The effectiveness of endoscopic submucosal dissection (ESD) is similar to that of surgery in the treatment of early lesions. The technique requires a high level of technical skill. Training on biologic models and the mastering of accessories facilitate ESD.
AimsThe aim was to evaluate the usefulness of the Endolifter in facilitating tissue exposure during ESD in an in vivo porcine model performed at the experimental surgery laboratory of the School of Medicine at the Universidad de São Paulo in Brazil.
Material and methodA study with an experimental design employing an in vivo porcine model was conducted on 5 Yorkshire pigs weighing 20-25kg. ESDs were performed using the Endolifter. Mucosal layer dissection was carried out with a dual knife and IT knife and all the endoscopic procedures were performed by a single expert endoscopist.
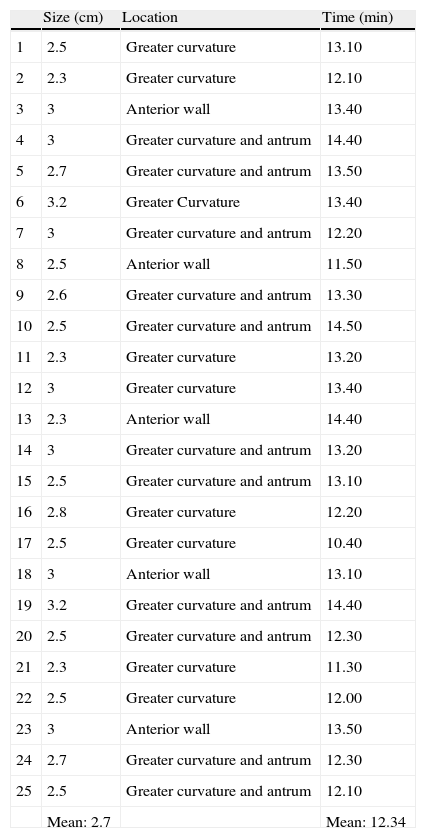
ResultsA total of 25 ESDs were performed, with a technical success rate of 100%. The mean dissection time was 12.34min (range: 10.40-14.50 min) and the mean lesion size was 2.7cm (range: 2.3-3.2cm). There were no episodes of bleeding or perforations during the procedures.
ConclusionsThe Endolifter enables rapid and effective ESDs to be carried out. It is an applicable and easy-to-use device that can be manipulated by a single operator.
La disección endoscópica de la submucosa (DES) tiene una efectividad similar a la cirugía en el tratamiento de las lesiones incipientes. La técnica requiere un alto nivel de destreza técnica. El entrenamiento en modelos biológicos y el perfeccionamiento de accesorios facilita la DES.
ObjetivosEl objetivo fue evaluar la utilidad del Endolifter para facilitar la exposición tisular durante la DES en un modelo in vivo porcino. Fue realizado en el laboratorio de cirugía experimental de la Escuela de Medicina de la Facultad de Sao Paulo, Brasil.
Material y métodoDiseño experimental en modelo porcino in vivo. Se incluyeron un total de 5 cerdos Yorkshire con un peso de 20-25kg. Las DES se realizaron usando el dispositivo Endolifter. Se empleó un Dual-Knife y el IT-Knife para la disección de la capa submucosa. Todos los procedimientos endoscópicos fueron realizados por un único endoscopista experto.
ResultadosSe realizaron un total de 25 DES, con una tasa de éxito técnico del 100%. El tiempo promedio de la disección fue de 12.34min (10.40-14.50min) y el tamaño promedio de las lesiones fue de 2.7cm (2.3-3.2cm). No se presentó hemorragia ni perforaciones durante el procedimiento.
ConclusionesEl Endolifter permite realizar DES de manera rápida y eficaz. Es factible, de fácil uso y puede ser empleado por un solo operador.
Endoscopic submucosal dissection (ESD) is as effective as surgery in the treatment of early lesions and is minimally invasive and less costly. It is a modification of the endoscopic mucosal resection technique in which the diathermy snare is substituted by a cutting instrument (needle knife).1,2
ESD enables en bloc extraction of superficial tumors, offering important advantages over other endoscopic resection techniques: a higher cure rate, en bloc resection, complete resection, and fewer cases of local recurrence.3–5
The technique is carried out in 3 stages: 1) injection of a fluid that increases the submucosal space by separating the muscle layer, 2) a circumferential cut outside the line marking the borders of the lesion, and 3) dissection that consists of removing the tumor with the needle knife.2
ESD indications in gastrointestinal cancer are: well-differentiated intestinal-type intramucosal cancer with no ulceration, under 2cm if it is raised and under 1cm if it is flat or depressed. These indications have been modified by the National Cancer Center in Japan to include well-differentiated intramucosal cancer regardless of size and well-differentiated intramucosal cancer with ulcerous scarring < 30mm.2,6
However, the technique requires a high level of technical skill, longer procedure duration, and there is a greater complication probability, mainly due to the lack of adequate tissue layer exposure and a deficient submucosal space (cushion).
These difficulties can be reduced with continuous training on experimental animals or other models, developing skills and shortening the learning curve.
The most serious technical complications are perforation and delayed bleeding.7 The overall complication rate in experienced centers is 1.9% (bleeding 1.5%, perforation 0.5%).8,9
The aim of the present study was to evaluate the usefulness of an endoscopic accessory (EndoLifter Olympus Medical Corporation, Tokyo, Japan) in facilitating tissue exposure (the submucosal space) during endoscopic dissection in an in vivo porcine model.
MethodsThis is an experimental design conducted on an in vivo porcine model.
It was performed at the experimental surgery laboratory of the School of Medicine of the Universidad de Sao Paulo, Brazil, and was approved by the University Bioethics Committee in accordance with Resolution N° 592 and article 32 of the 1998 Federal Law N° 9.605 (Environmental Crimes Law) dealing with animal protection (the Research on Animals Law, approved September 9, 2008).
AnimalsA total of 5 Yorkshire pigs (2 females and 3 males) weighing 20-25kg were included. They were isolated 3 days prior to the study, during which time they underwent health tests applied by the laboratory's veterinary service. All the animals were fed the same diet and had access to water ad libitum.
Preoperative care and anesthesiaThe pigs were in a fasting state 24h prior to the endoscopic procedure. Mechanical ventilation was administered through orotracheal intubation and general anesthesia was induced intravenously with ketamine (5mg/kg) and thiopental (10-30mg/kg).
MaterialsThe EndoLifter (Olympus Medical Corp., Tokyo, Japan) is a novel grasping forceps mounted on a transparent plastic cylinder at the distal end of the endoscope. The grasping forceps is connected to a piece of looped metal in the form of a cube, directly below its jaws, that is attached to the hood (Fig. 1). The grasping forceps is able to rotate in front of the endoscope as it advances (Fig. 2), allowing the resected mucosa to be lifted and exposed at the same time as the submucosal space, facilitating dissection. The transparent cap extends 0.3cm toward the front portion of the endoscope. The piece that is the cube-shaped loop attached to the cap extends 1.5cm from the plastic cover. The closed mouth of the grasping forceps is 0.3cm long. When it is completely extended and open, the tip of the grasping forceps extends approximately 1.8cm from the end of the cap. The maximum diameter of the EndoLifter grasping forceps above the hood is 14.95mm and its maximum aperture is 8mm. It was designed to be adapted to a standard gastroscope with a 9.8mm inner diameter channel.
TechniqueESDs were performed using the EndoLifter device mounted on a standard gastroscope (Olympus 145, Medical Systems, Brazil), an IT Knife, (Olympus, Medical Corporation, USA), a Dual Knife, (Olympus, Medical Corporation, USA), and an electrosurgical generator (ERBE Elektromedizin, GmbH) with a blend 2 effect at 75W. The gastroscope was introduced through the mouth of the pig, inspecting the esophagus and stomach. Gastric lavage was done with 0.9% normal saline solution until the gastric cavity was free of solid residuals. Five ESDs of target lesions were carried out. They were created through hood suctioning, and on only one occasion, the later injection of a solution made up of mannitol (10%) + 0.9% normal saline solution + carmine indigo + adrenaline 1:10,000, while at the same time creating the submucosal cushion (endoscopic injector with a disposable 23-gauge needle [Olympus Medical Corporation]). The target lesions were situated along the greater curvature, the anterior wall of the stomach, and the pyloric antrum. The circumferential incisions were done with the Dual Knife and cautery, and the submucosal layer was dissected with the IT Knife with the help of the EndoLifter for lifting the tissue to be resected. All the endoscopic procedures were performed by a single expert endoscopist. Once the endoscopic dissections were finished, the animals were euthanized. The surgical specimens were fixed in formalin immediately after their resection and sent for histopathologic study.
VariablesDissection procedure time (min) (evaluated from the beginning of the circumferential incision to the end of the dissection). Continuous quantitative variable.
Size (mm) of the resected tissue. Discrete quantitative variable.
Complications. a) Bleeding: defined as the presence of any amount of blood at the moment of submucosal dissection. b) Perforation: defined as the loss of continuity of the gastric wall that communicates the interior of the cavity with the peritoneum or other intra-abdominal viscera.
ResultsTwenty-five ESDs were performed in vivo on the porcine stomach. The technical success rate was 100%. In all the lesions, the disease-free margins were outside of the line marking the lesion borders. The mean dissection time was 12.34min (10.40-14.50min) and the mean size of the lesions was 2.7cm (2.3-3.2cm) (Table 1). There was no bleeding or perforation in any of the endoscopic procedures. The EndoLifter facilitated the dissection by enabling the specimen to be held in place and manipulated during the resection, thus exposing the submucosal space and reducing the need to constantly inject the submucosa; an average of 2 to 3ml of submucosal injection per lesion was used.
Results.
| Size (cm) | Location | Time (min) | |
| 1 | 2.5 | Greater curvature | 13.10 |
| 2 | 2.3 | Greater curvature | 12.10 |
| 3 | 3 | Anterior wall | 13.40 |
| 4 | 3 | Greater curvature and antrum | 14.40 |
| 5 | 2.7 | Greater curvature and antrum | 13.50 |
| 6 | 3.2 | Greater Curvature | 13.40 |
| 7 | 3 | Greater curvature and antrum | 12.20 |
| 8 | 2.5 | Anterior wall | 11.50 |
| 9 | 2.6 | Greater curvature and antrum | 13.30 |
| 10 | 2.5 | Greater curvature and antrum | 14.50 |
| 11 | 2.3 | Greater curvature | 13.20 |
| 12 | 3 | Greater curvature | 13.40 |
| 13 | 2.3 | Anterior wall | 14.40 |
| 14 | 3 | Greater curvature and antrum | 13.20 |
| 15 | 2.5 | Greater curvature and antrum | 13.10 |
| 16 | 2.8 | Greater curvature | 12.20 |
| 17 | 2.5 | Greater curvature | 10.40 |
| 18 | 3 | Anterior wall | 13.10 |
| 19 | 3.2 | Greater curvature and antrum | 14.40 |
| 20 | 2.5 | Greater curvature and antrum | 12.30 |
| 21 | 2.3 | Greater curvature | 11.30 |
| 22 | 2.5 | Greater curvature | 12.00 |
| 23 | 3 | Anterior wall | 13.50 |
| 24 | 2.7 | Greater curvature and antrum | 12.30 |
| 25 | 2.5 | Greater curvature and antrum | 12.10 |
| Mean: 2.7 | Mean: 12.34 |
The ESD learning curve is related to the acquisition of manual skill. Novel accessories continue to emerge for facilitating dissection technique. One of the main difficulties at the time of dissection is the lack of traction-contraction; another is inadequate vision of the submucosal tissue, especially when the resected mucosa tends to collapse over the surgical area, making it difficult to maintain adequate visualization of the dissection area and of the blood vessels; this increases the risk for bleeding and perforation, which are the 2 main complications of the procedure.
Different accessories have been developed for carrying out traction-contraction. Kondo et al.10 developed percutaneous traction assisted by endoscopic mucosal resection using the IT Knife, resulting in a technique that not only is complicated due to its invasiveness, but also requires various operators. Gotoda et al.11 described the technique of submucosal dissection for early-stage cancer, employing a magnetic anchoring system. With this technique, all tumors were dissected with no complications. But even though there were no complications due to prolonged magnetic field exposure, the technical difficulties involved in the moving of and access to the large modules of these systems, reduce the feasibility of its use in daily clinical practice. Imaeda et al.12 described a technique in which external forceps were used to perform traction-contraction, introducing 2 grasping forceps after submucosal injection, manipulating the tissue to be resected. The procedure turned out to be cumbersome and difficult to reproduce.
There is still a need for an easy-to-manage device that provides aid during submucosal dissection. The EndoLifter is an alternative that appears to fill this gap, given that it is easy to use and is of great help at the moment of tissue layer exposure. When using the EndoLifter it is important to maintain the grasping forceps at the optimal angle to and distance from the operating field. In our simulation, the radius employed was approximately 2cm from the tip of the endoscope, which enabled us to adequately manipulate the surgical specimen. The use of this device also made it unnecessary to apply multiple submucosal injections that were used to limit the dissection layer, and prevented a deeper dissection and perforation. Being able to grasp and lift the surgical specimen was another advantage, making it possible to have traction and accurately direct the instruments toward the desired area within the operating field. This facilitates the dissection, minimizing the use of cautery, and subsequently reducing potential complications, such as delayed perforation due to muscle layer necrosis from excess coagulation.
Some endoscopists recur to displacing the surgical specimen upwards with the tip of the endoscope as an alternative, in order to observe the submucosal layer; with the EndoLifter, the need for this complicated and risky procedure is eliminated because it ensures adequate vision of the layer to be dissected. No prolonged training is necessary to be able to use the EndoLifter, given that its management is not different from that of the traditional forceps utilized in endoscopic procedures, nor does it involve a different spatial orientation.
ConclusionsThe EndoLifter enables endoscopic submucosal dissection to be performed rapidly and effectively. It is a feasible accessory that is easy to use and can be managed by a single operator.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Sato-Uemura R, Christiano-Sakai M, Duarte-Jordão R, Guimarães-Horneaux de Moura E, Velázquez-Aviña J, Sobrino-Cossío S, et al. Endolifter, una nueva herramienta para una segura y rápida disección endoscópica submucosa. Revista de Gastroenterología de México. 2014;79:161–165.