (even in patients with positive SARS-CoV-2 infection). No type of biologic therapy should be suspended, unless there are signs of COVID-19. Regarding patients with IBD activity: the oral and/or topical 5-ASA dose should be optimized in cases of disease relapse. Budesonide MMX should be considered in cases of mild-to-moderate activity, to avoid systemic steroid use. Systemic steroids should be avoided whenever possible because doses above 20 mg per day have an immunosuppressive effect, which could increase susceptibility to any type of infection, including COVID-19. The combined use of thiopurines with steroids and/or tumor necrosis factor (TNF) monoclonal antibodies should also be avoided because those combinations can increase the risk for infections, including COVID-19. Finally, biologic treatment with anti-TNF-alpha agents or any other mechanism of action, such as anti-integrins or anti-interleukins, should be suspended if patients become infected with SARS-CoV-2. The drugs can be restarted once the infectious process is resolved.
Hasta este momento, la evidencia disponible sugiere que los pacientes con enfermedad inflamatoria intestinal (EII) no tienen un mayor riesgo de desarrollar COVID-19. En pacientes con remisión de la EII: los 5-aminosalicilatos (5-ASA) no incrementan el riesgo de infección y se deben continuar sin necesidad de suspenderlos o disminuir la dosis; los fármacos inmunomoduladores como las tiopurinas y metotrexato deben continuarse sin modificar las dosis (a considerar en el paciente con infección por SARS-CoV-2 positiva); y en relación con cualquier tipo de terapia biológica, no deberá suspenderse a menos que existan datos de COVID-19. En aquellos pacientes con actividad de la EII: se deberá optimizar la dosis de 5-ASA en administración oral y/o tópica en caso de recaída de la enfermedad; considerar el uso de budesonida MMX en casos de actividad leve a moderada con el fin de evitar el uso de esteroides sistémicos; los esteroides sistémicos deben evitarse en la medida de lo posible, ya que dosis mayores de 20 mg al día ejercen un efecto inmunosupresor el cual pudiera aumentar la susceptibilidad a cualquier tipo de infección, incluyendo COVID-19; evitar el uso combinado de tiopurinas con esteroides y/o agentes anti-TNF ya que puede incrementar el riesgo de infecciones, incluyendo COVID-19; y finalmente, el tratamiento biológico con agentes en contra del factor de necrosis tumoral alfa (anti-TNF alfa) o de cualquier otro mecanismo de acción como antiintegrinas o antiinterleucinas deberán suspenderse en caso de documentarse infección por SARS-CoV-2 y podrán reiniciarse en cuanto haya cedido el proceso infeccioso.
In December of 2019, a new type of pneumonia caused by a novel member of the family Coronaviridae, called SARS-CoV-2, was identified in the city of Wuhan, China.1 The phylogenic analysis showed it to be a different virus, with an ∼80% similarity to the nucleotides of SARS-CoV-1.2 The disease caused by SARS-CoV-2 is characterized by dry cough, fever, dyspnea, fatigue, and lymphopenia.3–6 The more severe cases (apparently up to 15-20% of infected patients) can become more complicated, with infected patients developing interstitial pneumonia that can clinically lead to acute respiratory distress syndrome (ARDS), as well as death.7 Since the initial outbreak, the epidemic has rapidly propagated worldwide, and on January 30, 2020, the World Health Organization (WHO) declared the disease, now called COVID-19, an international public health emergency, and on March 11, 2020, declared it a pandemic. The epidemiologic panorama is constantly evolving and data from April 6, 2020, show that 184 countries are affected, with more than 1,213,973 confirmed cases and 67,841 confirmed deaths worldwide.8
Given the lack of specific antiviral therapies, the current treatment for COVID-19 consists mainly of support measures, despite the fact that several compounds are being studied for treating this potentially fatal infection.
The COVID-19 pandemic is most certainly conditioning the treatment strategy of inflammatory bowel disease (IBD), given that it is pertinent to think that there is an increased risk for infection, compared with the general population, due to a general deterioration of the immune system that is typical of autoimmune diseases, combined with the iatrogenic effect produced by the use of corticosteroids and immunosuppressive drugs.9 However, at present, the risk of infection with SARS-CoV-2 in patients with IBD and immunosuppressive treatment does not appear to differ from that of the general population. Since the beginning of the pandemic, few cases of patients with IBD plus SARS-CoV-2 infection have been reported. The majority of those cases have had favorable clinical outcomes, suggesting a potential “protective” effect from the anti-inflammatory therapy utilized in those patients, without being able to establish actual causality.10
In the present review, we critically analyze the evidence on the positive or negative effects of the medications commonly used in the treatment of IBD, as well as the management of the disease in its different grades of activity, to optimize the approach to patients with IBD in the current setting (COVID-19 disease).
Inflammatory bowel disease and risk of SARS-CoV-2 infectionCoronaviruses are the largest viruses with a positive-sense single-stranded RNA genome. On the one hand, the host immune response is essential for resolving the SARS-CoV-2 infection, but it can also be crucial for the pathogenesis of the main clinical manifestations of the disease. Angiotensin-converting enzyme 2 (ACE2) has been identified as the host cell entry receptor for the envelope glycoprotein of SARS-CoV-2.11 ACE2 is a type I membrane protein expressed in the cells of the kidney, heart, gastrointestinal tract, blood vessels, and more importantly, the AT2 alveolar epithelial cells of the lung, which are particularly prone to viral infection.12 The infection with SARS-CoV-2 leads to the negative regulation of ACE2 expression, resulting in excess angiotensin II production by the ACE-related enzyme. Stimulation of the type 1a angiotensin II receptor (AGTR1A) has been suggested to increase pulmonary vascular permeability, which could explain the increase in lung damage when ACE2 expression decreases.13 Due to that mechanism of action, individuals that have diabetes mellitus or hypertension that take ACE inhibitors or angiotensin receptor blockers have been postulated to possibly have an increased risk for COVID-19 infection, as well as increased disease severity.14 At present, there is little evidence to support that hypothesis and the European Society of Cardiology recently published a statement highly recommending the continuation of those treatments, in spite of the current pandemic.15
The analysis of the distribution of SARS-CoV-2 in the different biologic samples of patients with COVID-19 showed that up to 50% of stool samples were positive.16–18 In addition, stool samples remained positive in more than one-fifth of the patients, after having had negative results in respiratory samples.17 The findings might explain why some patients with COVID-19 experience gastrointestinal symptoms and would imply that SARS-CoV-2 can be propagated through the fecal route. ACE2 expression increases in the inflamed intestine of patients with IBD.19 Furthermore, the proteomic analysis of tissue samples from patients with IBD revealed significantly higher ACE2 expression in Crohn’s disease (CD) than in ulcerative colitis (UC).20 As stated above, together with binding to ACE2, the fusion of the coronavirus envelope with the host cell membranes is critical for establishing successful infection. That process is mediated by a specific fusion21 that is activated through a proteolytic excision induced by trypsin-like proteases of the host cell. That mechanism has been reported to be overexpressed in IBD.22 Those observations suggest that the inflamed bowel in patients with IBD is an optimal door through which the virus enters into human tissue. However, no evidence has been found that would suggest that COVID-19 affects patients with IBD more frequently than the general population, and no patient with IBD and infected by SARS-CoV-2 has yet to be reported in the referral centers for IBD care in Wuhan.18
In vitro studies have shown that soluble ACE2 may act as a competitive interceptor of SARS-CoV-2 by preventing the binding of the viral particle to the ACE2 expressed in the surface of the cell.23 In particular, there is an increased level of ACE2 in the peripheral blood of patients with IBD,24 increasing the possibility that said isoform could contribute to limiting infection with SARS-CoV-2.
Even though SARS-CoV-2 is detectable in stool,16 there is no clear evidence that the ACE2 content in the ileum and colon influences entry and replication of the virus in the intestinal cells, and as a result, facilitates its transmission by a pathway other than the respiratory route. SARS-CoV-2 might need additional factors, not yet identified, that promote cell binding, to guarantee host cell infection. That proposal is viable because there is evidence supporting the rapid propagation of SARS-CoV-2 through the respiratory route, in spite of the modest expression of ACE2 in the upper respiratory tract.25
Another aspect relevant to COVID-19 infection in IBD is related to the current therapy employed for treating the disease, given that many patients are taking immunomodulators (e.g., azathioprine, methotrexate) to stay in remission, as well as to prevent complications associated with IBD. The use of such compounds has been associated with a greater risk of infections because they block the intracellular signals needed by the host to fight against pathogens.26 On the other hand, it is notable that the suppression of the inflammatory response driven by effector cytokines in IBD (e.g., using cytokine blockers) could be beneficial, not only for attenuating the continuous inflammation of the mucosa, but also for preventing COVID-19-associated pneumonia.
The currently available evidence suggests that patients with IBD are not at greater risk for developing COVID-19 and should continue taking their medications as prescribed. Patients that take immunosuppressants should be carefully controlled to detect signs and/or symptoms suggestive of COVID-19. In addition, patients above 60 years of age and/or with comorbidities (e.g., coronary disease, hypertension, diabetes mellitus, pulmonary disease, cerebrovascular diseases) should adhere to the general prevention indications (staying home, avoiding public gatherings, frequent handwashing, not touching the face without having disinfected the hands, etc.).18,27,28
General considerations in inflammatory bowel disease managementInflammatory bowel disease in remission27–30- o
The 5-aminosalicylates (5-ASAs) do not increase the risk for infection. They should be continued with no need for suspension or dose reduction.
- o
Immunomodulators (thiopurines and methotrexate) could be associated with an increased risk for viral infections, but their suspension or reduced dose is not recommended, given the risk for exacerbating IBD.
- o
Biologic therapy with anti-TNF-alpha, vedolizumab, and ustekinumab should be continued and drug administration regimens should not be modified for the purpose of preventing infection with SARS-CoV-2.
- o
All patients undergoing immunosuppressive therapy with calcineurin inhibitors, such as oral cyclosporine or tacrolimus, should continue their use.
- o
Treatment with tofacitinib should not be suspended, nor should the dose be reduced, for the purpose of preventing infection with SARS-CoV-2.
- o
If the patient develops COVID-19 disease, the recommendation is to suspend treatment based on systemic steroids (doses greater than or equal to 20 mg of prednisone daily), immunomodulators, therapies with anti-TNF-alpha, ustekinumab, and tofacitinib, with the exception of the 5-aminosalicylates, and most likely, vedolizumab.
- o
- o
Oral and/or topical 5-ASA dose should be optimized in cases of disease relapse.
- o
Consider using budesonide MMX in cases of mild-to-moderate activity, to avoid systemic steroid use.
- o
Systemic steroids should be avoided as much as possible because doses above 20 mg per day have an immunosuppressive effect that could increase the risk for infection with SARS-CoV-2.
- o
Avoid the combined use of thiopurines with steroids and/or anti-TNF agents because it can increase the risk for infections, including SARS-CoV-2.
- o
In cases that have tested positive for SARS-CoV-2 or have developed COVID-19 disease, the recommendation is to suspend systemic steroids, immunomodulators, anti-TNF therapy, ustekinumab, and tofacitinib. They can be restarted 14 days after the infectious process has ended.
- o
The decrease in circulating CD4+ T lymphocytes in peripheral blood has been associated with a longer elimination time of the virus (SARS-CoV-2) and a more severe course of the disease (COVID-19), therefore it can be reasonable to suspend thiopurines in suspected cases of infection with SARS-CoV-2. That indication should be considered on an individual case basis.
- o
COVID-19 disease progresses (toward recovery or death) in approximately 3-4 weeks. In theory, temporary interruption of immunosuppressive therapy should not have an impact on the risk for an IBD flare.
- o
In cases of severe relapse of UC, the risk of developing infection with SARS-CoV-2 would be lower with the use of cyclosporine or infliximab, than with intravenous systemic steroids.
- o
In patients with UC that have uncontrolled symptoms, the oral dose of 5-ASA should be optimized ± the addition of topical 5-ASA (rectal).
CorticosteroidsCorticosteroid use should be avoided as much as possible, and patients whose dose of prednisolone is ≥ 20 mg per day should be closely monitored. The gradual reduction of prednisone (10 mg/week) should be considered, whenever possible, and the risks of extending steroid exposure in general should be balanced against reducing the dose too quickly. Consider using budesonide MMX (9 mg/day for 8 weeks) or beclomethasone (5 mg/day for 4 weeks) for relapse in UC patients (important to evaluate after 2 weeks). Exclusive enteral nutrition should always be preferred in patients with CD relapse. Consider using budesonide (9 mg/day for 8 weeks) for active CD in the terminal ileum or the ileocecal location.
Immunomodulators (azathioprine, 6-mercaptopurine, methotrexate)Beginning monotherapy with an immunomodulator is not recommended. Therapy combined with biologic treatment should be carried out with extreme care, and the risk-benefit of each case evaluated individually. Older adult patients (> 65 years of age) or young adult men under 30 years of age, or those with a significant comorbidity that are in sustained remission with thiopurines, should be considered for monotherapy after adequate discussion with the IBD medical team.
As stated above, the decrease of circulating CD4+ T lymphocytes in peripheral blood has been associated with a longer elimination time of the virus (SARS-CoV-2) and a more severe course of the disease (COVID-19), making it reasonable to suspend thiopurines in suspected cases of SARS-CoV-2 infection.
Anti-TNF therapy (adalimumab, infliximab, golimumab)Consider beginning monotherapy (thus, consider adalimumab to promote at-home attention and reduce the risk for infliximab-related immunogenicity). Utilize early therapeutic monitoring of medications when possible, emphasizing those that are appropriate for later combined immunosuppression, when necessary. Anti-TNF therapy should not be suspended in patients with IBD, to prevent infection with SARS-CoV-2. The doses and administration intervals should be maintained. At present, the administration of intravenous infusions has been shown to be safe in hospitals where patients with COVID-19 disease are being treated.
A different regimen can be considered for patients that start a new biologic product. In such cases, subcutaneous medications may be preferred, together with a remote educational program for the patient and home delivery service. Due to the lack of scientific evidence on the elective change in patients with UC, therapy with infliximab should not be changed to adalimumab or golimumab.
Anti-IL-12/23p40 therapy (ustekinumab)There is no evidence of greater risk for infection with SARS-CoV-2. An advantage of ustekinumab is the single administration of the intravenous induction dose, followed by subcutaneous maintenance dosing.
Anti-α4β7 integrin therapy (vedolizumab)There is no evidence of greater risk for SARS-CoV-2 infection. It is unlikely that it increases the risk for complications of COVID-19, although applying the information from existing trials regarding COVID-19 should be done with caution. The GEMINI study showed that randomized patients undergoing treatment with vedolizumab vs. placebo stayed in remission up to week 24, thus administration of the drug could be continued in patients with IBD.
Janus kinase inhibitors (tofacitinib)There is no evidence of a greater risk for SARS-CoV-2 infection. However, patients undergoing treatment with tofacitinib require rigorous monitoring. If SARS-CoV-2 infection or the development of COVID-19 disease is reported, the drug should be suspended for up to 2 weeks after the infection has resolved.
Surgery and inflammatory bowel diseaseElective surgeries in patients with IBD have been postponed at the majority of hospital centers. Whenever possible, emergency treatment of perianal sepsis should be performed as an outpatient procedure. Complex IBD surgery should be postponed whenever possible and its scheduling should be regularly reviewed at meetings with the multidisciplinary team of IBD specialists. Emergency procedures (e.g., subtotal colectomy in severe UC, bowel resection to control stricturing disease in CD) will continue to be handled as part of routine care. As in cases of active disease, the selection of postoperative therapy to prevent recurrence should be considered in the context of the COVID-19 pandemic.30,31
Opportune surgery is the other cornerstone of IBD care and the suspension of elective surgery in patients with IBD that were indicated for a procedure (allowing only oncologic cases to undergo elective surgery or emergency surgery) is very concerning, given that it could result in a higher number of emergency presentations and more complications, as a consequence of the delay in surgical treatment.27,30 In the region of Milan, Italy, where elective surgery (including for IBD) has been substantially reduced or detained in the last 3 weeks, with the prospect of more weeks of delay or cancellation, there is important concern about possible disease progression and worse surgical outcomes, once they are performed, in patients with IBD.27
Patient follow-up and healthcare unit reorganizationThe interruption of the routine check-ups in medical follow-up has caused anxiety in both the patients with IBD and the treating medical personnel. However, patients should be reminded that said interruption is temporary and that remote consultations can help the patients and medical care providers avoid potential follow-up loss when clinical problems present. That approach has also been started by IBD specialists in China during the COVID-19 outbreak.27
The current travel restrictions and recommendations of the health authorities to stay home have caused great concern in relation to the functioning of clinics and outpatient infusion centers for the administration of biologic therapy. At present, those centers have remained functioning in different countries, under strict norms to prevent outbreaks of infection, such as having control points at the entrance of the hospital where, before entering, the patient is examined and asked if he/she has had cough or fever or been in contact with persons having those symptoms within the 2 weeks prior to his/her hospital visit; when receiving their infusions, patients are seated at a distance of 1-2 m from each other; and surgical masks are worn by the patients and the personnel of the clinic, and the latter wear latex gloves.27
The guidelines for performing endoscopic procedures in the context of the current pandemic have been established by different gastrointestinal endoscopy societies.32,33 Surveillance procedures for IBD should be postponed. Each specific case of IBD that could require an endoscopic procedure should be carefully evaluated to determine priority. Alternative disease evaluation methods, including the use of biomarkers, radiology, and capsule endoscopy, should be considered.30
The capacity to carry out laboratory and imaging studies may become reduced. Nevertheless, each hospital center should individually discuss the operation of their clinical analysis and x-ray laboratories. Limitations may vary between hospital centers during the pandemic, affecting the study of patients with IBD.30
With respect to clinical trials, many have already been put on hold by their sponsors. If that is not the case, the examination of the participants, as well as their recruitment and follow-up, should be reviewed at the local level to determine the suitability of the current clinical situation. The benefits of substituting surgery and/or corticosteroid use with test medications, that under different circumstances would not be available, must be compared with the risk inherent in personal visits and the unknown effects of the test medications during the course of COVID-19. Whenever possible, test visits should be carried out remotely and research that requires hospital care should be postponed unless it is clinically important. The relevant regulatory agencies should be advised of amendments to the protocol and immediate participant protection must be resolved with the lead coordinators of the clinical trials, given that formal approval can be significantly delayed. Sponsors should consider reducing administrative tasks, during the time of expanded healthcare team responsibilities due to the pandemic, considering the fact that many research team members will be redistributed to provide direct clinical care.30,34
Inflammatory bowel disease experience at other centersThe study group of Ping An et al. in Wuhan, China, affirms that, from the time the first case of COVID-19 was reported, the healthcare personnel of the center remained alert and began to send out educational and instructive information concerning preventive measures for COVID-19. There were 318 registered IBD patients (204 with UC and 114 with CD) 20 days before Wuhan went into lockdown. All the patients received the opportune warnings and took the appropriate preventive actions, and from December 8, 2019 to February 18, 2020, none of the patients with IBD had presented with COVID-19 infection.35
Norsa et al.36 reported an IBD follow-up of 522 patients in Bergamo, Italy. Fifty-nine percent of the patients were in treatment exclusively with 5-ASAs; 22% received immunosuppressive therapy with thiopurines, methotrexate, steroids, or others; and 22% were treated with biologic therapy (infliximab, adalimumab, vedolizumab, ustekinumab, and golimumab). Up to March 23, 2020, the authors concluded that none of their patients with IBD were affected by SARS-CoV-2-related pneumonia and advised all their patients to continue their customary treatment regimen.
In Milan, Italy, Fiorino et al.37 instructed all their patients with IBD to continue with their current medical therapies, especially if they were in remission. They recognized the controversial nature of steroid use during COVID-19 but stated that low doses and short-term steroids were not associated with a worse outcome, even in patients with critical COVID-19 pneumonia. Although it is known that thiopurines and JAK inhibitors can decrease the number of activated T cells, Fiorino et al. did not recommend suspending the treatments with those agents in patients in remission and advised them to strictly follow the preventive indications. Those authors did not provide data in favor of or against the use of monoclonal antibodies.
At the IBD clinic of the Instituto Nacional de Ciencias Médicas y Nutrición in Mexico City, the team of Yamamoto-Furusho et al. has postponed face-to-face consultations in IBD patients in remission and provided follow-up by telephone to make a treatment adjustment or provide guidance about COVID-19 in relation to their disease. On the other hand, patients with clinical signs of moderate-to-severe activity that are very well selected are given a face-to-face appointment to rule out an infectious process as the cause of relapse, or to adjust medical treatment. Surgical procedures in IBD have been postponed until the end of phases 2 and 3 of the COVID-19 contingency plan.
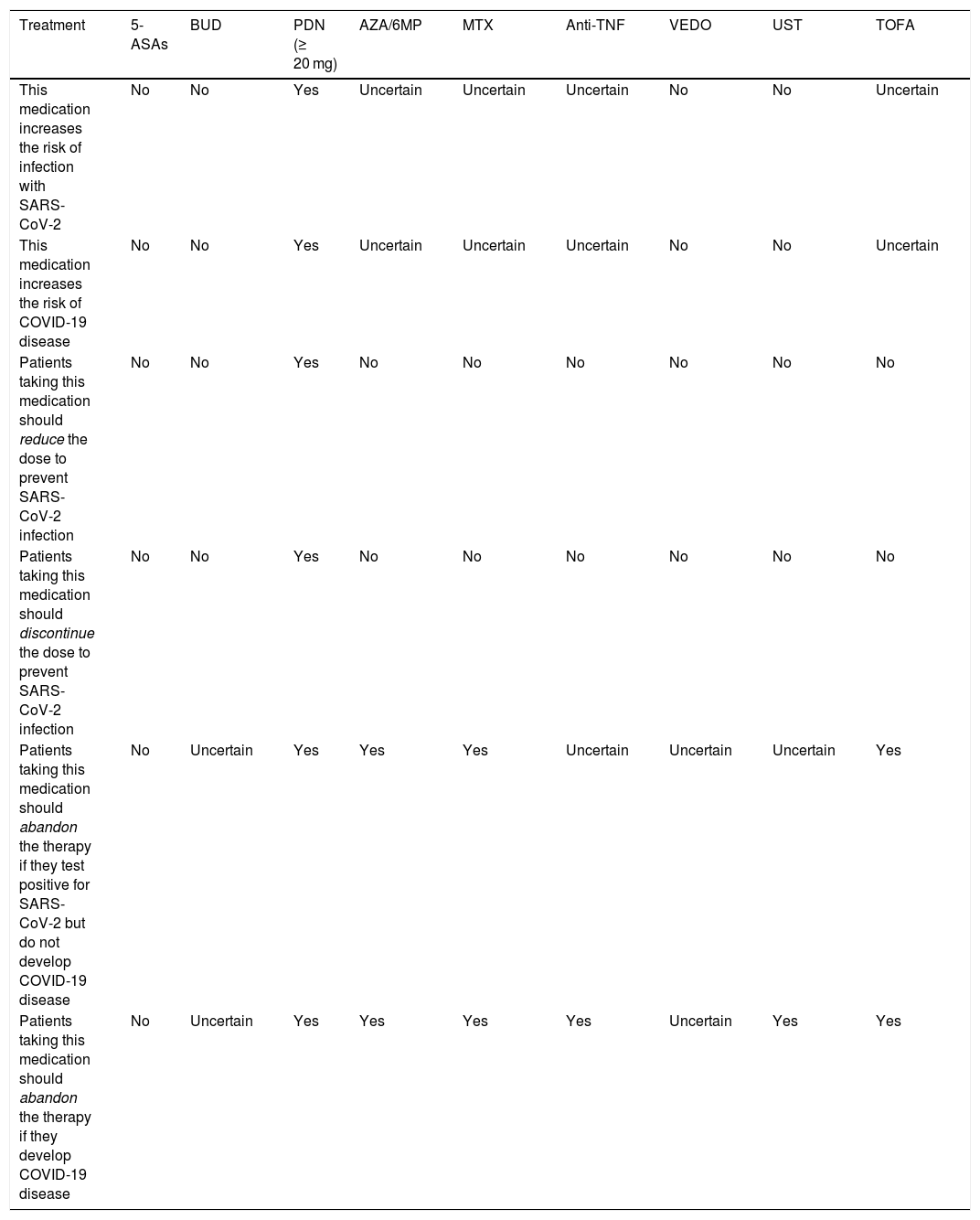
Finally, Table 1 summarizes the recent recommendations of the International Organization for the Study of Inflammatory Bowel Diseases.38
Medication use recommendations for treating IBD in the context of SARS-CoV-2 or COVID-19.
| Treatment | 5-ASAs | BUD | PDN (≥ 20 mg) | AZA/6MP | MTX | Anti-TNF | VEDO | UST | TOFA |
|---|---|---|---|---|---|---|---|---|---|
| This medication increases the risk of infection with SARS-CoV-2 | No | No | Yes | Uncertain | Uncertain | Uncertain | No | No | Uncertain |
| This medication increases the risk of COVID-19 disease | No | No | Yes | Uncertain | Uncertain | Uncertain | No | No | Uncertain |
| Patients taking this medication should reduce the dose to prevent SARS-CoV-2 infection | No | No | Yes | No | No | No | No | No | No |
| Patients taking this medication should discontinue the dose to prevent SARS-CoV-2 infection | No | No | Yes | No | No | No | No | No | No |
| Patients taking this medication should abandon the therapy if they test positive for SARS-CoV-2 but do not develop COVID-19 disease | No | Uncertain | Yes | Yes | Yes | Uncertain | Uncertain | Uncertain | Yes |
| Patients taking this medication should abandon the therapy if they develop COVID-19 disease | No | Uncertain | Yes | Yes | Yes | Yes | Uncertain | Yes | Yes |
Anti-TNF: anti-tumor necrosis factor; AZA: azathioprine; BUD: budesonide; COVID-19: coronavirus disease; MTX: methotrexate; PDN0: prednisone; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TOFA: tofacitinib; UST: Ustekinumab; VEDO: vedolizumab; 5-ASAs: 5-aminosalicylates; 6MP: 6-mercaptopurine.
Source: Rubin et al.38.
The COVID-19 pandemic has modified hospital care schemes, affecting the diagnosis, treatment, and follow-up of different pathologies, including IBD. The multidisciplinary teams that treat patients with IBD must adapt the current patient approach strategies to ensure the minimum standard of quality of care. Conventional treatment (with the exception of steroids at doses above 20 mg per day), biologic therapy, and small molecule inhibitors should not be suspended in patients with IBD, to prevent SARS-CoV-2 infection. In patients that test positive for SARS-CoV-2 or develop COVID-19 disease, therapy with steroids above 20 mg per day and biologic therapy based on anti-TNF drugs, ustekinumab, and tofacitinib will need to be suspended; vedolizumab is an exception because it has a local mechanism of action.
Ethical considerationsNo patients participated in the present study, nor were patient data utilized, therefore patient informed consent was not needed. Likewise, given that no interventions, maneuvers, or information management were carried out, the study was considered low risk and did not require review or approval from the local ethics committee. Nevertheless, the study follows the current research norms and guarantees the confidentiality of personal and identification data, as well as the anonymity of the participants (all health workers that participated voluntarily). The present article contains no personal information that can identify the participants.
Financial disclosureNone.
Conflict of interestDr. Jorge de Léon and Dr. Carlos Hurtado declare they have no conflict of interest.
Dr. Jesús Kazuo Yamamoto Furusho is a member of the Advisory Board, an opinion leader, and speaker for Abbvie Laboratories Mexico, Abbvie (international), Takeda (international), Takeda Mexico, Pfizer (international and regional), Janssen Cilag (international) and Janssen Cilag Mexico. He is an opinion leader and speaker for Farmasa, Ferring, and Farmasa Schwabe and a research consultant for UCB Mexico. He has received research funding from Shire, Bristol Myers Squib, Pfizer, Takeda, and Celgene laboratories.
Please cite this article as: de León-Rendón JL, Hurtado-Salazar C, Yamamoto-Furusho JK. Aspectos y consideraciones generales en la enfermedad inflamatoria intestinal durante la pandemia por COVID-19. Revista de Gastroenterología de México. 2020;85:295–302.