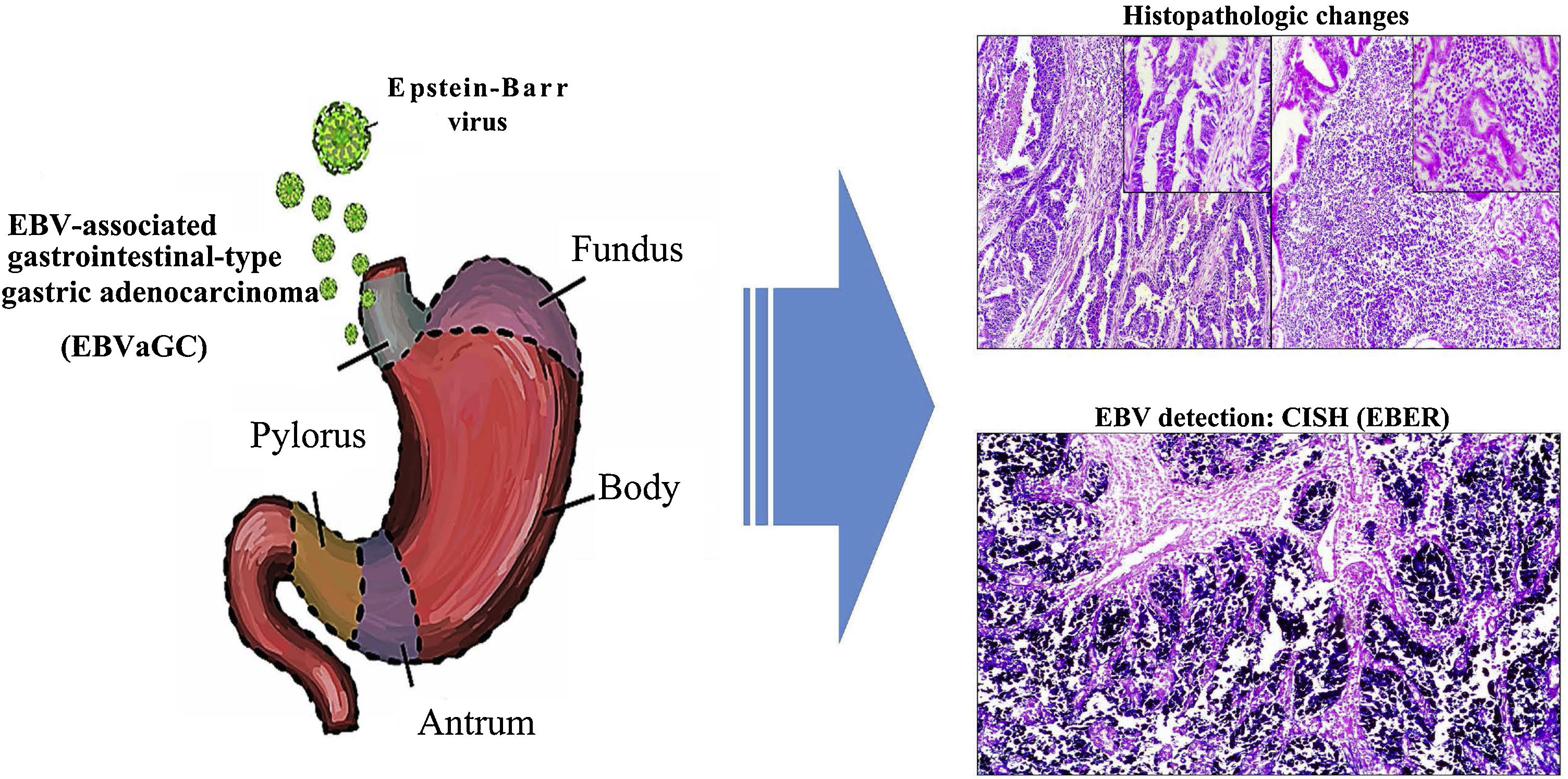
Epstein-Barr virus (EBV) infection is an etiologic factor in EBV-associated gastric carcinoma (EBVaGC). The aim of our study was to describe the clinical and histopathologic characteristics of EBV infection in intestinal-type gastric adenocarcinoma samples.
Material and methodsOf 180 paraffin-embedded gastrectomy samples, 28 were studied. Chromogenic in situ hybridization was performed to detect EBV. Sociodemographic and histopathologic data were obtained from the patients’ clinical histories.
ResultsA total of 21.4% of the samples were positive for EBV. The predominant morphologic characteristic was the lace pattern, with dense inflammatory infiltration. Fifty percent of the EBVaGC+ patients were men, and the median age of the positive patients was 59 years (range: 50–75); 77.2% of the EBVaGC− patients were men, and the median age of the negative patients was 66 years (range: 34–89). Helicobacter pylori infection was associated with 10.7% of the EBVaGC+ patients and 53.6% of the EBVaGC− patients. In the EBVaGC+ patients, the cardia was the most frequent tumor location (17.9%), 7.1% had histologic grades 2 and 3, and 17.9% presented with Borrmann classification type III. In the EBVaGC− patients, the cardia and fundus were the most frequent tumor locations (71.4%), 35.7% had histologic grade 2, and 39.3% and 21.4% presented with Borrmann classification type III and IV, respectively.
ConclusionsThe present study describes the clinical and histopathologic characteristics associated with EBVaGC positivity. Those data may aid in the selection of cases that are candidates for analysis through molecular methods aimed at identifying EBV infection in intestinal-type gastric adenocarcinoma.
La infección por virus de Epstein-Barr (VEB) es un factor etiológico de un subgrupo de cáncer gástrico (CaGVEB). El objetivo del estudio fue caracterizar la clínico-histopatología de la infección por VEB en muestras de adenocarcinoma gástrico de tipo intestinal.
Material y métodos28 de 180 muestras de gastrectomías parafinizadas fueron estudiadas, se realizó hibridación in situ cromogénica para reconocimiento del VEB. Se obtuvieron datos sociodemográficos e histopatológicos de historias clínicas.
Resultados21.4% de las muestras fueron positivas para VEB. La característica morfológica predominante fue patrón en encaje con denso infiltrado inflamatorio. De los pacientes CaGVEB+ 50% eran hombres, mediana de edad 59 años (rango 50–75), y de los CaGVEB−, 77.2% eran hombres, mediana de edad 66 años (rango 34–89). 10.7% de los CaGVEB+ y 53.6% de los CaGVEB− se asociaron a infección por Helicobacter pylori. Entre los CaGVEB+, la localización del tumor más frecuente fue cardias (17.9%) y entre los CaGVEB− fue cardias y fondo (71.4%). En la clasificación según grado histológico, los CaGVEB+ se asociaron a grados 2 y 3 (7.1%), y los CaGVEB- a grado 2 (35.7%). Para la clasificación Borrmann, 17.9% de los CaGVEB+ presentaron clasificación III, mientras que 39.3% y 21.4% de CaGVEB− presentaron clasificación III y IV, respectivamente.
ConclusionesEste estudio presenta características clínico-histopatológicas asociadas a CaGVEB+ que pueden contribuir con la selección de casos candidatos a ser estudiados por métodos moleculares dirigidos a la identificación de la infección por virus de Epstein-Barr en adenocarcinoma gástrico de tipo intestinal.
Gastric cancer (GC) is one of the most frequent malignant neoplasms. According to the 2018 GLOBOCAN project, GC held fifth place in incidence, with 1,033,701 cases, and third place in mortality (782,685 deaths). In Colombia, a total of 7419 new cases of GC and 5505 deaths are reported1. In the Colombian Southwest, where the GC rate is high2, the Department of Cauca held fourth place in incidence, with 26.8 cases in men and 16.9 in women (age-adjusted rate per 100,000 person-years) for the time frame of 2007–2011 and a total of 1426 deaths between 2007 and 20133,4.
Among the elements related to GC are genetic factors5, biologic factors, such as infection by the Helicobacter pylori (H. pylori) bacterium and the Epstein-Barr virus (EBV)6, and lifestyle-associated factors7,8. In addition, the presence of premalignant lesions in the gastric mucosa, such as atrophy of the gastric mucosa or intestinal metaplasia, whether caused by infection or autoimmune or atrophic gastritis, has also been related to progression to a carcinogenic process9.
Based on its histopathologic characteristics, GC can be classified as intestinal-type gastric adenocarcinoma or diffuse-type gastric adenocarcinoma, according to the Lauren classification10, or as papillary, tubular, or mucinous types and poorly cohesive carcinomas, according to the classification proposed by the World Health Organization11. Alternatively, the Cancer Genome Atlas proposed a molecular classification divided into four subtypes: Epstein-Barr virus-associated gastric cancer (EBVaGC), microsatellite instability, chromosomal instability, and genomically stable tumors12.
EBVaGC presents in 2–20% of the worldwide population13,14, and reports vary from 75,000 to 90,000 cases per year15. At the histologic level, EBVaGC is preferentially associated with intestinal-type gastric adenocarcinoma16,17. It is characterized by marked intratumoral or peritumoral immune cell infiltration, which in turn, is classified into 3 subtypes: lymphoepithelioma-like carcinoma (LELC), conventional adenocarcinoma, and Crohn’s disease-like lymphocytic reaction (CLR)18,19. A study on 123 cases of EBVaGC showed that 43.1% were cases of typical LELC, 42.3% were CLR, and 14.6% were conventional adenocarcinoma18.
In the early stages, EBVaGC is also characterized histologically by the presence of a “lace pattern” that is predominant in LELC and less frequently seen in conventional-type adenocarcinoma19. EBVaGC is predominantly located in the cardia and fundus of the stomach, where the mucosa can present with atrophy and intestinal metaplasia, as a consequence of the virus20.
Currently, the gold standard in GC therapy is surgical resection, improved by standardized lymph node dissection. However, EBVaGC has a distinct tumorigenic profile, given that its positivity for EBV is known to be a favorable prognostic factor and thus could potentially be used as a biomarker in GC for the development of targeted therapeutic intervention21–23.
The aim of the present work was to determine the frequency of EBV infection in intestinal-type gastric adenocarcinoma samples in patients in the Department of Cauca, Colombia, with the awareness that, at present, no studies related to the theme have been conducted in the Colombian Southwest24,25. Our results could have important clinical implications, given that searching for EBV in patients with GC is not commonly carried out in medical practice, and doing so could facilitate the formulation of differentiated prevention and treatment strategies.
Materials and methodsType of studyA retrospective cross-sectional study was conducted.
Sample selectionGiven that, according to background information, EBVaGC is related to a greater frequency of intestinal-type gastric adenocarcinoma, 180 cases of that type of adenocarcinoma were studied in patients diagnosed within the time frame of 2013 and 2017. Their data came from a hospital pathology laboratory sample bank. The sample selection process was carried out by a specialist in anatomic pathology. Based on the inflammatory response patterns of the host that are associated with the presence of EBV infection (LELC, conventional adenocarcinoma, CLR), 28 gastrectomy samples were selected that met the criteria stated below.
Inclusion criteriaSamples of patients that, through pathology studies, were diagnosed with intestinal-type gastric adenocarcinoma, that met the histologic pattern criteria described above, for suspecting EBV infection, and that were adequately conserved in paraffin blocks for analysis, utilizing molecular biology techniques, were included in the study.
Exclusion criteriaPatients whose clinical histories reported immunosuppressive or antimicrobial therapies, the use of H2 receptor blockers, proton pump inhibitors, or nonsteroidal anti-inflammatory drugs 30 days before surgery, a confirmed HIV (AIDS) diagnosis, and patients that did not present with the histopathologic patterns associated with EBV infection were excluded from the study.
Clinical history analysisA survey-type instrument was designed for collecting the related information in the clinical histories of the 28 selected patients. The variables analyzed were: age, sex, risk factors associated with GC, Borrmann classification, tumor location, histologic tumor grade, histopathologic findings, TNM classification (malignant tumor classification through T: size of the original tumor, N: nearby lymph nodes affected, and M: distant metastasis, according to the American Joint Committee on Cancer)26, tumor clinical stage and size, and the result of in situ hybridization and immunohistochemistry for identifying the latent membrane protein 1 (LMP-1) for EBV detection.
EBV identification through CISHThe paraffin-embedded sample sections were cut on microtomes and placed on slides for their later installation in automated Ventana (Benchmark XT) equipment. Epstein-Barr virus-encoded small RNAs (EBER) in situ hybridization with the EBER 1 DNP probe (Ventana, cat # 760-1209A, Tucson, AZ) and the ISH iView Blue Plus anti-DNP detection system (Ventana, Tucson, AZ) were utilized to detect EBV, employing the standard staining protocol and recommendations for the INFORM EBER Probe in BenchMark XT instruments. The resulting slides were read by two specialists in pathologic anatomy in a double-blinded manner.
EBV identification through immunohistochemistryTo immunohistochemically identify the 60 kDa latent membrane protein 1 (LMP-1), encoded by the BNLF1 gene of the virus, the anti-LMP-1 mouse monoclonal primary antibody (CS1-4) (Sigma–Aldrich Co. LLC – USA) was employed, using the Cell Marque (Sigma–Aldrich Co. LLC – USA) detection kit, following the manufacturer’s instructions.
Statistical analysisThe data were tabulated, using the Microsoft Excel (version 2013) program. A descriptive statistical analysis was then carried out through the cross-tabulation of variables, subclassifying the samples as positive or negative for EBV infection. A bivariate analysis was performed between the variables of interest, utilizing the chi-square test, and the significance criterion was defined as a probability value below 0.05 (p < 0.05). The analyses were carried out using the IBM SPSS version 24 program.
Ethical considerationsThe present study meets the current bioethical research regulations and was approved by the ethics committee of the Hospital Universitario San José de Popayán-Colombia, where the paraffin-embedded gastric samples were obtained (Approval Act N°6, May 19, 2016), as well as by the ethics committee of the Universidad del Cauca, with identification code 4487. Given that the data from the clinical histories were tabulated and managed using numerical codes, preserving patient data anonymity, informed consent was not requested for the publication of this article.
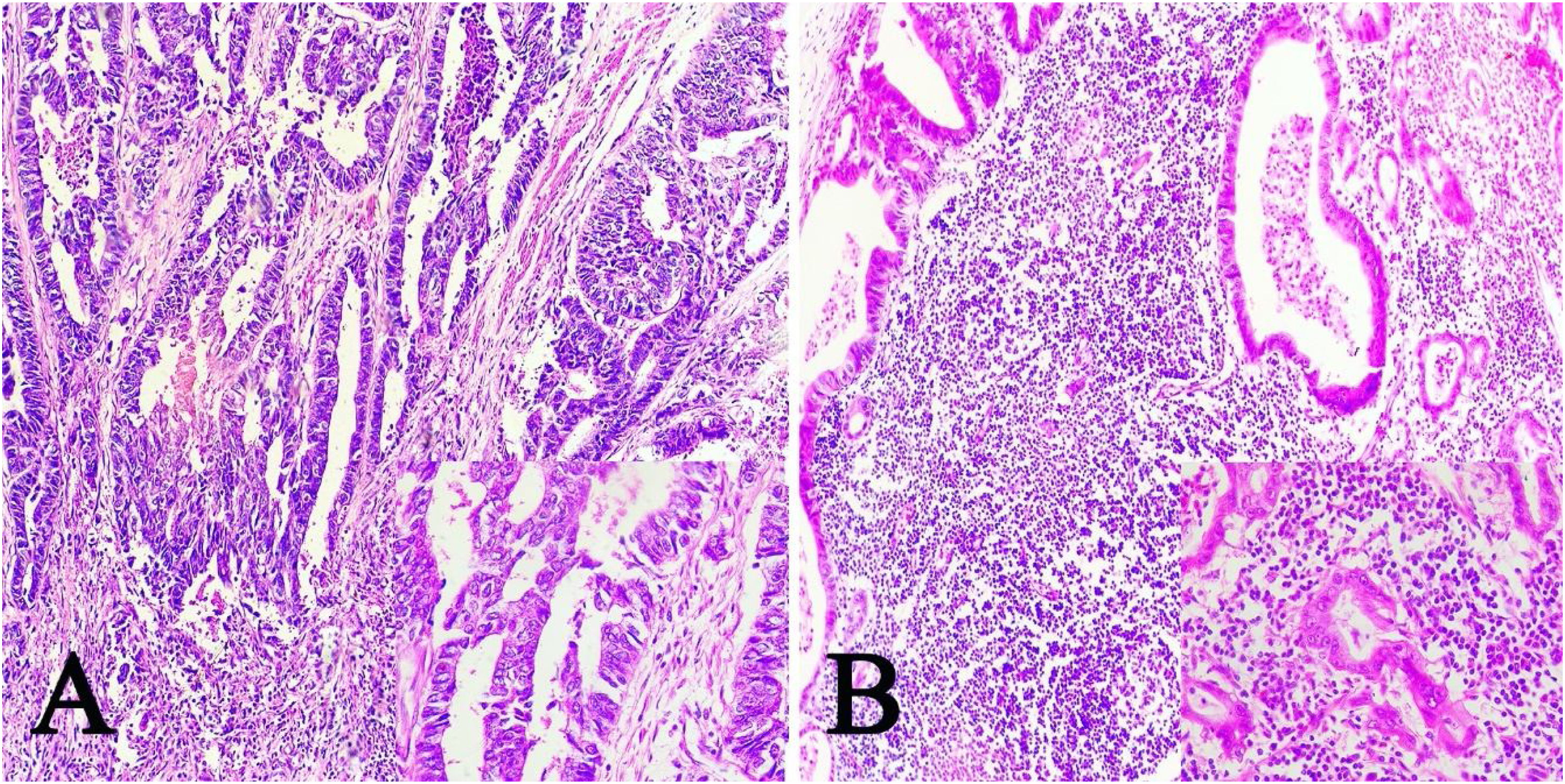
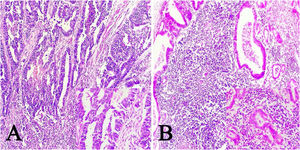
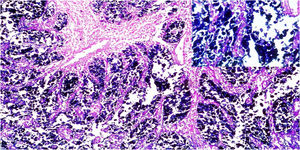
ResultsOf the 28 samples selected, the predominant histopathologic pattern was the lace pattern that shows irregularly anastomosed tubules and cords associated with moderate-to-dense lymphocytic infiltration (Fig. 1A), followed by the LELC-type pattern (Fig. 1B). None of the other histopathologic patterns associated with EBV infection were found.
Microscopic images of intestinal-type gastric adenocarcinoma A) A lace pattern, with mild peritumoral mononuclear inflammatory infiltration (×10 magnification), lower right square: ×40 magnification. B) Lymphoepithelioma-like gastric adenocarcinoma, with lymphoid accumulations (magnification ×10), lower right square: ×40 magnification of neoplastic cells surrounded by lymphoid-like cells. Hematoxylin & eosin staining.
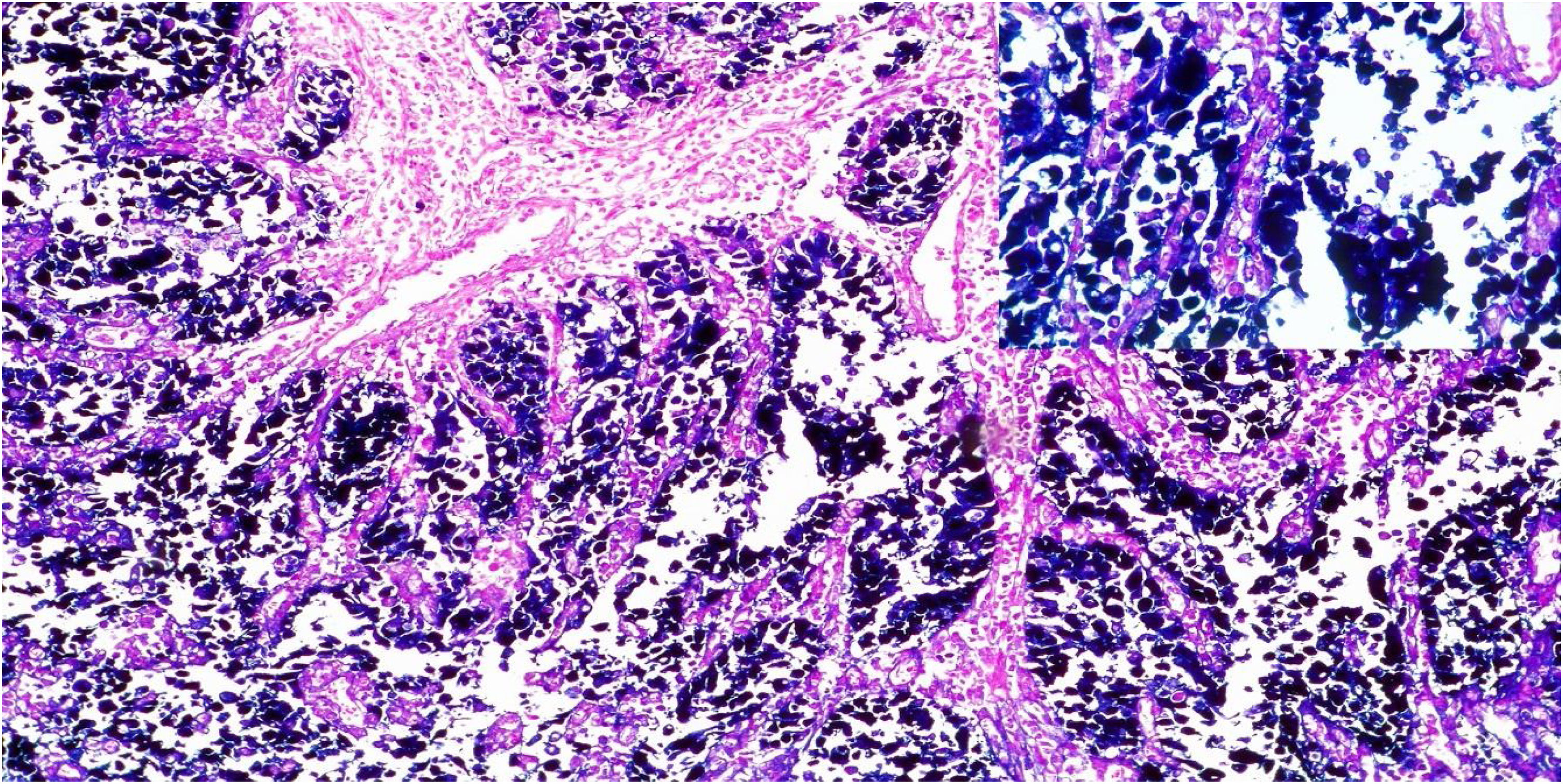
A total of 21.4% of the 28 biopsies analyzed were positive for EBV infection (EBV+), through the CISH technique, upon detecting the EBER gene messenger RNA (mRNA). The hybridization signal was observed in the nucleus of all the neoplastic cells, and no signal was found in the stromal cells or in the peritumoral lymphocytes (Fig. 2).
Microscopic images of chromogenic in situ hybridization for detecting Epstein-Barr virus in gastric adenocarcinoma samples. Nuclear reactivity in in situ hybridization for RNA encoded by the Epstein-Barr virus (×10 magnification), upper square, ×40 magnification, contrast with eosin.
Of the EBV+ patients, 50% were men, 50% were women, and their median age was 59 years, ranging from 50 to 75 years. Of the patients negative for EBV infection (EBV−), 77.3% were men, 22.7% were women, and their median age was 66 years, ranging from 34 to 89 years. No significant differences were found upon analyzing the sociodemographic data of age and sex between the EBV+ and EBV− patients (data not shown).
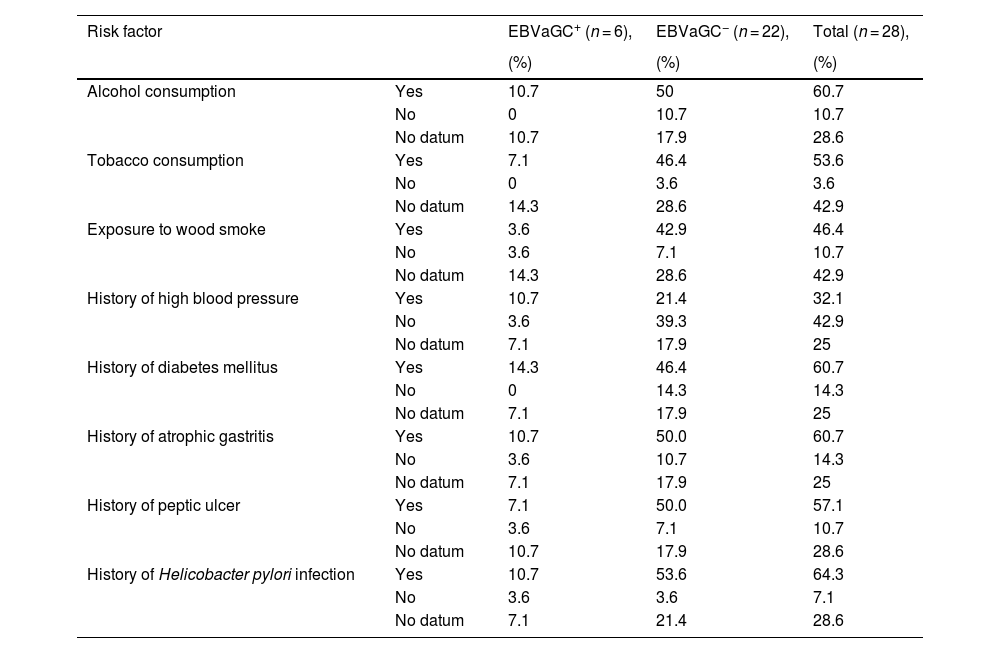
Regarding the risk factors related to GC recorded in the clinical histories, the variables with greater frequency in the EBV+ patients were alcohol consumption (10.7%), a history of high blood pressure (10.7%), a history of diabetes mellitus (14.3%), a history of atrophic gastritis (10.7%), and a history of H. pylori infection (10.7%). In the EBV− patients, the variables with greater frequency were alcohol consumption (50%), tobacco consumption (46.4%), exposure to wood smoke (42.9%), a history of diabetes mellitus (46.4%), a history of atrophic gastritis (50%), a history of peptic ulcer (50%), and a history of H. pylori infection (53.6%). Upon statistically comparing the risk factor data between EBV+ and EBV− patients, there were no significant differences (Table 1).
Identification of the risk factors for Epstein-Barr virus-associated gastric cancer in patients with intestinal-type gastric adenocarcinoma.
| Risk factor | EBVaGC+ (n = 6), | EBVaGC− (n = 22), | Total (n = 28), | |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| Alcohol consumption | Yes | 10.7 | 50 | 60.7 |
| No | 0 | 10.7 | 10.7 | |
| No datum | 10.7 | 17.9 | 28.6 | |
| Tobacco consumption | Yes | 7.1 | 46.4 | 53.6 |
| No | 0 | 3.6 | 3.6 | |
| No datum | 14.3 | 28.6 | 42.9 | |
| Exposure to wood smoke | Yes | 3.6 | 42.9 | 46.4 |
| No | 3.6 | 7.1 | 10.7 | |
| No datum | 14.3 | 28.6 | 42.9 | |
| History of high blood pressure | Yes | 10.7 | 21.4 | 32.1 |
| No | 3.6 | 39.3 | 42.9 | |
| No datum | 7.1 | 17.9 | 25 | |
| History of diabetes mellitus | Yes | 14.3 | 46.4 | 60.7 |
| No | 0 | 14.3 | 14.3 | |
| No datum | 7.1 | 17.9 | 25 | |
| History of atrophic gastritis | Yes | 10.7 | 50.0 | 60.7 |
| No | 3.6 | 10.7 | 14.3 | |
| No datum | 7.1 | 17.9 | 25 | |
| History of peptic ulcer | Yes | 7.1 | 50.0 | 57.1 |
| No | 3.6 | 7.1 | 10.7 | |
| No datum | 10.7 | 17.9 | 28.6 | |
| History of Helicobacter pylori infection | Yes | 10.7 | 53.6 | 64.3 |
| No | 3.6 | 3.6 | 7.1 | |
| No datum | 7.1 | 21.4 | 28.6 |
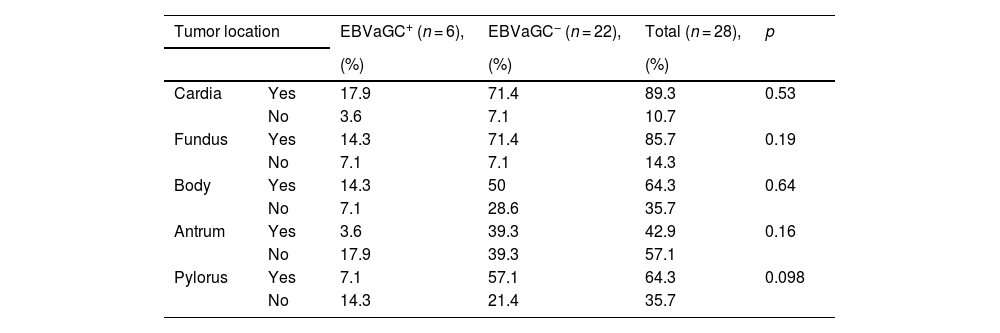
With respect to tumor location in the stomach, in the EBV+ patients, the most frequent location was the cardia (17.9%), followed by the fundus and body (14.3% for each), and in the EBV− patients, the most frequent location was the cardia and fundus (71.4% for each), followed by the pylorus (57.1%) and body (50%) (Table 2).
Tumor location in Epstein-Barr virus-associated gastric cancer in patients with adenocarcinoma.
| Tumor location | EBVaGC+ (n = 6), | EBVaGC− (n = 22), | Total (n = 28), | p | |
|---|---|---|---|---|---|
| (%) | (%) | (%) | |||
| Cardia | Yes | 17.9 | 71.4 | 89.3 | 0.53 |
| No | 3.6 | 7.1 | 10.7 | ||
| Fundus | Yes | 14.3 | 71.4 | 85.7 | 0.19 |
| No | 7.1 | 7.1 | 14.3 | ||
| Body | Yes | 14.3 | 50 | 64.3 | 0.64 |
| No | 7.1 | 28.6 | 35.7 | ||
| Antrum | Yes | 3.6 | 39.3 | 42.9 | 0.16 |
| No | 17.9 | 39.3 | 57.1 | ||
| Pylorus | Yes | 7.1 | 57.1 | 64.3 | 0.098 |
| No | 14.3 | 21.4 | 35.7 | ||
p value, chi-square test.
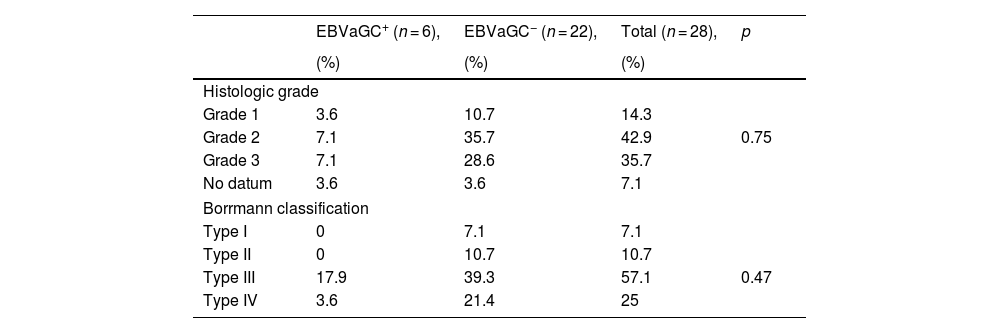
According to the histologic grade classification, the EBV+ patients had histologic grades 2 and 3 (7.1%), whereas the EBV− patients had histologic grade 2 (35.7%). In the Borrmann classification, the majority of the EBV+ patients had the type III classification (17.9%), whereas 39.3% of the EBV− patients had type III and 21.4% had type IV (Table 3).
Histologic classification of biopsies of Epstein-Barr virus-associated gastric cancer in patients with intestinal-type gastric adenocarcinoma.
| EBVaGC+ (n = 6), | EBVaGC− (n = 22), | Total (n = 28), | p | |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| Histologic grade | ||||
| Grade 1 | 3.6 | 10.7 | 14.3 | |
| Grade 2 | 7.1 | 35.7 | 42.9 | 0.75 |
| Grade 3 | 7.1 | 28.6 | 35.7 | |
| No datum | 3.6 | 3.6 | 7.1 | |
| Borrmann classification | ||||
| Type I | 0 | 7.1 | 7.1 | |
| Type II | 0 | 10.7 | 10.7 | |
| Type III | 17.9 | 39.3 | 57.1 | 0.47 |
| Type IV | 3.6 | 21.4 | 25 | |
p value, chi-square test.
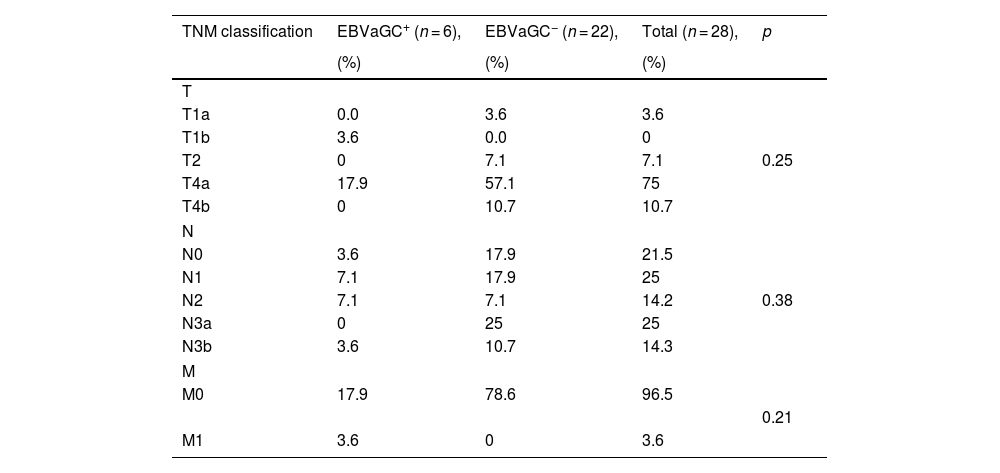
With respect to the TNM classification that evaluated the clinical stage of the disease, 17.9% of the patients with EBV+ biopsies and 57.1% with EBV− biopsies were in stage T4a. In the N analysis, 14.2% of the EBV+ patients were in stages N1 and N2, whereas in the EBV− patients, 25% were in stage N3a, 17.9% were in stage N0, and 17.9% were in stage N1. Regarding metastasis, 17.9% of the EBV+ patients were in stage M0 and 3.6% were in stage M1, whereas 78.6% of the EBV− patients were in stage M0 (Table 4).
TNM classification of Epstein-Barr virus-associated gastric cancer tumors in patients with intestinal-type gastric adenocarcinoma.
| TNM classification | EBVaGC+ (n = 6), | EBVaGC− (n = 22), | Total (n = 28), | p |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| T | ||||
| T1a | 0.0 | 3.6 | 3.6 | |
| T1b | 3.6 | 0.0 | 0 | |
| T2 | 0 | 7.1 | 7.1 | 0.25 |
| T4a | 17.9 | 57.1 | 75 | |
| T4b | 0 | 10.7 | 10.7 | |
| N | ||||
| N0 | 3.6 | 17.9 | 21.5 | |
| N1 | 7.1 | 17.9 | 25 | |
| N2 | 7.1 | 7.1 | 14.2 | 0.38 |
| N3a | 0 | 25 | 25 | |
| N3b | 3.6 | 10.7 | 14.3 | |
| M | ||||
| M0 | 17.9 | 78.6 | 96.5 | |
| 0.21 | ||||
| M1 | 3.6 | 0 | 3.6 | |
p value, chi-square test.
In the present study, of the 180 patients diagnosed with intestinal-type gastric adenocarcinoma, 15.5% had the predominant histopathologic features reported for EBV infection, such as the lace pattern, characterized by the connection and fusion of neoplastic glands27. That description coincides with previous studies that found the lace pattern in 38% of cases of EBVaGC28.
LELC was the other histologic pattern found. It is characterized by a heavy lymphocytic infiltration, a better immune response against carcinogenic cells, a lower cancer stage, a lower incidence of metastasis, and therefore, a better prognosis, compared with GCs that are not associated with EBV infection and have less lymphocytic infiltration29,30. Wang et al.31 described the case of a patient diagnosed with lymphoepithelioma-like GC and found that the tumor was composed of epithelial cell nests surrounded by a large quantity of plasma cells and lymphocytes, confirming the findings common to that histologic pattern. Tang et al. also reported similar descriptions32.
Of the 28 patients, 21.4% were positive in the in situ hybridization analysis, concurring with the positivity range of 2–20% that has been reported in the literature14,33. In addition, hybridization occurred only in the tumor cells. That exclusive distribution has been explained34 by the fact that the carcinogenesis due to EBV is a late event in the process of well differentiated and/or moderately differentiated gastric carcinogenesis, given that there is evidence supporting the absence of EBV in gastric dysplasia and superficial carcinomas35.
Furthermore, the total negativity for LMP-1 in the study samples concurs with that reported by other authors34,36,37 and confirms that negative LMP-1 does not rule out the presence of EBV. The immunohistochemical identification of EBV is highly specific, but not very sensitive, because LMP-1 is only expressed in the type II latency of the virus. In gastric adenocarcinoma, the latent EBV pattern corresponds mainly to latency I, followed by latency II, which is why the ideal method, or gold standard, is in situ hybridization for EBER 1, that is transcribed in both latencies38.
Regarding the sociodemographic characteristics of the study patients, the results showed that 50% of the EBV+ samples were in men, in contrast with that described in the literature stating that male sex is predominant in the disease39,40. The age range was wide (50–75 years), concurring with that reported in the meta-analysis by Lee et al., in 2009, showing that the association between EBV and GC presented in patients ranging from 50 to 68 years of age39.
In the present study, the most frequent tumor location in the samples of intestinal-type carcinoma predominated in the proximal and middle regions of the stomach, especially the cardia, similar to that reported by other authors24,41,42, and the data compiled in the 2009 meta-analysis by Lee et al. that included 48 articles. They found a high association of EBVaGC positivity with location in the cardia and body, with an odds ratio (OR) of 1687 (95% CI 1.330–2.139) and 2144 (95% CI 1.614–2.848), respectively, suggesting that those parts of the stomach have a more adequate environment for EBV infection39. In a 2010 meta-analysis utilizing data from 22 articles, Li et al. found more EBV positivity in the cardia and less in the pylorus43.
Consistent with previous studies, the most frequent grades of differentiation in EBVaGC+ cases were grades 2 and 3, corresponding to moderately and poorly differentiated cell grades22,44,45. That histopathologic grading shows that EBV-infected cells originally developed a differentiated histopathology and then progressed to a poorly differentiated one, in the more advanced stages of the disease, demonstrating that the grade in this type of cancer is intermediate-to-high. The EBV− biopsies showed greater distribution in grade 2 disease, followed by grade 3 and then grade 1, similar to that reported by Van Beek et al. in 200422. In those cases, the moderately differentiated cells demonstrated that they are predominantly intermediate-grade cancers.
According to the macroscopic characteristics, based on the Borrmann classification, the EBVaGC+ cases showed a predominance of type III, similar to that previously reported46,47, signifying that the tumor is ulcerated and infiltrating48. In the EBVaGC− cases, types III and IV were more frequent and those tumors are distinguished by aggressive clinical and pathologic characteristics that include invasion of the serosa (T3 and T4) and higher metastasis rates in the lymph nodes48.
According to the TNM classification, the EBVaGC+ cases showed high percentages in T4a, N1, N2, and M0, indicating that despite the fact that EBVaGC+ cases invade neighboring structures (T4a), they have limited extension into the lymph nodes. Thus, EBVaGC+ patients that have less lymph node involvement have less residual disease, indicating a better prognosis22,46,49. On the other hand, EBVaGC− patients had a greater frequency of T4a, N3a, and M0, and unlike EBVaGC+ patients, the negative group presented with a higher number of lymph nodes with metastasis (N3), indicating that having greater lymph node involvement results in a greater probability of metastatic processes to other organs, thus reducing the median specific 5-year survival rate50.
According to the information compiled in relation to the risk factors associated with GC, the data reported in the clinical histories showed that a high percentage of the patients with GC, regardless of EBV positivity status, consumed alcohol and tobacco, were exposed to wood smoke, and had histories of atrophic gastritis, peptic ulcer, and H. pylori infection, all of which are frequent risk factors for the disease51,52. Close to 60% of the patients with GC had a history of diabetes mellitus and around 30% presented with high blood pressure. However, there is no evidence relating those diseases to the development of GC. They are comorbidities that could be associated with the mean age of the study patients (65 years, range: 34–89 years)53. Of the previously described factors, tobacco use has been considered a risk factor in EBVaGC+ patients, which coincides with our results54.
The most frequent risk factor in the patients with GC was H. pylori infection (64.3%). A history of infection was found in 10.7% of the EBVaGC+ patients and 53.6% of the EBVaGC− patients. That has also been reported by authors, such as Wu et al., in 2000, who detected the presence of H. pylori DNA through polymerase chain reaction (PCR) testing, reporting a positivity of 36.4% and 68.3% in EBVaGC+ and EBVaGC− samples, respectively. They concluded that there was no statistically significant relation between EBVaGC and H. pylori infection6. Likewise, in 2006, Luo et al., through PCR, found that 46.15% of the EBVaGC+ cases were positive for the bacterium, compared with 81.40% of the EBVaGC− cases55.
The association between EBV and H. pylori in patients with GC is controversial. Some studies suggest that there is some type of cooperation between EBV and H. pylori, in which the presence of one of those microorganisms can promote the growth of the other and vice versa, a fact that could also increase its virulence. Even though the mechanisms of that synergy are not completely understood, there is evidence that, during the course of H. pylori and EBV coinfection, the number of immune cells at the infection site increases, potentiating gastric inflammation and tissue damage56,57.
The results of the present study show that the cases of EBVaGC detected in patients from the Department of Cauca have the epidemiologic, clinical, and histopathologic characteristics congruent with the results of other studies58,59, and confirm that the EBER-CISH technique enables the adequate detection of EBV infection in patients with GC.
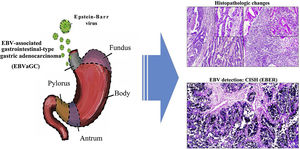
In the Department of Cuca, routine laboratory testing for identifying EBV in patients diagnosed with intestinal-type gastric adenocarcinoma is not carried out. The results of our study can hopefully serve to increase awareness in pathologists, so that, based on the histopathologic patterns of the immune response of the host against the tumor, cases can be selected that would be candidates for molecular diagnosis through in situ hybridization, to detect EBV. Thus, the cancer could be subclassified, contributing not only to the understanding of the disease, but also to the proposal of clinical trials in that subgroup of patients, in the search for therapies that aid in improving their survival12 (Fig. 3).
Graphical summary. Epstein-Barr virus (EBV) infection is an etiologic factor in EBV-associated gastric cancer (EBVaGC). That type of cancer is differentiated by a histologic lace pattern, characterized by the connection and fusion of neoplastic glands. Those histopathologic parameters facilitate the selection of cases that are candidates for molecular studies, such as in situ hybridization, which could contribute to the understanding of the disease and be useful in the evaluation of clinical and pathologic determining factors in regions with a high incidence of gastric cancer.
Andrés Vidal Realpe: data acquisition, analysis, and interpretation; drafting of the article.
Rosa Amalia Dueñas-Cuellar: study concept and design; data interpretation; drafting of the article, critical review of the intellectual content.
Victoria Eugenia Niño Castaño: data analysis and interpretation; drafting of the article.
Diana Lorena Mora-Obando: study concept and design; critical review of the intellectual content.
José de Jesús Arias Agudelo: data acquisition.
Harold Jofre Bolaños: critical review of the intellectual content, final approval of the version presented herein.
Financial disclosureThe present work was funded by the Office of the Vice President of the Universidad del Cauca, Call XI-2016, Project ID4487. That office did not participate in any way in the study design, the collection, analysis, and interpretation of the data, the writing of the article, or the decision to send the article for publication.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Vidal-Realpe A, Dueñas-Cuellar RA, Niño-Castaño VE, Mora-Obando DL, Arias-Agudelo JJ, Bolaños HJ. Características clínico-patológicas del adenocarcinoma gástrico asociado al virus de Epstein-Barr en una región de alta incidencia de cáncer gástrico en Colombia. Rev Gastroenterol Méx. 2023;88:256–266.