Hepatocellular carcinoma (HCC) is the fifth most common neoplasm worldwide and hepatitis C virus (HCV) is the leading cause, conditioning a 3 to 7% risk per year in patients with liver cirrhosis (LC).1 In the era of pegylated interferon (PEG-IFN), the sustained virologic response (SVR) was about 50%, and in cirrhotic patients that achieved SVR, the incidence of HCC decreased to 0.5-1% annually.2
The introduction of direct-acting antiviral (DAA) therapy resulted in a reported SVR above 90%, with a positive impact on the course of HCC, reducing the adverse effects in patients with a more advanced disease stage.3 Despite the promising results of DAA therapy, some reports suggest it may increase the risk for HCC occurrence and recurrence,4 hence our decision to present the following 2 cases (Table 1).
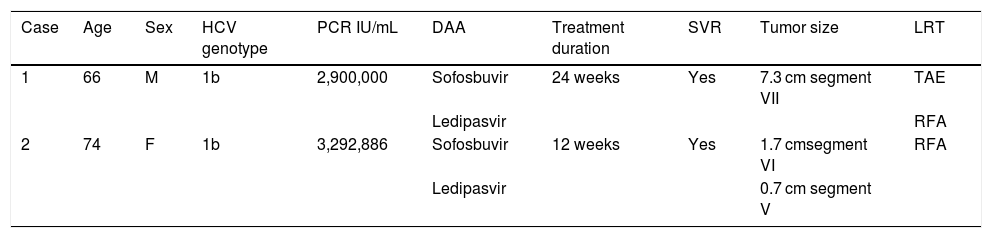
Summary of clinical cases.
| Case | Age | Sex | HCV genotype | PCR IU/mL | DAA | Treatment duration | SVR | Tumor size | LRT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | 1b | 2,900,000 | Sofosbuvir | 24 weeks | Yes | 7.3 cm segment VII | TAE |
| Ledipasvir | RFA | ||||||||
| 2 | 74 | F | 1b | 3,292,886 | Sofosbuvir | 12 weeks | Yes | 1.7 cmsegment VI | RFA |
| Ledipasvir | 0.7 cm segment V |
DAA: direct-acting antiviral; F: female; HCV: hepatitis C virus; LRT: locoregional therapy; M: male; PCR: polymerase chain reaction; RFA: radiofrequency ablation; SVR: sustained virologic response; TAE: transarterial embolization.
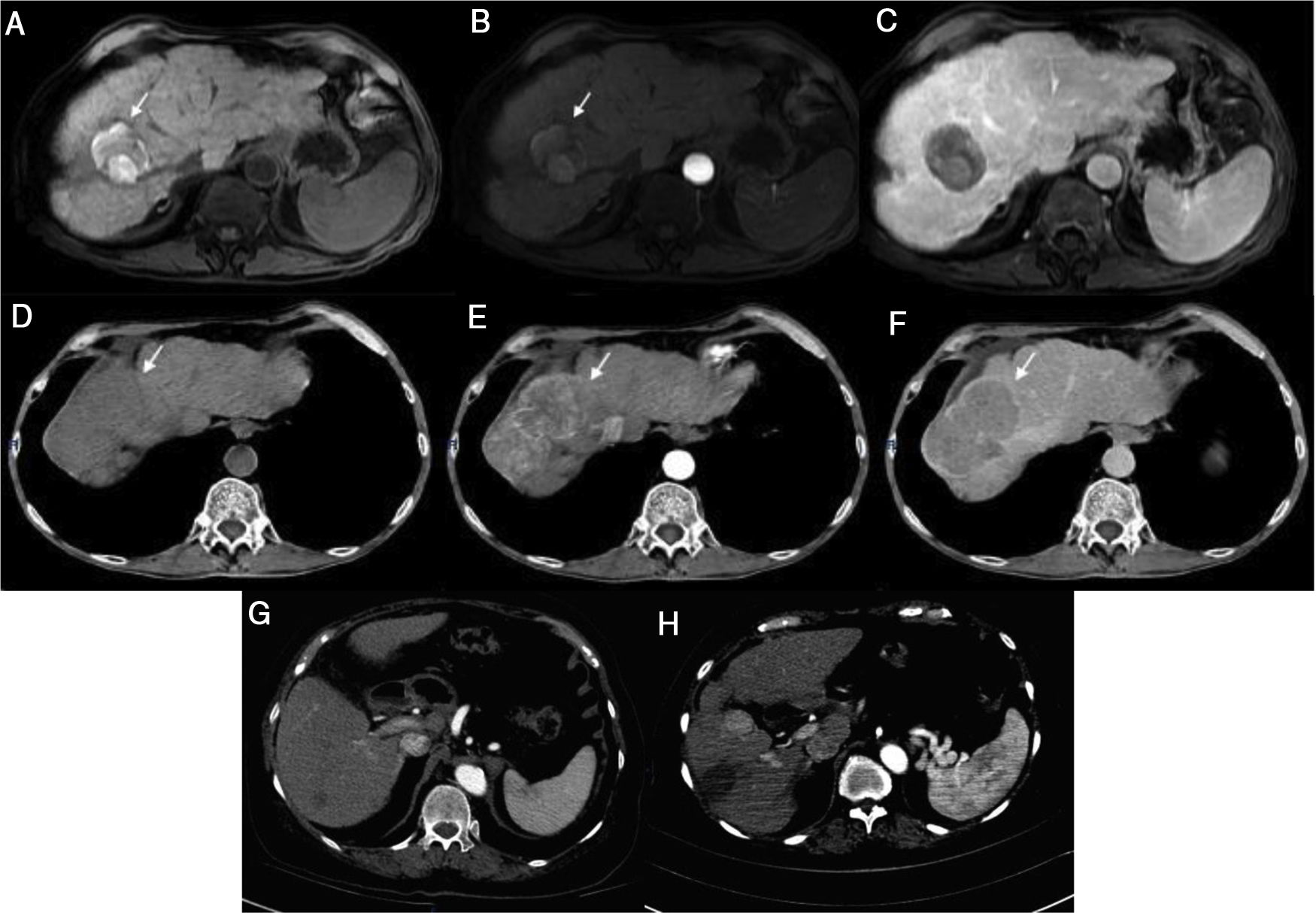
A 66-year-old man was diagnosed with LC secondary to HCV in 2014. A magnetic resonance imaging (MRI) study performed that same year revealed a 7.3 cm lesion in segment VII, displaying typical HCC behavior, for which he underwent transarterial embolization (TAE). In 2015, he again required TAE, after which he had complete radiologic response (CRR) (Fig. 1a-c) and remained under surveillance. In March 2017, the patient achieved SVR with DAA therapy. A control imaging study performed after SVR showed that the lesion had progressed, with vascular invasion (Fig. 1d-f).
Case 1) Magnetic resonance imaging (MRI): T1 FatSat image shows hyperintensity of the tumor in segment VII secondary to necrosis with no arterial enhancement and no new lesions observed in the venous phase (a-c). The tumor seen on the previous MRI now shows activity and an increase in size (d-f). Case 2) Computed tomography (CT): 7 mm lesion with enhancement and a 17 mm lesion with typical behavior (g). Control image 2 months post-radiofrequency ablation shows the increase in size of the lesion from 7 mm to 25 mm, with a typical image of HCC (h).
A 74-year-old woman was diagnosed with LC secondary to HCV in 2016. An ultrasound study performed before she started DAA therapy showed no focal lesions. After achieving SVR, she presented with acute cholecystitis. A tomography scan revealed 2 lesions, one measuring 7 mm that displayed typical HCC behavior, and the other measuring 1.7 cm that had contrast medium enhancement (Fig. 1g). A biopsy was performed on the larger lesion, and HCC was reported. The patient underwent radiofrequency ablation (RFA), and 2 months later, the lesion had increased in size from 7 mm to 2.5 cm (Fig. 1h).
Despite the fact that the development and recurrence of HCC observed in our case reports appear to be associated with DAA therapy, evidence from recent studies has shown no such association. In a cohort study on 33,137 patients, Mun et al. evaluated the risk of de novo HCC developing after antiviral treatment and concluded that there were no significant differences in HCC risk after DAA treatment.5 Those results were consistent for de novo cases and recurrence in a systematic review and meta-analysis by Rutledge et al. that included 138 studies (n = 177,512).6
The impact of DAA on liver transplantation has been evaluated in cohorts at European and Latin American centers, and in both of those studies, no evidence of higher waitlist progression or post-transplantation recurrence was reported.7,8 The controversy derived from previous analyses appears to be related to study methodology.
Today, the benefit of treating patients with DAAs is clear and has been associated with a decrease in all-cause mortality.9 Predictive scoring systems have been developed with the aim of stratifying the risk for HCC in patients that have undergone DAA therapy.10
Ethical considerationsThe present scientific letter meets the current bioethical research regulations. It was authorized by the ethics committee of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”. The patients cannot be recognized or identified through the images or data contained in the article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Tapia-Sosa R, Hernández-Cabral F, Gabutti A, Páez-Zayas VM, García-Juárez I. Carcinoma hepatocelular asociado con el uso de la terapia antiviral de acción directa para virus de hepatitis C: reporte de dos casos. Revista de Gastroenterología de México. 2021;86:197–199.