Nonalcoholic fatty liver disease (NAFLD) is a metabolic liver disorder commonly attributed to fatty acid deposition that can induce hepatic necroinflammation, defined as nonalcoholic steatohepatitis (NASH). It is strongly associated with obesity. Laparoscopic sleeve gastrectomy (LSG) is a favorable surgical modality for the treatment of morbid obesity.
AimOur study evaluated the impact of LSG on patients with NAFLD and morbid obesity, 3 months after the operation, through clinical and biochemical characteristics, clinico-biochemical indices, and imaging parameters.
Patients and methodsMorbidly obese patients with NAFLD ± NASH underwent LSG. They were thoroughly evaluated clinically (body weight, body mass index, waist circumference) and biochemically (transaminases and triglycerides), as well as through the fatty liver index (FLI), the hepatic steatosis index (HSI), and ultrasound elastography imaging studies (liver stiffness measurement [LSM] and the controlled attenuation parameter [CAP]), before and 3 months after the LSG.
ResultsTwenty-six obese patients with NAFLD underwent LSG that resulted in a significantly high reduction in all the parameters analyzed, except for liver transaminases.
ConclusionLSG is considered an efficient surgical modality for the treatment of morbidly obese patients with NAFLD.
La enfermedad de hígado graso no alcohólico (EHGNA) es un trastorno metabólico del hígado atribuido al depósito de ácido graso que puede inducir necroinflamación hepática, definida como esteatohepatitis no alcohólica (EHNA). Está asociada de manera importante con la obesidad. La gastrectomía laparoscópica en manga (GLM) es una modalidad quirúrgica favorable para el tratamiento de la obesidad mórbida.
ObjetivoNuestro estudio evaluó el impacto de la GLM en pacientes con EHGNA y obesidad mórbida, 3 meses posterior a operación, por medio de características clínicas y bioquímicas, índices bioquímicos y parámetros de imagenología.
Pacientes y métodosPacientes con obesidad mórbida con EHGNA con o sin EHNA se sometieron a una GLM. Fueron evaluados clínicamente (peso, IMC, circunferencia de cintura) y bioquímicamente (transaminasas y triglicéridos), al igual que con el índice de hígado graso (IHG), el índice de esteatosis hepática (IEH), y estudios de imagen de elastografía por ultrasonido (medición de rigidez hepática [MRH] y el parámetro de atenuación controlada [PAC]), previo a la GLM y 3 meses posoperatorio.
ResultadosVeintiséis pacientes obesos con EHGNA se sometieron a una GLM que resultó en una reducción significativa de todos los parámetros analizados, excepto por las transaminasas hepáticas.
ConclusiónLa GLM es considerada una modalidad quirúrgica eficiente para el tratamiento de pacientes con obesidad mórbida y EHGNA.
Nonalcoholic fatty liver disease (NAFLD) is rapidly growing and becoming one of the most evident causes of global hepatic disease.1 Histopathologically, it is characterized by the deposition of hepatic triglycerides and free fatty acids.2 It encompasses a wide spectrum of liver injury, with some cases progressing to nonalcoholic steatohepatitis (NASH), fibrosis, and consequently, to hepatocellular failure and carcinoma.3 The increasing incidence of NAFLD is strongly associated with the prevalence of obesity and its subsequent metabolic disorders.4
Overweight and obesity are defined by the BMI cutoff values of 25-30 kg/m2 and >30 kg/m2, respectively, and are major public health problems due to their rising prevalence in populations, including children.4
Bariatric surgeries (BSs) are more effective than conventional modalities for weight loss in cases of morbid obesity.5 They result in significant and durable weight loss, improvement of obesity-induced comorbidities, and better survival.6 Laparoscopic sleeve gastrectomy (LSG) is a promising treatment option for patients with morbid obesity.7 It is an effective procedure that induces significant weight reduction in the mid-term follow-up.8
The fatty liver index (FLI) is a reliable discriminator for diagnosing NAFLD, but waist circumference (WC) is simpler and more accessible, and its application is comparable.9 It is significantly correlated with NAFLD.10–11 The hepatic steatosis index (HSI) is also a simple, efficient screening test for NAFLD that can be utilized for selecting individuals for liver ultrasonography and determining the need for lifestyle modifications.12
Transient elastography (TE), with or without the controlled attenuation parameter (CAP) is a noninvasive imaging study for the accurate assessment of hepatic fibrosis and steatosis, and thus, is a reliable alternative to biopsy.13–14 Its advantages are its relatively lower cost compared with liver biopsy, and its diagnostic efficiency, particularly in the higher grades of liver fibrosis.15 The CAP is excellent for detecting significant hepatic steatosis16 because of its ease of measurement, operator-independence, and its simultaneous use with the liver stiffness measurement (LSM) for fibrosis.17 It can detect even low grades of steatosis.18
The present study aimed to evaluate the impact of LSG on morbidly obese NAFLD patients 3 months after the procedure through clinical (body measurements) and biochemical (transaminases, triglycerides) factors, hepatic fat indices (FLI and HSI), and imaging (FibroScan® and CAP) parameters.
Materials and methodsA prospective study was conducted on obese patients that underwent LSG at the Kasr Ainy Teaching Hospital, Faculty of Medicine, Cairo University. Preoperative assessment included a thorough personal, medical, and surgical history, basic laboratory testing, and nutritional counseling.
The inclusion criteria were adult patients (≥18 years of age), either sex, morbid obesity (BMI ≥ 40 kg/m2 alone, or BMI ≥ 35 kg/m2 with comorbidities, such as hypertension and diabetes mellitus [DM]).
The exclusion criteria were chronic liver disease, other than NAFLD, such as infectious diseases, autoimmune diseases, alcohol use disorder, metabolic diseases, etc. Gastritis, hiatal hernia, and gastroesophageal reflux disease were excluded by upper endoscopy to avoid de novo onset or post-LSG worsening.19–20
The patients included in the study underwent: 1) a complete personal and medical history interview to determine any comorbidities (DM, hypertension, ischemic heart disease, etc.); 2) a thorough clinical examination including the body measurements of BMI (with the overweight/pre-obese [BMI = 25-29.9 kg/m2], class I [BMI = 30-34.9 kg/m2], class II [BMI = 35-39.9 kg/m2] and class III/extreme obesity [BMI ≥ 40 kg/m2] categories)21 and WC; and 3) the investigative work-up performed one week before the LSG and 3 months after the procedure. It included a) biochemical tests (i. liver biochemical profile: aspartate transaminase [AST], alanine aminotransferase [ALT], and gamma glutamyl transferase [γ-GT], ii. serum triglycerides); b) liver fat indexes (i. FLI22 = logistic [0.953 * ln (TG) + 0.139 * BMI + 0.718 + ln (γ-GT) + 0.053 + WC-15.745] * 100, where logistic [x] = 1/ [1 + e_x] denotes the logistic function and ln the natural logarithm. Values < 30 rule out, and values ≥ 60 rule in steatosis, ii. HSI12 = 8 × ALT/AST + BMI + 2 [if DM]; + 2 [if female]; with values < 30 ruling out steatosis and values ≥ 36 determining steatosis); c) tests to exclude non-NAFLD chronic liver disease (i. hepatitis viral seromarkers [preoperatively only] for hepatitis B and C [HBsAg, HBcAb, HCV Ab], ii. autoantibodies, e.g., ANA, ASMA, AMA); and d) imaging studies: abdominal ultrasound (US), and transient elastography (TE), i.e., Fibroscan, using Fibroscan 502F01406®: a) liver stiffness measurement (LSM): a 50-MHz wave is passed into the liver from a small transducer on the end of an US probe that can measure the velocity of the shear wave (in meters per second) as it passes through the liver. The shear wave velocity is then converted into liver stiffness, which is expressed in kilopascals (kPa), and b) CAP: it measures ultrasonic attenuation in the liver at 3.5 MHz, using signals acquired by the FibroScan®, based on vibration-controlled transient elastography (VCTE™). An XL probe was used in thickened abdominal wall cases, i.e., skin liver distance (SLD) ≥ 45 mm, otherwise an X probe was used.23–24 The CAP was measured only on validated measurements, according to the same criteria used for LSM and on the same signals, ensuring that liver ultrasonic attenuation was simultaneously obtained, in the same volume of liver parenchyma as the LSM. The final CAP value, which ranged from 100 to 400 decibels per meter (dB/m), was the median of the individual measurements; and 4) written statements of informed consent for the performance of LSG, explaining its possible complications, were obtained and no personal data that could identify the patients were included in the study.
Statistical analysisData were coded and entered using the SPSS® version 25 program. Statistical tests were carried out to analyze the usefulness of TE and the other study parameters, as well as the impact of LSG. The data were summarized using mean, standard deviation, median, minimum, and maximum for the quantitative data and frequency (count) and relative frequency (percentage) for the categorical data. The non-parametric Wilcoxon signed rank test was used for comparing the serial measurements in each patient. The chi-square test was performed to compare the categorical data, but the Fisher’s exact test was used when the expected frequency was less than 5. Statistical significance was set at a p value below 0.05.
Ethical considerationsThe present study was conducted on humans and the subjects evaluated were obese patients that presented with NAFLD. The study was approved by the ethics committees of the Tropical Medicine and General Surgery departments of the Faculty of Medicine, Cairo University, and followed the principles laid out in the Declaration of Helsinki. Written statements of informed consent were signed by the patients prior to undergoing LSG.
ResultsThe present study included 26 NAFLD patients with morbid obesity that underwent LSG at the General Surgery Department, Kasr AlAiny Teaching Hospital, Faculty of Medicine, Cairo University, within the time frame of May 2016 through August 2018.
The mean patient age was 33.58 years (range: 19-63, median 34.5 years) and there was a predominance of females (21 patients; 80.8%). DM was the only comorbidity and was found in 6 patients (23.1%).
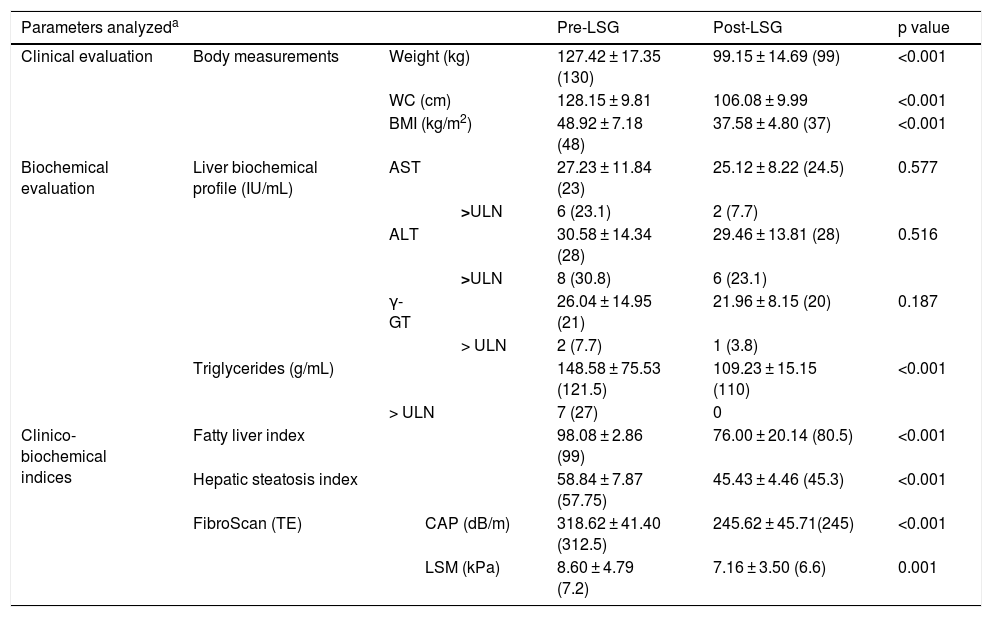
Table 1 shows the clinical, laboratory, and imaging evaluations of the patients, before LSG and 3 months after the procedure.
The main clinical, laboratory, and imaging evaluations in the study patients, before LSG and 3 months after the procedure.
| Parameters analyzeda | Pre-LSG | Post-LSG | p value | ||||
|---|---|---|---|---|---|---|---|
| Clinical evaluation | Body measurements | Weight (kg) | 127.42 ± 17.35 (130) | 99.15 ± 14.69 (99) | <0.001 | ||
| WC (cm) | 128.15 ± 9.81 | 106.08 ± 9.99 | <0.001 | ||||
| BMI (kg/m2) | 48.92 ± 7.18 (48) | 37.58 ± 4.80 (37) | <0.001 | ||||
| Biochemical evaluation | Liver biochemical profile (IU/mL) | AST | 27.23 ± 11.84 (23) | 25.12 ± 8.22 (24.5) | 0.577 | ||
| >ULN | 6 (23.1) | 2 (7.7) | |||||
| ALT | 30.58 ± 14.34 (28) | 29.46 ± 13.81 (28) | 0.516 | ||||
| >ULN | 8 (30.8) | 6 (23.1) | |||||
| γ-GT | 26.04 ± 14.95 (21) | 21.96 ± 8.15 (20) | 0.187 | ||||
| > ULN | 2 (7.7) | 1 (3.8) | |||||
| Triglycerides (g/mL) | 148.58 ± 75.53 (121.5) | 109.23 ± 15.15 (110) | <0.001 | ||||
| > ULN | 7 (27) | 0 | |||||
| Clinico-biochemical indices | Fatty liver index | 98.08 ± 2.86 (99) | 76.00 ± 20.14 (80.5) | <0.001 | |||
| Hepatic steatosis index | 58.84 ± 7.87 (57.75) | 45.43 ± 4.46 (45.3) | <0.001 | ||||
| FibroScan (TE) | CAP (dB/m) | 318.62 ± 41.40 (312.5) | 245.62 ± 45.71(245) | <0.001 | |||
| LSM (kPa) | 8.60 ± 4.79 (7.2) | 7.16 ± 3.50 (6.6) | 0.001 | ||||
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; CAP: controlled attenuation parameter; dB/m: decibel per meter; γ-GT: gamma glutamyl transferase; kPa: kilopascal; LSG: laparoscopic sleeve gastrectomy; LSM: liver stiffness measurement; TE: transient elastography; ULN: upper limit of normal WC: waist circumference.
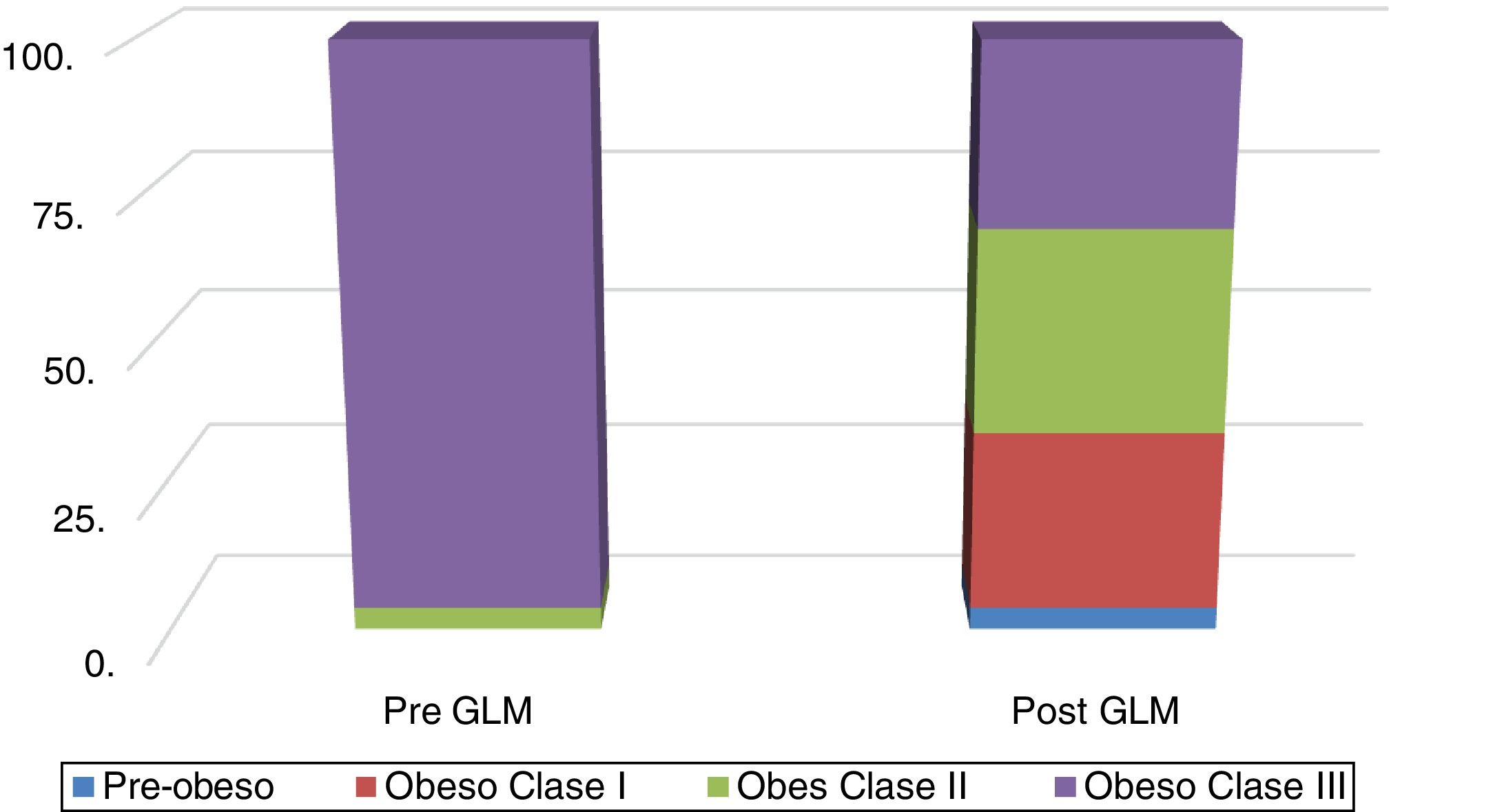
Fig. 1 shows the distribution of obesity classes, before and after LSG. The mean reduction of excess weight loss (EWL) was 22.2% and 23.2%, as measured by body weight and BMI, respectively.
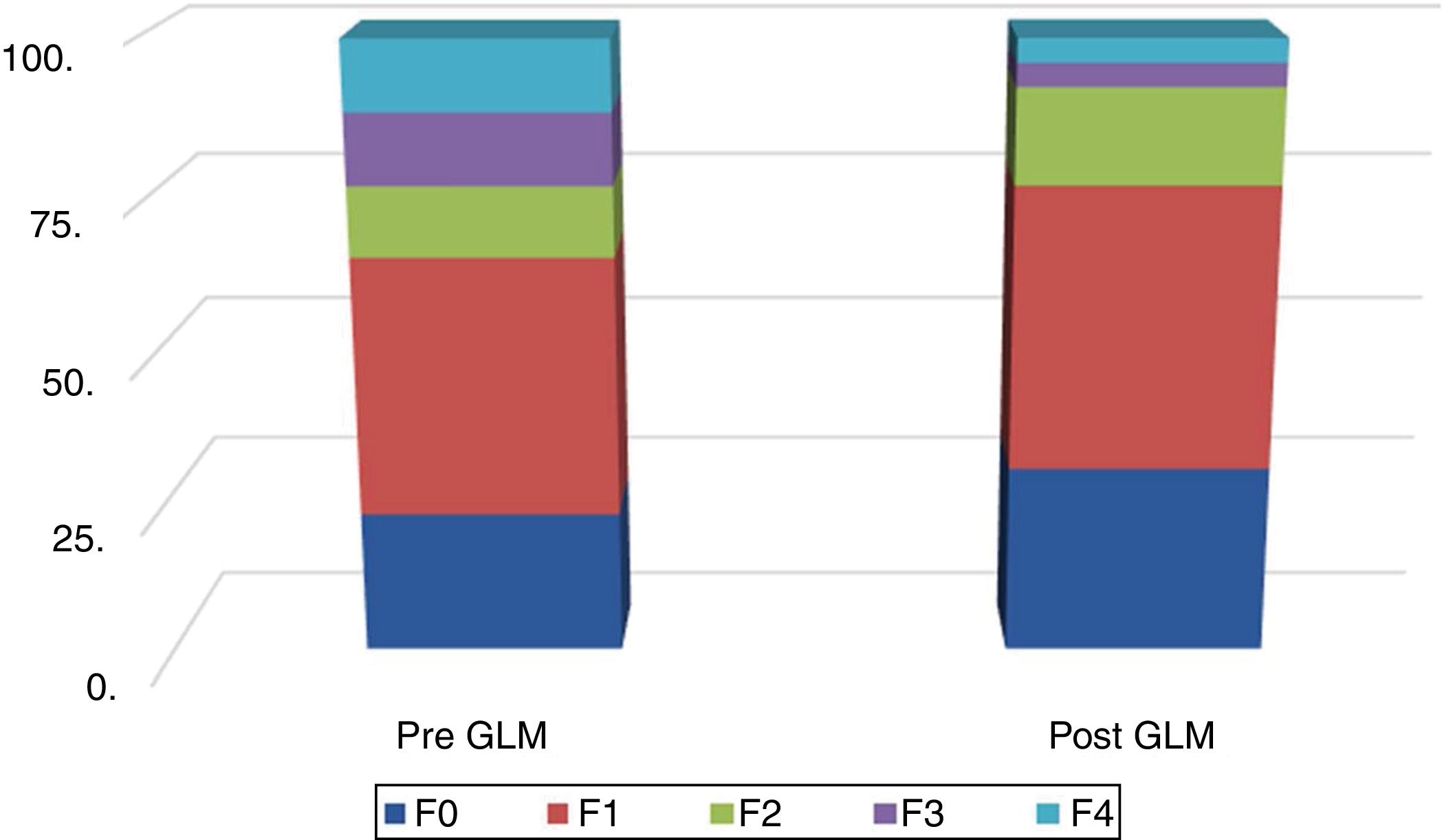
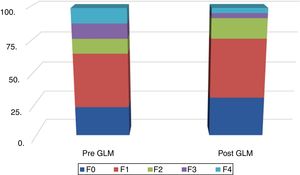
Fig. 2 shows the distribution of LSM scores, before and after LSG.
A broad, highly significant improvement was found in all the parameters analyzed, except for the hepatic enzymes. Moreover, none of the patients experienced any intraoperative or postoperative complications.
Discussion and conclusionsNAFLD is rising to a pandemic prevalence due to obesity and insulin resistance, and is becoming the most common indication for liver transplantation.2 It constitutes more than 75% of chronic liver disease,25 and is diagnosed by transaminases or US. It significantly increases the risk of type 2 DM and metabolic syndrome over a median 5-year follow-up period.26
On the other hand, obesity has been declared a global epidemic by the World Health Organization since 1997.27 Obesity is associated with various morbidities and with mortality but the relation varies between different ethnic groups.4
In the present study, 23.1% of the obese patients with NAFLD also presented with DM. Both DM and obesity are independent risk factors for NAFLD.12,28
LSG, which was first performed by Hess and Hess in 1988,29 is gaining in popularity as a primary bariatric procedure, particularly because of its safety and effectiveness.30 It was initially considered the first-stage procedure in biliopancreatic diversion or Roux-en-Y gastric bypass for high-risk patients,31 then later proved to be an efficient single BS for morbid obesity.32
Obese NAFLD patients were evaluated in the present study, utilizing clinical, biochemical, and imaging parameters, compared before LSG and 3 months after the procedure.
In the present analysis, obesity was central (mean WC = 128.15 ± 9.81 cm, median = 128 cm, range = 110-145 cm), according to all definitions utilized; for Americans (>102 cm in men and >88 cm in women),33 Europeans (≥94 cm in men and ≥80 cm in non-pregnant women), South Asian and Chinese men ( >90 cm), and Japanese men (>85 cm).34 The role of central/abdominal obesity in the development of metabolic syndrome and insulin resistance has been described since 1991.35 WC is a simple and accessible index, with discriminatory power in the diagnosis of NAFLD,11 and is one of the independent risk factors for NAFLD.28
NASH is a complex disease, associated with obesity, DM, and hyperlipidemia, but its presence is growing in average-weight subjects.36
Typically, NASH patients are middle-aged, obese, and female, and often have hyperglycemia (±overt DM) and/or hyperlipidemia, with other medical comorbidities, for which they are under long-term treatment.37 Patients in the present study were obese and predominantly female (80.8%). DM, together with hypertriglyceridemia, was found in only 2 patients. Nevertheless, NASH was present in only 23.1% of the patients, in terms of elevated transaminases, particularly ALT, as a marker of hepatitis activity.12,38–41 Serum ALT and γ-GT levels correlated with NAFLD.42 The percentage of NASH in our study was similar to that found by Browning et al., 2004, because aminotransferase levels tend to decrease over time, even with progressing fibrosis.43 Importantly, hepatic histologic examination via a liver biopsy was not one of the methodologies in our study, thus limiting the number of confirmed cases of NASH.
In the present study, 27% of the patients had hypertriglyceridemia, one with a markedly elevated level (451 mg/dl). Dyslipidemia, as reported by Binobaid et al, 2018, is one of the independent risk factors for NAFLD.28
The mean FLI in our study was 98.08 ± 2.86 (median: 99). Huang et al., 2015, have defined 30 as the optimal FLI cutoff value for diagnosing NAFLD in middle-aged and elderly Chinese patients.44 Sviklāne et al., 2018, confirmed its strong correlation with transaminases, with 90% sensitivity and 74% specificity, and a value ≥ 60 was associated with metabolic syndrome and nephropathy.45 Similarly, Zelber-Sagi et al., 2013, reported its strong correlation with the SteatoTest™.46
The mean HSI in our patients was 58.84 ± 7.87 (median = 57.75). The HSI is a reliable clinical and biochemical tool in diagnosing NAFLD.12 Sviklāne et al., 2018, found an adequate correlation between the HSI and WC, with 86% sensitivity and 66% specificity,45 whereas we recorded a value > 36, reaching a specificity of 92.4%.12
In the present study, high CAP and LSM values of 318.62 ± 41.40 (median: 312.5) and 8.60 ± 4.79 (median: 7.2), respectively, were recorded. TE became more economically feasible in advanced grades of hepatic fibrosis.47 Our recorded value reflected the severe grade of steatosis, similar to several previous results. The CAP, which is the modality reflecting the usefulness of TE in NAFLD, was significantly correlated with the percentage and grade of steatosis in NAFLD. The median CAP with significant steatosis was 317 (IQR: 284-339) for the confirmed cutoff value of 283 dB/m. The CAP had 76% sensitivity, 79% specificity, and positive and negative predictive values of 87% and 64%, respectively. However, it was higher in the patients with mild (F0-F1) fibrosis,17 and was associated with steatosis grade, BMI, and serum triglycerides. The median CAP for steatosis grades S0, S1, S2, and S3 were 184 dB/m, 305 dB/m, 320 dB/m, and 324 dB/m, respectively. The optimal CAP cutoff values for estimating steatosis grades S1, S2, and S3 were 263 dB/m, 281 dB/m, and 283 dB/m, respectively.16
LSG is an efficient BS for remarkable excess weight loss, a low complication rate, and short hospital stay.48 NAFLD that includes NASH could be an indication for BS. Weight loss is still the ideal tool for reducing hepatic steatosis, steatohepatitis, and fibrosis, by shifting to healthy dietary habits and attaining a physically active lifestyle, but unfortunately, not all patients respond satisfactorily to those changes. Thus, BS may be considered the best option, with its benefits diminishing the risks and providing the best course of action.49 In our study, all the preoperative parameters (clinical, biochemical, clinico-biochemical index, and imaging), except for the transaminases, significantly improved 3 months after surgery, concurring with previous studies that reported NAFLD resolution ranging from >50%50 to 90%.51
We reported a highly significant (p < 0.001) weight loss 3 months post-LSG, in terms of body weight, WC, and BMI, with a mean loss of 22.2%, 17.3%, and 23.25%, respectively, results concurring with those of many studies. Manco et al., 2016, reported 21.5% excess weight loss (% EWL) one year after LSG,49 whereas Diamantis et al. reported overall 59.3% EWL ≥ 5 years after the procedure.52 Royal Alexandra Hospital reported an EWL% for LSG that varied from 33% to 90% that was sustained, postoperatively, for up to 3 years. Its advantages were a low mortality rate, few major complications, a relatively short surgery duration, reduced hospital stay, and lower cost.7
Transaminase levels in our patients were comparable in the pre-LSG and post-LSG settings, in contrast with the results reported by Karcz et al.,53 in which there was a significant reduction. Our results could be attributed to the low prevalence of transaminase elevation, i.e., 23.1%, and the fact that there can be a paradoxical aggravation of steatosis, with or without steatohepatitis, in some post-BS cases,54–56 reflected in the highly significant reduction of the FLI and HSI, of 22.5% and 22.8%, respectively, concurring with that reported by Matter et al. 2005.54
We also found a highly significant (p < 0.001) reduction and normalization of serum triglycerides (the mean reduction was 26.5%), similar to the findings of Karcz et al., 2011, who recorded a mean triglyceride reduction of 37.5% at one year post-LSG.53
We evaluated NAFLD post-LSG, including its TE examination, which showed a highly significant regression, as assayed by CAP and LSM reported at 3 months after the procedure. BS is favored for improving the histologic and metabolic changes associated with NAFLD.57 NASH reversed completely in all patients and F0 disappeared in 90%.49
In addition, none of the patients experienced any of the complications of bleeding, gastric leakage or stricture, reflux esophagitis, or nutritional deficiencies.58 Nevertheless, Sasaki et al., 2014, recommended further long-term studies to confirm the true effects of BS as potential treatment for NASH.59
We conclude that LSG is an efficient BS for morbidly obese NAFLD patients.
Study limitationsThe number of patients included in the present study is a rather small sample for a pilot study addressing this topic. The study also encompassed a short period of time and there was a low level of surgical candidacy.
Author contributionsMohamed Abdelbary: lead author, designed the study designer, revised the manuscript, and approved the final submitted version.
Raghda Marzaban: carried out the literature search and drafted the manuscript.
Hadeel Eldeen, Marwa Khairy, and Ayman Yosry: carried out the FibroScan® study of the study patients, before and after surgery.
Mohamed Menesy: interpreted and analyzed the data.
Mohamed Fahmy and Amr Ayad: the surgeons that performed LSG on the study patients.
Bassem Mouheb: the young surgeon that evaluated the patients that were candidates for LSG, carried out the data acquisition, and assisted in the surgery.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Abdelbary MS, Marzaban R, Gamal Eldeen H, Khairy M, Menesy M, Fahmy MH, et al. La utilidad clínica de la elastografía transitoria como una herramienta de imagenología para evaluar el impacto a corto plazo de la gastrectomía laparoscópica en manga, en conjunto con parámetros clínicos y bioquímicos e índices clínico-bioquímicos en pacientes con enfermedad de hígado graso no alcohólico: un estudio piloto egipcio. Revista de Gastroenterología de México. 2021;86:125–132.
This original article was a contributory study between the Tropical Medicine and General Surgery Departments, Faculty of Medicine, Cairo University, Egypt.