The success rates of therapies for treating Helicobacter pylori vary greatly worldwide and the ideal treatment has yet to be clearly established.
AimsA systematic review was carried out to evaluate the effectiveness of current first and second-line therapies in treating H.pylori infection.
MethodsTwo researchers independently carried out Internet search engine reviews (PUBMED, EMBASE, MEDLINE) of clinical trials on adults published between 1990 and 2012 in both English and Spanish.
ResultsForty-three (n=8,123) clinical trials were evaluated that included first and second-line triple, quadruple, and sequential therapies. The eradication rates of the standard triple therapy are unacceptable (≤80%) in countries where H.pylori is highly resistant to clarithromycin and metronidazole. Administration of the standard triple therapy for more than 7days does not improve its effectiveness. No statistically significant differences were observed between the eradication rates of the quadruple therapy with bismuth and the standard triple therapy. Even though the sequential and concomitant therapies are equally successful regimens, the triple therapy with levofloxacin offers the best results as first and second-line treatment, but quinolone resistance can diminish its effectiveness. The triple therapy with levofloxacin and the sequential and concomitant treatments were superior to the standard triple regimen as first-line therapy.
ConclusionsCurrently there is no ideal first or second-line treatment for achieving 100% eradication. The therapeutic order should be carried out according to the initial treatment and local antimicrobial resistance studies.
Las tasas de éxito de las terapias para tratar el Helicobacter pylori varían ampliamente a nivel mundial. El tratamiento óptimo no ha sido claramente establecido.
ObjetivoSe realizó un revisión sistemática para evaluar la eficacia de las terapias actuales de primera y segunda línea en la infección por Helicobacter pylori.
MétodosDos investigadores realizaron la revisión independiente en motores de búsqueda electrónica (PUBMED, EMBASE, MEDLINE) de ensayos clínicos publicados entre 1990 y 2012, incluyendo adultos e idiomas inglés y español.
ResultadosSe evaluaron 43ensayos clínicos (n=8,123), que incluyen terapias triples, cuádruples y secuenciales, de primera y segunda línea. Las tasas de erradicación de la terapia triple estándar son inaceptables (≤80%) en países donde el H.pylori presenta alta resistencia a claritromicina y metronidazol. Un tiempo mayor a 7días no mejora la eficacia de la triple terapia estándar. No se observaron diferencias significativas entre las tasas de erradicación de la terapia cuádruple con bismuto y la triple terapia estándar. Aunque las terapias secuencial y concomitante son regímenes igualmente exitosos, la terapia triple con levofloxacino ofrece los mejores resultados como primera y segunda línea, pero la resistencia a quinolonas puede disminuir su eficacia. La triple terapia con levofloxacino, la secuencial y la concomitante fueron superiores al esquema triple estándar como régimen de primera línea.
ConclusionesActualmente no existe un tratamiento óptimo de primera o segunda línea que logre una erradicación del 100%. El orden terapéutico deberá realizarse acorde al tratamiento inicial y a estudios locales de resistencia antimicrobiana.
Infection due to Helicobacter pylori (H. pylori) affects approximately 50% of the worldwide population, especially in the developing countries. It has a great impact, reaching a prevalence of up to 90% in the adult population.1–5 This bacterium has a causal relation to mucosa-associated lymphoid tissue (MALT) lymphoma and gastroduodenal ulcer, as well as an associative relation to pathologies such as functional dyspepsia, gastritis, and gastric adenocarcinoma.6–8
Among the different therapies for treating H. pylori are the standard triple therapy, which is a proton pump inhibitor (PPI) and 2 out of 3 antibiotics (amoxicillin or clarithromycin or metronidazole/tinidazole), bismuth-containing quadruple therapy (PPI, bismuth, tetracycline, and metronidazole), levofloxacin-containing triple therapy (PPI, levofloxacin, and amoxicillin), sequential therapy (PPI plus amoxicillin followed by PPI, clarithromycin and metronidazole or tinidazole), and concomitant or quadruple therapy without bismuth, which is a PPI, clarithromycin, amoxicillin, and metronidazole.7,9 The standard triple therapy currently has unacceptable H. pylori eradication levels under the 80% intention-to-treat (ITT) rate. This is mainly due to the increased resistance to clarithromycin and metronidazole.7,9 According to the Maastricht IV consensus,10 the standard triple therapy, or the bismuth-containing quadruple therapy as an alternative, have been recommended as first-line treatment in regions with a resistance to clarithromycin under 15 to 20%, while the quadruple therapy with bismuth or the triple therapy with levofloxacin are recommended as second-line therapies. On the other hand, in regions in which clarithromycin resistance is greater than 15 to 20%, the indicated first-line therapeutic regimens include the bismuth-containing quadruple therapy, the sequential therapy, and the concomitant or quadruple therapy without bismuth, while the triple therapy with levofloxacin is used as second-line therapy.11–16
Taking into account the prevalence and potential clinical consequences of this chronic infection, it is of the utmost importance to identify the ideal treatment regimens that achieve excellent eradication percentages.1 However, the success rates vary greatly worldwide and there is no consensus on their effectiveness. No ideal first-line treatment or treatment after a failed first eradication attempt has been clearly determined, and there are discrepancies in relation to optimum duration, doses, and the medication to use in each line of therapy.9,17
The aim of the present study was to provide a systematic review of the international literature that includes randomized and non-randomized clinical trials in order to evaluate the eradication effectiveness of the current first and second-line therapeutic regimens in the management of adults presenting with H. pylori infection.
MethodsSearch strategyTwo researchers (J.D.Forero and M.Rey) independently searched the electronic databases (PubMed, EMBASE, MEDLINE) containing studies published within the time frame of 1990 to 2012, including randomized and non-randomized clinical trials on adults above the age of 18 years in both English and Spanish. The electronic bibliographic search was done using the following MeSH terms: Helicobacter pylori AND treatment OR therapy AND eradication AND first-line treatment AND second-line treatment.
Clinical trial selection criteria- 1.
Randomized and non-randomized clinical trials.
- 2.
Eradication rate evaluation in first-line treatments.
- 3.
Eradication rate evaluation in second-line treatments in the context of an initial therapeutic failure.
- 4.
Triple, quadruple, or sequential therapies with a variety of doses, durations, and medications employed.
- 5.
Patients with H. pylori infection with no previous treatment or those with a prior therapeutic failure with first-line regimens.
- 6.
Patients indicated for H. pylori infection treatment established according to the Maastricht IV Consensus.
- 7.
Infection demonstrated by at least one diagnostic test (urea breath test, stool antigen test, histologic test, rapid urease test or culture).
- 8.
Eradication confirmed by at least one diagnostic test carried out at a minimum of 4 weeks after having taken the last medication.
- 9.
An eradication effectiveness report in an intention-to-treat (ITT) or per-protocol (PP) analysis.
- 10.
A report of the number of treated patients and the number of cured patients.
The clinical trials that evaluated patients with multiple previous treatment failures were excluded, along with trials in which the different medications and therapy durations were not clearly indicated or when the number of treated and/or cured patients could not be clearly calculated. Resistance to antibiotics, adverse effects, or adherence to first or second-line treatments were not included in the systematic review.
Data extraction process and definitionsIn each study, the following variables were collected on a standardized format, summarizing the included articles:
- 1.
Author.
- 2.
Region of origin.
- 3.
Year.
- 4.
Study design.
- 5.
First-line regimens (doses and days).
- 6.
Second-line regimens (doses and days).
- 7.
Number of patients included.
- 8.
Eradication in ITT analysis, patients treated and cured.
- 9.
Eradication in PP analysis, patients treated and cured.
All the patients that had begun therapy and had initially been assigned with or without randomization were included in the ITT analysis. The PP analysis was carried out according to the final therapeutic regimen adhered to by the patient, regardless of the dose.
The methodological quality of the clinical trials included in the study was evaluated using the Jadad scale.18 This validated scale includes 3 evaluation directives: randomization, blinding, and a description of the patients that were removed from or that abandoned the study. Its score is from 0 to 5, a higher score representing a higher study quality. A randomized clinical trial is considered to be of poor quality if it has a score under 2.
Statistical analysisDescriptive statistics (the eradication proportion reported in the intention-to-treat [ITT] or per-protocol [PP] analysis) were used to summarize and describe the effectiveness data of the clinical trials. A p<0.05 was used to determine the statistical significance in all the therapeutic regimens included in the study.
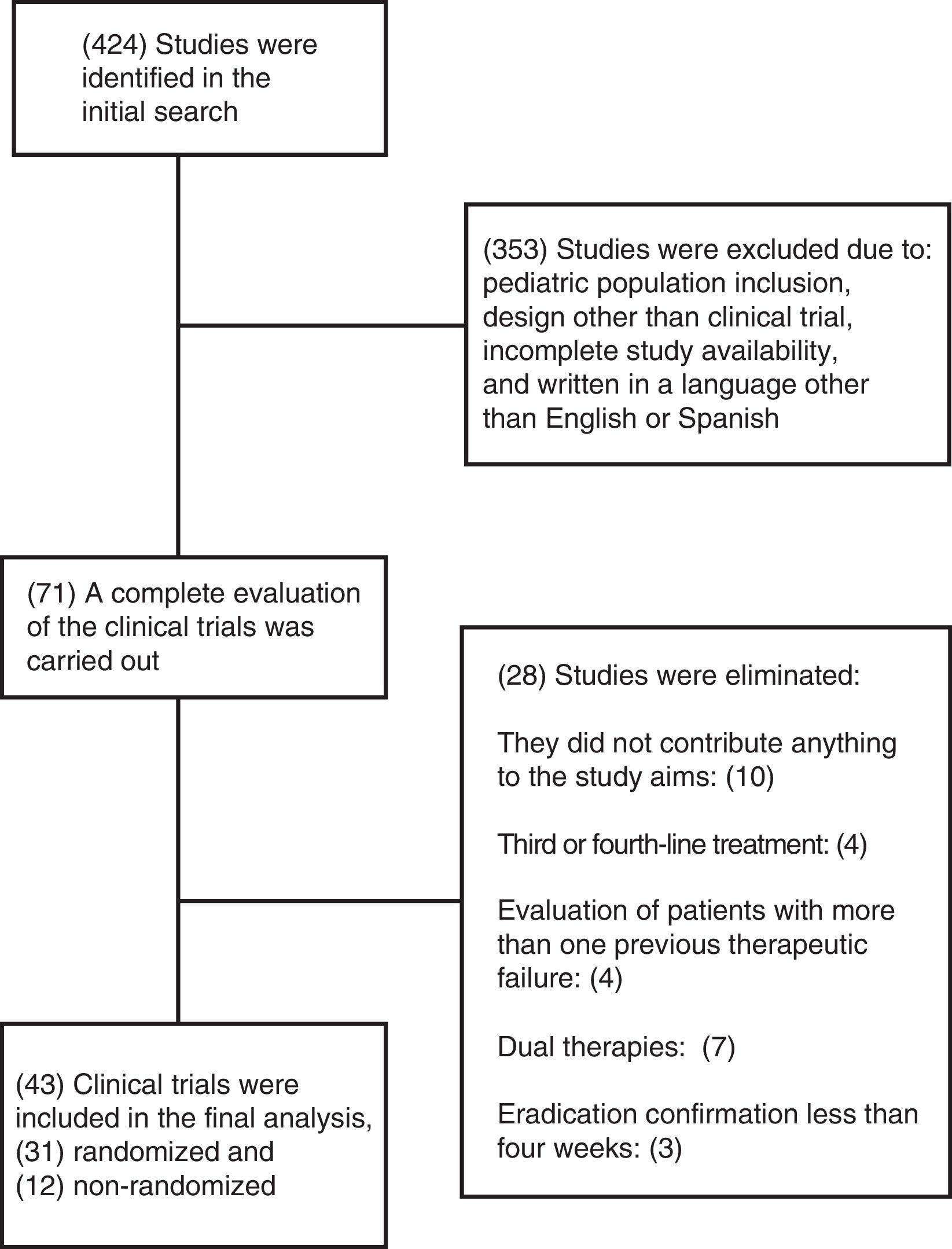
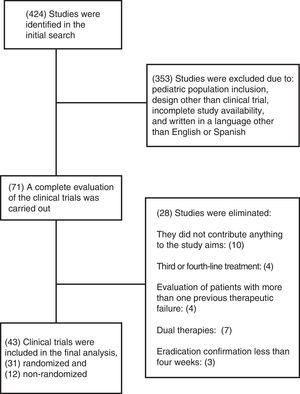
ResultsSummary of the included studiesThe initial search strategy identified 424 articles, 353 of which were excluded. Seventy-one potential clinical trials were identified, and after a thorough analysis, 28 were eliminated. The most common reasons for exclusion were studies that evaluated third or fourth-line eradication therapy regimens, the inclusion of patients with more than one previous failed therapy, dual therapies, eradication confirmation in a period of less than 4 weeks from the time of the last medication taken, or studies that did not fit the aims of the review. Forty-three clinical trials were included in the final analysis (figure 1).
Study descriptionsA total of 8,123 participants were included in the 43 clinical trials that fit the inclusion criteria of the systematic review; 31 were randomized clinical trials representing a total of 6,708 patients and 12 were non-randomized clinical trials with 1,415 patients. The clinical trial characteristics and results are summarized in Tables 1 and 2.
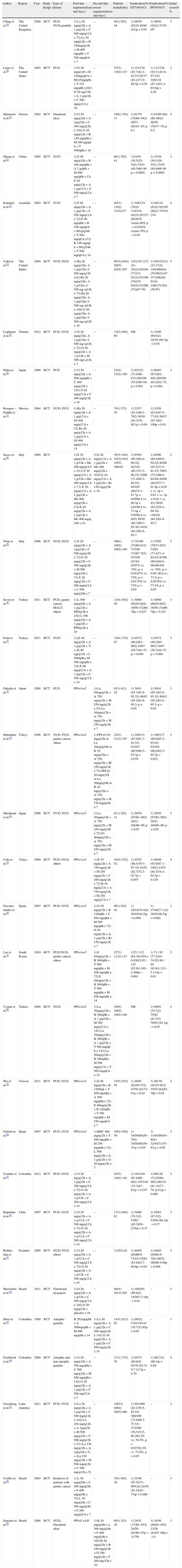
Randomized clinical experiments that evaluate first-line or second-line regimens for the treatment of Helicobacter pylori infection.
| Author | Region | Year | Study design | Type of disease | First-line regimens(doses and days) | Second-line rescue regimens(doses and days) | Patients included(n) | Eradication(%) (ITT)95%CI | Eradication(%) (PP)95%CI | Jadad score(0-5) |
| Ching et al.19 | The United Kingdom | 2008 | RCT | PUD, NUD,gastritis | 1) La 30 mg/q12h + A 1 g/q12h + C 500 mg/q12 h x 72) La 30 mg/q12h + B 240mg/q12h + M 400 mg/q8h + T 500 mg/q6 h x 7 | – | 941) 502) 44 | 1) 46/50 (92)2) 40/44 (91)p>0.05 | 1) 46/50 (92)2) 37/38 (97 | 3 |
| Laine et al.20 | The United States | 2003 | RCT | PUD | 1) O 20 mg/q12h + B 140mg/q6 h + M125mg/q6h + T 125 mg/q6h x102) O 20 mg/12h + A 1 g/q12h + C 500 mg/q12 h x 10 | – | 2751) 1382) 137 | 1) 121/138 (87.7)82.2–93.2114/137 (83.2)77.0–89.5p=0.29 | 1) 111/120 (92.5) (87.8–97.2)2) 108/124 (87.1)(81.2–93.0)p=0.16 | 3 |
| Mantzaris et al.21 | Greece | 2002 | RCT | Duodenal ulcer | 1) O 20 mg/q12h + A 1g/q12h + C 500 mg/q12h x 102) O 20 mg/q12h + B 120 mg/q6h + M 500 mg/q8 h + T 500/q6h x 10 | – | 1491) 782) 71 | 1) 61/78 (78)68–882) 46/71 (65)47–83; p 0.1 | 1) 61/69 (88)(80–96)2) 46/59 (78)77–79; p 0.2 | 4 |
| Zheng et al.22 | China | 2005 | RCT | NUD | 1) E 40 mg/q12h + B 440 mg/q6h + A 2 g/q6h + M 400 mg/q6h x 12) E 20 mg/q12h + A 1 g/q12 h + C 500 mg/q12 h x 7 | – | 801) 392) 41 | 1)15/39 (38.5)25–542) 33/41 (80.5)66–90 p=0.0002 | 1) 15/38 (39.5)26–552) 33/39 (84.6)66–90 p<0.0001 | 3 |
| Katelaris et al.23 | Australia | 2002 | RCT | NUD | 1) P 40 mg/q12h + A 1 g/q12h + C 500 mg/q12 h x 72) P 40 mg/q6h + B 108 mg/q6 h + M1g/q24h + T 500 mg/q6 h x73) B 108 mg/q6 h + M1g/24h + T 500 mg/q6 h x 14 | – | 4051) 1342) 1343)137 | 1) 104/134 (78)2) 110/134 (82)3) 95/137 (69)82% versus 69% p<0.0182% versus 78% p>0.05 | 1) 94/114 (82)2) 92/105 (88)3) 75/101 (74) | 4 |
| Vakil et al.24 | The United States | 2004 | RCT | NUD, PUD | 1) Ra 20 mg/q12h+ A 1 g/q12h+ C 500 mg/q12h x32) Ra 20 mg/q12h+ A 1 g/12h+ C 500 mg /q12h x 73) Ra 20 mg/q12h+ A 1 g/q12h+ C 500 mg /q12h x 104) O 20 mg/q12h+ A 1 g/q12h+ C 500 mg /q12h x 10 | – | 8031)1942) 2003) 2024) 207 | 1)51/187 (27)(21-34)2)150/194 (77)(71-83)3)153/196 (78)(72-84)4)151/206 (73)(67-79) | 1) 50/167(23)(23-37)2) 140/166(84)(79-90)3)147 /171(86)(81-91)4) 146/179 (82)(76-87) | 5 |
| Loghmari et al.25 | Tunisia | 2012 | RCT | PUD, NUD | 1) O 20 mg/q12h+ A 1 g/q12h+ C 500 mg /q12h x 72) O 20 mg/q12h + A 1 g/12h + M 500 mg/ q12h x 7 | – | 1201) 602) 60 | NR | 1) 32/46 (69.6)2) 19/39 (48.7)p<0.05 | |
| Habu et al.26 | Japan | 1998 | RCT | PUD | 1) O 20 mg/q12h + A 500 mg/q6h + C 400 mg/q12h x 142) O 20 mg/q12 h + C 400 mg/q12h x 14 | – | 2341) 1252) 109 | 1) 92/125 (73.6)66–812) 60/109 (55.0)46–64; p<0.001 | 1) 60/97 (87.6)81–942) 60/109 (61.9)52–72; p<0.001 | 3 |
| Bosques – Padilla et al.27 | Mexico | 2004 | RCT | NUD, PUD | 1) Ra 20 mg/q12h + A 1 g/q12 h + Of 400 mg/q12 h x 72) Ra 20 mg/q12h + A 1 g/q12 h + Of 400 mg/q12 h x 14 | – | 761) 372) 39 | 1) 23/37 (62.2)46.1–762) 36/39 (92.3)79–98.1p=0.04 | 1) 23/36 (63.9)47.5–77.62) 36/37 (97.3)85–100p=0.01 | |
| Sacco et al.28 | Italy | 2009 | RCT | 1) E 20 mg/q12h + A 1 g/12h + Mo 400 mg/q12 h x 10.2) E 20 mg/q12 h + A 1 g/12h + Mo 400 mg/q12 h x 7.3) E 20 mg/q12 h + A 1 g/q12h + Mo 400 mg/q12h x 5.4) E 20 mg/q12h + A 1 g/q12h + Mo 400 mg/q 24h x 10. | 5) E 20 mg/q12 h + A 1 g/q12h + Mo 400 mg/q12h x 10.6) E 20 mg/q12 h + A 1 g/q12h + Ri 150 mg/q12h x 10. | 3931) 942) 1023) 924) 1055) 306)19 | 1) 85/94 (90.4)84.4–96.42) 82/102 (80.3)71.5–86.33) 70/98 (71.4)62.3–80.54) 84/105 (80)72.3–87.7p<0.0590.4 vs. 80.3p<0.0190.4 vs. 71.4p<0.0590.4 vs. 805) 26/30 (86.7)69.7–95.36) 16/19 (84.2)61.6–95.3 | 1) 85/90 (94.4)89.6–99.22) 82/96 (85.5)77.5–91.43) 70/93 (75.2)66.4–84.04) 84/99 (84.8)77.7–91.9p<0.05 1 vs. 2p<0.01 1 vs. 3p<0.04 1 vs. 45) 26/28 (92.2)76.2–99.16) (16/19) (84.2)61.6–95.3 | 3 | |
| Nista et al.29 | Italy | 2006 | RCT | NUD, PUD | 1) E 20 mg/q12h + A 1g/q12h + C 500 mg/q12h x 72) E 20 mg/q12h + C 500 mg/q12h + M 500 mg/q12h x 73) E 20 mg/q12h + C 500 mg/q12 h + L 500 mg/q24h x 7 | – | 3001) 1002) 1003) 100 | 1) 75/100 (75)69-812) 72/100 (72)67-783) 87/100 (87)81-9387% vs. 75%; p<0.05;87% vs. 72%; p<0.0175% vs. 72% p>0.05 | 1) 75/95 (79)73-852) 72/93 (77.4)71.4-84.43) 87/96 (90.6)84.6-96.690.6% vs. 79%, p<0.05; 90.6 vs. 77.4; p<0.0579% vs. 77.4% p>0.05 | 3 |
| Seven et al.30 | Turkey | 2011 | RCT | PUD, gastric cancer, MALT, others | 1) L 500 mg/q24h + A 1 g/q12h + PPI/q12h x 102) L 500 mg/q12h + A 1 g/q12h + PPI/q12h x 10 | – | 1101) 602) 50 | 1) 36/60 (60)54-642) 36/50 (72)66-78p=0.427 | 1) 36/60 (60)54-642) 36/50 (72)66-78p=0.427 | 3 |
| Polat et al.31 | Turkey | 2011 | RCT | NUD | 1) (E 40 mg/q12h + A 1 g/q12h x 7) + (E 40 mg/q12h + L 500/q6h+ M 500 mg/q8h x 7)2) E 40 mg/q12 h + A 1 g/q12h + C 500 mg/q12 h x 14 | – | 1391) 722) 67 | 1) 65/72 (90.2)83–962) 34/67 (50.7)44–57; p=0.000 | 1) 65/72 (90.2)83–962) 34/67 (50.7)44–57; p=0.000 | 3 |
| Fukuda et al.32 | Japan | 2006 | RCT | PUD | PPI+A+C | 1) La 30mg/q12h + A 750 mg/q12h + M 250 mg/q12h x 52) La 30mg/q12h + A 750 mg/q12h + M 250 mg/q12h x 7 | 831) 412) 42 | 1) 39/41 (95.1)61.6–95.32) 40/42 (95.2)61.6–95.3; p>0.05 | 1) 39/41 (95.1)61.6–95.32) 40/42 (95.2)61.6–95.3; p>0.05 | 3 |
| Matsuhisa et al.33 | Tokyo | 2006 | RCT | NUD, PUD, gastric cancer, others | PPI+A+C | 1) PPI (O 20 mg/q12h or La 30mg/q24h or R 10 mg/q12h) + A 750 mg/q12h + M 250 mg/q12h x 72) PPI (O 20 mg/q12h or La 30mg/q24h or R 10 mg/q12h) + A 750 mg/q12h + M 750 mg/q24h x 7 | 2281) 1212) 107 | 1) 106/121 (87.6)81.7–93.52) 93/107 (86.9)80.5–93.3p=0.876 | 1) 106/117 (90.6)85.3–95.92) 93/105 (88.6)82.5–94.7p=0.621 | 3 |
| Murakami et al.34 | Japan | 2006 | RCT | NUD, PUD | PPI+A+C | 1) La 30mg/q12h + A 750 mg/q12h + M 250 mg/q12h x 72) Fa 40mg/q12h + A 750 mg/q12h + M 250 mg/q12h x 7 | 611) 302) 31 | 1) 29/30 (97)83–992) 29/31 (94)80–99; p>0.05 | 1) 29/30 (97)83–992) 29/31 (94)80–99; p>0.05 | 3 |
| Ueki et al.35 | Tokyo | 2009 | RCT | PUD, NUD, others | PPI+A+C | 1) R 10 mg/q12h + A 750 mg/q12h + M 250 mg/q12h + C 200 mg/q12h x 72) R 10 mg/q12 h + A 750 mg/q12h + M 250 mg/q12 h x 7 | 1041) 522) 52 | 1) 45/52 (88.5)79.7–97.32) 43/52 (82.7)72.7–92.7p=0.407 | 1) 46/49 (93.9)87.1–1002) 43/51 (84.3)74.3–94.3p=0.129 | 4 |
| Navarro-Jarabo et al.36 | Spain | 2007 | RCT | PUD, NUD | PPI+A+C | 1) O 20 mg/q12h + B 120/q6h + T 500 mg/q6h + M 500 mg/q8h x 72) O 20 mg/q12h + A 1 g/q12h + Ri 150 mg/q12h x 7 | 991) 542) 45 | 1) 38/54(70.4)2) 20/45(44.4)p=0.009 | 1) 37/48(77.1)2) 20/43(46.5)p=0.002. | 3 |
| Lee et al.37 | South Korea | 2010 | RCT | PUD,NUD, gastric cancer, others | PPI+A+C | 1) E 20mg/q12h + B 300/q6h + T 500 mg/q6h + M 500 mg/q8h x 72) E 20mg/q12h + B 300/q6h + T 500 mg/q6h + M 500 mg/q8h x 14 | 2271) 1122) 115 | 1)72 / 112 (64.3)0,504 a 0,8302) 95 / 115 (82.6)1.165–2.499p=0.001 | 1) 71 / 92 (77.2)44–74.92) 88 / 94 (93.6)1.213–5.113p=0.01 | 3 |
| Uygun et al.38 | Turkey | 2008 | RCT | NUD | PPI+A+C | 1) La 30mg/q12h + B 300/q6h + A 1 g/q12h + M 500 mg/q12 h x 142) La 30mg/q12h + B 300/q6h + A 1 g/q12h + T 500 mg/q6 h x 143) La 30mg/q12h + B 300/q6h+ M 500 mg/q12 h + T 500 mg/q6 h x 14 | 3001) 1002) 1003) 100 | NR | 1) 68/91 (74.7)2) 75/92 (81.5)3) 78/95 (82.1)p>0.05 | 3 |
| Wu et al.39 | Taiwan | 2011 | RCT | PUD, NUD | PPI+A+C | 1) E 40 mg/q12h + B 120/6qh + T 500 mg/q6h + A 500 mg/q6h x 72) E 40mg/q12h + B 120/q6h + T 500 mg/q6h + M 250 mg/q6 h x 7 | 1201) 622) 58 | 1) 38/62 (62)50-752) 47/58 (81)71-91p=0.02 | 1) 49/ 59 (83)74-922) 35/55 (64)52-76p=0.01 | 4 |
| Gisbert et al.40 | Spain | 2007 | RCT | PUD, NUD | PPI+A+C | 1) RBC 400 mg/q12h + T 500 mg/q6h + M 250 mg/q6h x 52) L 500 mg/q12h + A 1 g/q12h + O 20 mg/q12h x 7 | 1001) 502) 50 | 1) 34/50(68)59–792) 34/50(68)59–79 p>0.05 | 1) 31/45(69)54–802) 32/45(71)57–82 p>0.05 | 3 |
| Castaño et al.41 | Colombia | 2012 | RCT | PUD, NUD | 1) O 20 mg/q12h + A 1 g/q12h + C 500 mg/q12 h x 72) O 20 mg/q12h + A 1 g/12h + C 500 mg/q12 h x 14 | – | 2931) 1492) 144 | 1) 101/149 (67.8)68–882) 107/144 (74.3)47–83;p=0.247 | 1) 98/136 (72.05)80–962) 99/122 (81.14)77–79; p 0.2p=0.086 | 3 |
| Riquelme et al.42 | Chile | 2007 | RCT | PUD, NUD | 1) O 20 mg/q12h + A 1 g/12 h + C 500 mg/q12 h x 72) O 20 mg/q12h + A 1 g/12 h + C 500 mg/q12 h x 14 | – | 1311) 692) 62 | 1) 54/69 (78.3)2) 53/62 (85.5)9%-23%p=0.37 | 1) 54/63 (87.5)2) 53/60 (88.3)p=0.37 | 3 |
| Robles-Jara et al.43 | Ecuador | 2008 | RCT | NUD, PUD, others | 1) O 20 mg/q12h + A 1 g/12 h + C 500 mg/q12 h x 72) O 20 mg/q12h + A 1 g/12h + C 500 mg/q12 h x 10 | – | 1) 652) 62 | 1) 44/65 (68)66.9-73.82) 52/62 (83.8)82.7-89.6p=0.03 | 1) 44/65 (68)66.6-782) 46/52 (88)86.4-98p=0.008 | 3 |
| Mazzoleni et al.44 | Brazil | 2011 | RCT | Functional dyspepsia | 1) O 20 mg/q12h + A 1 g/12h + C 500 mg/q12 h x 102) O 20 mg/q12h + placebo x 10 | – | 4041) 2012) 203 | 1) 164/201 (88.6)2) 14/203 (7.4)p<0.01 | – | 5 |
| Mera et al.45 | Colombia | 1999 | RCT | Atrophic gastritis | B 262mg/q6h + A 500mg/q8h + M 400 mg/q8h x 14 | 1) La 30 mg/q12h + A 1 g/q12h + C 500 mg/q12h x 142) O 30 mg/q12h + A 1 g/q12h + C 500 mg/q12h x 14 | 1431) 812) 62 | 1) (69)2) (70)114/143 (79.7)72-85p>0.05 | – | 3 |
| Fischbach et al.46 | Colombia | 2009 | RCT | Atrophic and non-atrophic gastritis | 1) O 20 mg/q12h + A 500 mg/q8h + C 500 mg/q12h + M 500 mg/q8h x 142) O 20 mg/q12h + A 1 g/q12h + C 500 mg/12 h x 7 | – | 1511) 752) 76 | 1) 65/75 (86.8)2) 65/76 (85.3)-9.7-12.7p=0.79 | 1) (96.7)2) (86.3)p=0.04 | 4 |
| Greenberg et al.47 | Latin America | 2011 | RCT | PUD, NUD | 1) La 30 mg/q12h + A 1 g/q12h + C 500 mg/q12h x 142) La 230 mg/q12h + A 1g/q12h + M 500 mg/q12h + C 500 mg/q12h x 53) (La 230 mg/q12h + A 1g/q12h x 5) + (La 230 mg/q12h + M 500 mg/q12h + C 500 mg/q12h x 5) | – | 14631) 4882) 4893) 486 | 1) 401/488 (82.2)78.5-85.52) 360/489 (73.6)69.5-77.53) 372/486 (76.5)72.5-80.282.2% vs. 76.5%, p=0.03782.2% vs. 73.6%, p>0.05 | – | 3 |
| Coelho et al.48 | Brazil | 2003 | RCT | Relatives of patients with gastric cancer | 1) L 30 mg/q24h + C 500 mg/q24h + F 400 mg/q24h x 72) L 30 mg/q24h + C 500 mg/q24h + F 200 mg/q24 h x 7 | – | 791) 402) 39 | 1) 35/40 (87.5)(73–96%)2) 24/39 (61.5)(45–77)p=0.006 | – | 3 |
| Sanches et al.49 | Brazil | 2008 | RCT | NUD, Duodenal ulcer | PPI+C+A/F | 1) R 20 mg/q24h + L 500 mg/q24h + F 400 mg/q24h x 102) R 20 mg/q12h + B 240 mg/q12h + D 100 mg/q12h + F 200 mg/12h x 10 | 601) 312) 29 | 1) 24/31 (77)62–932) 24/29 (83)68–97p=0.750 | 1) 24/30 (80)65–952) 23/28 (82)67–96p=1.0 | 3 |
A: amoxicillin; Az: azithromycin; B: bismuth; C: clarithromycin; q12h: twice a day; q6h: four times a day; q8h: three times a day; q24h: once a day; NUD: non-ulcer dyspepsia; PUD: peptic ulcer disease; RCT: randomized clinical trial; NRCT: non-randomized clinical trial; E: esomeprazole; Fa: famotidine; F: furazolidone; PPI: proton pump inhibitor; ITT: intention-to-treat; La: lansoprazole; L: levofloxacin; Me: metronidazole; Mo: moxifloxacin; NR: not reported; O: omeprazole; Of: ofloxacin; P: pantoprazole; PP: per-protocol; R: rabeprazole; Ri: rifabutin; T: tetracycline; Ti: tinidazole.
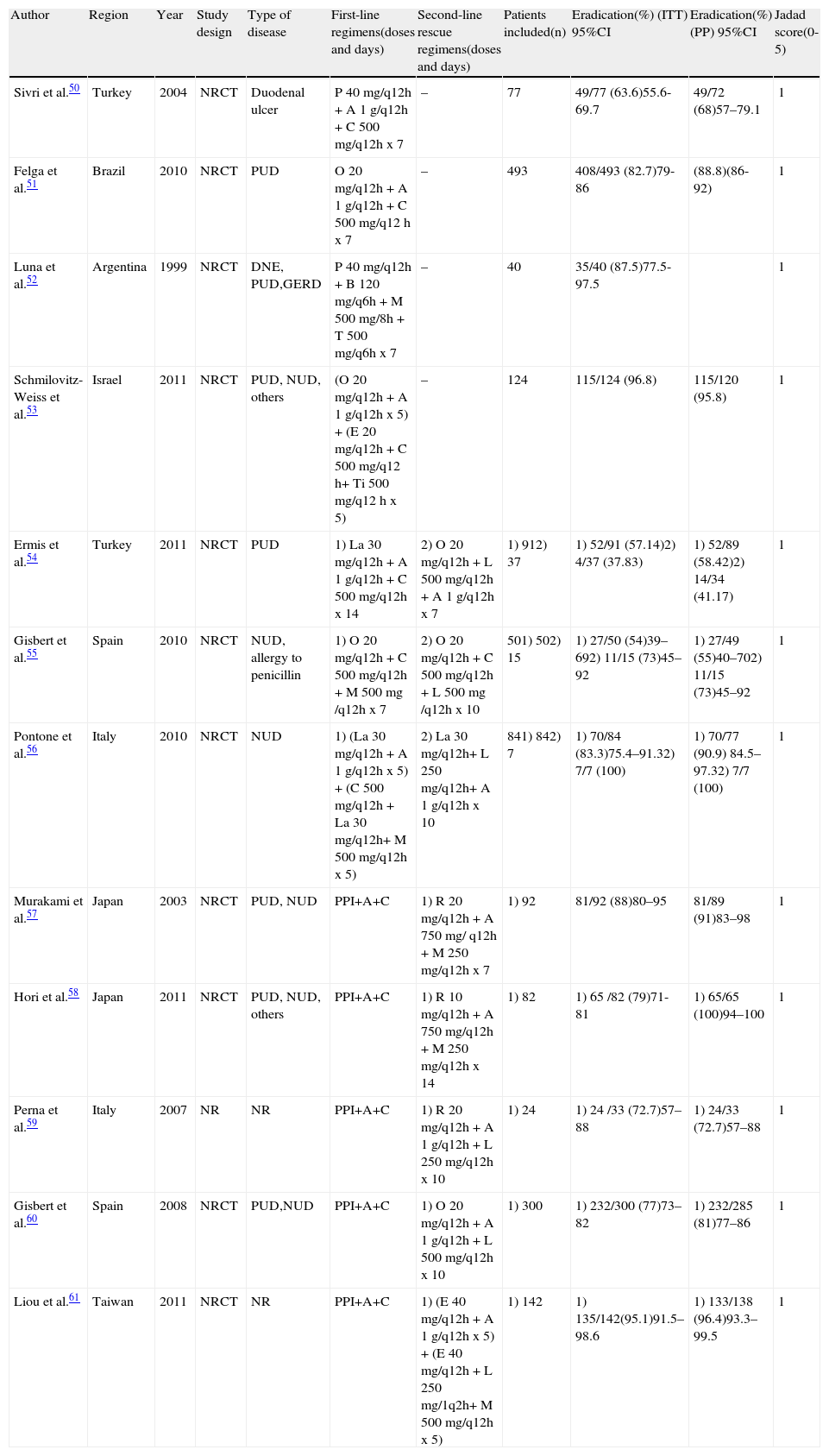
Non-randomized clinical experiments that evaluate first-line or second-line regimens for treating Helicobacter pylori infection.
| Author | Region | Year | Study design | Type of disease | First-line regimens(doses and days) | Second-line rescue regimens(doses and days) | Patients included(n) | Eradication(%) (ITT) 95%CI | Eradication(%) (PP) 95%CI | Jadad score(0-5) |
| Sivri et al.50 | Turkey | 2004 | NRCT | Duodenal ulcer | P 40 mg/q12h + A 1 g/q12h + C 500 mg/q12h x 7 | – | 77 | 49/77 (63.6)55.6-69.7 | 49/72 (68)57–79.1 | 1 |
| Felga et al.51 | Brazil | 2010 | NRCT | PUD | O 20 mg/q12h + A 1 g/q12h + C 500 mg/q12 h x 7 | – | 493 | 408/493 (82.7)79-86 | (88.8)(86-92) | 1 |
| Luna et al.52 | Argentina | 1999 | NRCT | DNE, PUD,GERD | P 40 mg/q12h + B 120 mg/q6h + M 500 mg/8h + T 500 mg/q6h x 7 | – | 40 | 35/40 (87.5)77.5-97.5 | 1 | |
| Schmilovitz-Weiss et al.53 | Israel | 2011 | NRCT | PUD, NUD, others | (O 20 mg/q12h + A 1 g/q12h x 5) + (E 20 mg/q12h + C 500 mg/q12 h+ Ti 500 mg/q12 h x 5) | – | 124 | 115/124 (96.8) | 115/120 (95.8) | 1 |
| Ermis et al.54 | Turkey | 2011 | NRCT | PUD | 1) La 30 mg/q12h + A 1 g/q12h + C 500 mg/q12h x 14 | 2) O 20 mg/q12h + L 500 mg/q12h + A 1 g/q12h x 7 | 1) 912) 37 | 1) 52/91 (57.14)2) 4/37 (37.83) | 1) 52/89 (58.42)2) 14/34 (41.17) | 1 |
| Gisbert et al.55 | Spain | 2010 | NRCT | NUD, allergy to penicillin | 1) O 20 mg/q12h + C 500 mg/q12h + M 500 mg /q12h x 7 | 2) O 20 mg/q12h + C 500 mg/q12h + L 500 mg /q12h x 10 | 501) 502) 15 | 1) 27/50 (54)39–692) 11/15 (73)45–92 | 1) 27/49 (55)40–702) 11/15 (73)45–92 | 1 |
| Pontone et al.56 | Italy | 2010 | NRCT | NUD | 1) (La 30 mg/q12h + A 1 g/q12h x 5) + (C 500 mg/q12h + La 30 mg/q12h+ M 500 mg/q12h x 5) | 2) La 30 mg/q12h+ L 250 mg/q12h+ A 1 g/q12h x 10 | 841) 842) 7 | 1) 70/84 (83.3)75.4–91.32) 7/7 (100) | 1) 70/77 (90.9) 84.5–97.32) 7/7 (100) | 1 |
| Murakami et al.57 | Japan | 2003 | NRCT | PUD, NUD | PPI+A+C | 1) R 20 mg/q12h + A 750 mg/ q12h + M 250 mg/q12h x 7 | 1) 92 | 81/92 (88)80–95 | 81/89 (91)83–98 | 1 |
| Hori et al.58 | Japan | 2011 | NRCT | PUD, NUD, others | PPI+A+C | 1) R 10 mg/q12h + A 750 mg/q12h + M 250 mg/q12h x 14 | 1) 82 | 1) 65 /82 (79)71-81 | 1) 65/65 (100)94–100 | 1 |
| Perna et al.59 | Italy | 2007 | NR | NR | PPI+A+C | 1) R 20 mg/q12h + A 1 g/q12h + L 250 mg/q12h x 10 | 1) 24 | 1) 24 /33 (72.7)57–88 | 1) 24/33 (72.7)57–88 | 1 |
| Gisbert et al.60 | Spain | 2008 | NRCT | PUD,NUD | PPI+A+C | 1) O 20 mg/q12h + A 1 g/q12h + L 500 mg/q12h x 10 | 1) 300 | 1) 232/300 (77)73–82 | 1) 232/285 (81)77–86 | 1 |
| Liou et al.61 | Taiwan | 2011 | NRCT | NR | PPI+A+C | 1) (E 40 mg/q12h + A 1 g/q12h x 5) + (E 40 mg/q12h + L 250 mg/1q2h+ M 500 mg/q12h x 5) | 1) 142 | 1) 135/142(95.1)91.5–98.6 | 1) 133/138 (96.4)93.3–99.5 | 1 |
A: amoxicillin; Az: azithromycin; B: bismuth; C: clarithromycin; q12h: twice a day; q6h: four times a day; q8h: three times a day; q24h: once a day; NUD: non-ulcer dyspepsia; PUD: peptic ulcer disease; RCT: randomized clinical trial; NRCT: non-randomized clinical trial; E: esomeprazole; Fa: famotidine; F: furazolidone; PPI: proton pump inhibitor; ITT: intention-to-treat; La: lansoprazole; L: levofloxacin; Me: metronidazole; Mo: moxifloxacin; NR: not reported; O: omeprazole; Of: ofloxacin; P: pantoprazole; PP: per-protocol; R: rabeprazole; Ri: rifabutin; T: tetracycline; Ti: tinidazole.
Geographically, the different studies were conducted in the United States, the United Kingdom, and Australia (4); Latin America (11); Italy (4); Spain (4); Turkey, Tunisia, Israel, and Greece (6); Japan, Taiwan, China, and South Korea (12). The treatments were first and second-line, with a duration of 1 to 14 days. All the randomized studies were prospective and open studies.
Randomized clinical trialsThree clinical trials conducted in the United Kingdom, the United States, and Greece compared the standard triple regimen using clarithromycin with the bismuth-containing quadruple therapy as first-line treatments. In the United Kingdom, the eradication rates in the ITT analysis of the standard triple regimen versus the bismuth-containing quadruple therapy of 7-day duration were 92 and 91%, respectively; in the United States the ITT analysis resulted in rates of 83.2 and 87.7%, respectively, with a 10-day treatment.19,20. In Greece, with a treatment of 10 days, the ITT eradication percentage for the triple therapy was 78% and it was 65% for the quadruple therapy.21 In China, the PP analysis of one-day quadruple therapy (PPI, bismuth, metronidazole, and amoxicillin) compared with 7-day standard triple therapy with clarithromycin, resulted in effectiveness rates of 39.5 and 84.6%, respectively.22 In Australia, a random comparison of 14-day triple therapy with bismuth, 7-day quadruple therapy with bismuth, and 7-day standard triple therapy with clarithromycin in 405 patients produced ITT eradication rates for each therapy of 69, 82, and 78%, respectively. The bismuth-containing quadruple therapy and the standard triple therapy had similar eradication rates; however, the bismuth-containing quadruple therapy was superior to the triple therapy with bismuth.23
Three clinical trials conducted in the United States, Japan, and Tunisia compared the eradication rates of different regimens of the standard triple therapy as first-line treatment. In the United States, the effectiveness of the standard triple therapy with rabeprazole for 3, 7, and 10 days and omeprazole for 10 days had eradication percentages in the ITT analysis as follows: 3 days 27%, 7 days 77%, and 10 days with rabeprazole 78% and 10 days with omeprazole 73%. There was no statistically significant difference between the 7-day regimen with rabeprazole and the 10-day regimens with rabeprazole and omeprazole.24 In Tunisia, the ITT eradication rates of the classic triple regimen with clarithromycin versus metronidazole of 7-day duration were 48.7 and 69.6%, respectively.25 In Japan, the ITT success rates of the standard triple therapy (omeprazole, amoxicillin, and clarithromycin) compared with the dual therapy (omeprazole and clarithromycin) of 14-day duration were 73.6 and 55%, respectively.26
Five randomized clinical trials from Mexico, Italy, and Turkey were reviewed, comparing the eradication rates of therapies based on quinolones. In the Mexican population the triple therapy with ofloxacin as first-line therapy at a dose of 800mg per day for 14 days, compared with a duration of 7 days, produced eradication rates in the ITT analysis of 92.3 and 62.2%, respectively, with a statistically significant difference.27 In Italy, as a first-line regimen, the standard triple therapy with moxifloxacin at a dose of 800mg per day and durations of 5, 7, and 10 days, and 400mg of moxifloxacin for 10 days, had eradication rates in the ITT analysis as follows: 5 days 71.4%, 7 days 80.3%, 10 days with 800mg of moxifloxacin per day 90.4%; and 10 days, 80% with 400mg of moxifloxacin per day. There was a statistically significant difference between the triple therapy with moxifloxacin at a 10-day dose of 800mg per day and the other therapeutic regimens.28 Additionally, in Italy the eradication rates of the triple therapies (PPI, clarithromycin, and metronidazole) versus (PPI, amoxicillin, and clarithromycin) versus (PPI, levofloxacin, and amoxicillin) for 7 days reached the respective ITT eradication rates of 75, 72, and 87%, whereas the triple therapy with levofloxacin showed a statistically significant difference in relation to the other therapeutic regimens.29 In Turkey, the effectiveness as first-line treatments of the 2 triple therapies with levofloxacin at different doses (500g/day versus 1g/day) had ITT success rates of 60 and 72.7%, respectively, even though the difference between the 2 groups was not statistically significant.30 Also in Turkey, the standard triple therapy with clarithromycin versus the sequential therapy with levofloxacin for 14 days reached an ITT success rate of 50.7 and 90.2%, respectively.31
Nine randomized clinical trials conducted internationally, with a total of 1,322 patients, compared the effectiveness of different second-line regimens after a first failed eradication attempt with the standard triple therapy using clarithromycin, obtaining the following results: In Japan, the eradication rates in the ITT analysis of the standard triple therapy with metronidazole for 5 and 7 days were 95.1 and 95.2%, respectively; with doses of metronidazole of 500mg/day versus 750mg/day, the success rates for each regimen in the ITT analysis were 87.3 and 86.9%.32,33 Other Japanese studies compared the eradication rates of the standard triple therapy with metronidazole and the triple therapy with famotidine, and the concomitant or quadruple therapy without bismuth for 7 days; the eradication rate in the ITT analysis for the standard triple therapy with metronidazole was 97% versus 94% for the triple therapy with famotidine; in the ITT/PP analyses for the standard triple therapy with metronidazole the eradication rate was 88.5%-93.9% versus 82.7%-84.3% for the concomitant or quadruple therapy without bismuth.34,35 In Spain, the bismuth-based quadruple therapy versus the rifabutin-based triple therapy, both of 7-day duration, achieved eradication percentages in the ITT analysis of 70.4 and 44.4%, respectively.36 In South Korea, the 7-day bismuth-containing quadruple therapy versus the same 14-day therapy showed effectiveness in the ITT analysis of 64.3 and 82.6% respectively.37 In Turkey, the 14-day quadruple therapy with a PPI and bismuth plus 2 different antibiotics (amoxicillin-metronidazole versus amoxicillin-tetracycline versus metronidazole-tetracycline) produced eradication rates in the PP analysis of 74.1, 81.5, and 82.1%, respectively, with no statistically significant differences between the therapeutic regimens.38 In Taiwan, the standard bismuth-based quadruple therapy (PPI, bismuth, tetracycline, and metronidazole) versus the quadruple therapy (PPI, bismuth, tetracycline, amoxicillin), both of 7-day duration, had the respective eradication rates in the ITT analysis of 81 and 62%.39 And finally, in the Spanish population, the triple therapy with ranitidine bismuth citrate (RBC), tetracycline, and metronidazole versus the triple therapy with levofloxacin were effective in 69 and 71%, respectively, with no statistically significant differences.40
Of the Latin American randomized clinical experiments included in the study, the following eradication rates for the clarithromycin-based standard triple therapy as first-line treatment were obtained: in Colombia, the effectiveness of 7-day versus 14-day treatment reached ITT eradication rates of 67.8 and 74.3%, respectively;41 in Chile, 7-day versus 14-day treatment had rates of 78.3 and 85.5%;42 in Ecuador, 7-day versus 10-day treatment, 68.3 and 83.8%;43 and in Brazil, the standard triple therapy with clarithromycin versus a control group (PPI plus placebo), both of 10-day duration, had rates of 88.6 and 7.4%, respectively.44 As second-line therapy, in Colombia, the 14-day clarithromycin-based triple therapy with lansoprazole versus omeprazole reached eradication rates of 69 and 70% in each respective regimen, with no statistically significant difference.45 The standard triple therapy, compared with the 7-day concomitant therapy versus 14 days as first-line therapies, had the respective eradication rates of 86.3 versus 96.7% in the PP analysis.46 Additionally, in a study conducted in different Latin American countries, the eradication rates of the standard triple therapy for 14 days, the concomitant therapy for 5 days, and the sequential therapy for 10 days were 82.2, 73.6, and 76.5% in the ITT analysis.47 Finally, in Brazil, the triple therapy with furazolidone (PPI plus clarithromycin) for 7 days had significantly higher ITT eradication rates with 400mg per day of furazolidone compared with 200mg per day of furazolidone (87.5% versus 61.5%); and as a second-line regimen, the triple therapy with furazolidone (PPI, levofloxacin, and furazolidone), administered once a day for 10 days, compared with the quadruple therapy with furazolidone (PPI, bismuth, doxycycline, and furazolidone), administered twice a day, had eradication rates in the ITT analysis of 77 and 83%, respectively.48,49
Non-randomized clinical trialsAs first-line treatments, the eradication rates of the clarithromycin-based triple therapy of 7-day duration was evaluated in 2 clinical trials: In the first one, the cure percentage was 68% in the PP analysis and 63.6% in the ITT analysis;50 in the second one, the eradication rate in the ITT analysis was 82.7% and 88.8% in the PP analysis.51 The 7-day bismuth-containing quadruple therapy had 87.5% effectiveness in the ITT analysis52 and the 10-day sequential therapy had 95.8% effectiveness in the PP analysis (115/124) and 92.7% effectiveness in the ITT analysis (115/120).53
Three non-randomized clinical trials also evaluated first-line treatment eradication rates (triple therapies with clarithromycin, metronidazole, and sequential therapies), followed by the levofloxacin-based triple therapy as second-line treatment, obtaining the following results: the standard clarithromycin-based triple therapy for 14 days reached an efficiency rate of 57.14% in the ITT analysis and one of 37.83% in the second-line levofloxacin-based triple therapy for 7 days.54 The triple therapy with clarithromycin and metronidazole attained eradication in 27 out of 50 cases, with an effectiveness of 54% in the ITT analysis, and as rescue treatment, the levofloxacin-based triple therapy achieved eradication in 11 of the 15 cases (73%).55 The effectiveness of the sequential therapy as first-line therapy reached 83.3% eradication in the ITT analysis and 90.9% eradication in the PP analysis; as second-line therapy, the levofloxacin-based triple therapy of 10-day duration had 100% eradication in both the ITT and PP analyses.56
Finally, 5 non-randomized clinical trials evaluated the eradication rates of different second-line therapies after failed standard clarithromycin-based triple therapy with the following results: The standard triple therapy with metronidazole of 1-week duration had an 88% eradication rate in the ITT analysis and a 91% eradication rate in the PP analysis;57 with a 2-week duration the eradication rate was 96% in the ITT analysis and 100% in the PP analysis.58 The effectiveness of the 2 triple therapies with levofloxacin of 10-day duration was 72.7% in both the ITT and PP analyses59 for the first study, whereas in the second study, the eradication rate was 81% in the PP analysis and 77% in the ITT analysis.60 The sequential therapy with levofloxacin for a duration of 10 days achieved an eradication rate of 95.1% in the ITT analysis and 96.4% in the PP analysis.61
DiscussionThe effectiveness of the different available therapeutic regimens, both first-line and second-line, included in the present systematic review for defining the ideal treatment for infection due to H. pylori varies significantly worldwide and there is currently no ideal therapeutic strategy for eradicating H. pylori infection in 100% of the cases. The proposal based on the standard triple therapy has not provided satisfactory results. Its first disadvantage is the resistance to antimicrobial drugs, especially to clarithromycin and metronidazole, and therefore many infected persons throughout the world do not benefit from these regimens. The sequential and concomitant regimens are good alternative treatments. The triple therapy with levofloxacin, as second-line treatment, is an alternative that has had excellent eradication results. Combinations that include rifabutin and furazolidone are also promising therapeutic possibilities.
Based on the observations of our review, the standard triple therapy as a first-line regimen of 1-week duration shows sub-optimal eradication rates in countries such as Turkey, Colombia, Ecuador, Japan, and the United States.24,26,41,43,50 However, in other countries such as Brazil and the United Kingdom, the eradication rates were higher;19,51 these results were similar to those obtained in the countries with low rates of resistance to clarithromycin (lower than 15 to 20%). Moreover, with therapies lasting longer than 7 days, greater eradication effectiveness was not found in countries that included the United States, Colombia, and Chile.24,41,42 These results are equivalent to those observed in the meta-analysis conducted by Fuccio et al.,62 that did not find a higher eradication rate in therapies of more than 7-day duration; likewise, in 3 randomized clinical trials conducted by Kim et al.,63 and Zagari et al.,64 the 2-week regimens did not have higher eradication rates than those of 1-week duration. Therefore, our review suggests that a duration greater than 7 days does not improve the performance of the standard triple therapy and that the resistance to antibiotics is the principal cause of eradication failure.18
On the other hand, the triple therapies with amoxicillin and the triple therapies with metronidazole for 7 days were equally effective, but the H. pylori eradication rates were unacceptable.29 Even though the triple therapies at high and low doses of metronidazole are considered effective, there were no significant differences in their effectiveness with respect to dose.33 There were also no differences in the 7-day triple therapies with clarithromycin in relation to the PPIs employed: pantoprazole and omeprazole, lansoprazole and omeprazole, or rabeprazole and omeprazole.24,25,45,50 The eradication rates, even though they were significantly higher with the triple therapy with clarithromycin, are still poor with respect to the dual therapy with clarithromycin, which achieved a better, but still unacceptable rate, according to the previously discussed criteria.26,65 Based on the results obtained from the different clinical trials included in this study, conducted in different geographic zones, it is suggested that this latter therapy reaches unacceptable eradication rates for cure (< 80%) in countries with growing rates of resistance to clarithromycin and metronidazole.12 Nevertheless, these results cannot be generalized worldwide, given that this therapeutic option is still adequate in countries that have H. pylori strains with a low resistance to clarithromycin (< 15-20%) and metronidazole (< 40%).10
In the presence of the growing increase in resistance to clarithromycin and the sub-optimum eradication rates offered by the standard triple therapy, new rescue therapies have emerged, such as the bismuth-containing quadruple therapy. In 3 studies included in our review, this therapy given for 7 days has obtained acceptable effectiveness in the ITT analyses and good effectiveness when given for 10 or 14 days.20,23,37,39,52 Our findings are adequately correlated with those of a meta-analysis conducted by Fischbach et al.66 in which they conclude that the quadruple therapies of 2-week duration improve effectiveness in relation to those of 1-week duration. In our review, the eradication rates of the quadruple therapy versus the standard triple therapy as first-line treatments were similar and there was no statistically significant difference between these 2 therapeutic regimens of 7 or 10-day duration.19–21,23 Likewise, our results are equivalent to those demonstrated in 2 meta-analyses conducted by Gene et al.,67,68 in which the quadruple therapy and the triple therapy reached similar eradication rates in the ITT analysis (79 and 80%; p=1).21 Despite the heterogeneity of the regimens included in this review, it can be concluded that the administration of treatments with a prolonged duration (greater than or equal to 10 days) and at high doses of bismuth would enable more effective eradication rates to be achieved.69
On the other hand, the standard triple therapy with metronidazole as second-line treatment, after the therapeutic failure of standard triple therapy with clarithromycin, obtained ideal eradication levels in our review; in particular, 4 clinical trials conducted in Japan show good eradication percentages in 5 days in the ITT analysis and excellent ones in 7 days.32,34,58 This therapy also shows itself to be effective as a second-line regimen at doses of metronidazole of 500 or 750mg/day, with good success rates in the ITT analyses for both regimens, even reaching up to a 93.5% eradication rate.33 The previous results could be explained by taking into account the geographic location in which the clinical experiments were carried out, given that the resistance rates to metronidazole are low in Japan (approximately 4%), whereas the resistance to clarithromycin is high (approximately 27.7%); this is above the recommended resistance limit for using clarithromycin (< 15-20%), but it is still within the accepted resistance limit for using metronidazole (< 40%).70 Nevertheless, when the standard triple therapy with clarithromycin fails, this antibiotic should not be included in the second-line treatment, because its secondary resistance would increase drastically.71
In our review, as first-line treatment the 10-day sequential therapy offers superior eradication rates with excellent effectiveness in the ITT analysis, compared with the 7-day standard triple therapy that has shown poor effectiveness.31 The sequential therapy provides good cure rates, above 90%,13 concurring with the results of 3 clinical trials conducted in Israel, Taiwan, and Italy that are included in the present review. It was used as first-line therapy and reached effective ITT eradication rates that were excellent and good in the latter two countries, respectively.53,56,61 Similar results have been reported in the systematic reviews carried out by Moayedi,72 Jafri et al.,73 and Grigorios et al.74
Another effective alternative to the standard triple therapy is concomitant or quadruple therapy without bismuth. In our review, 2 studies with concomitant therapy showed greater eradication in the PP analyses, excellent versus acceptable compared with the standard triple therapy.35,46 Likewise, meta-analyses conducted by Essa et al.75 and Georgopoulos et al.,76 obtained similar results to those of the present review.13 However, contrary to the previously obtained results, a Latin American study showed that the 14-day standard triple therapy was more effective than the 5-day concomitant therapy or the 10-day sequential therapy, with acceptable, unacceptable, and poor eradication rates, respectively.1,47 The discrepancies with the previous results could be explained by geographic variations and patterns of resistance to clarithromycin. As the authors of the Latin American study explained, the population included in the clinical experiment had a low prevalence of clarithromycin resistance and therefore achieved better results with the standard triple therapy.47,77,78
As clarithromycin replacement, therapy with quinolones associated with amoxicillin in the regular doses and a PPI twice a day has been suggested. In our review, the triple therapy with levofloxacin with a duration of 7 to 14 days was evaluated in 7 clinical trials and achieved poor to excellent eradication rates in the ITT analyses; effectiveness improved with the longer treatments.27,29,30 Additionally, as first-line therapy, this treatment was more effective than both the triple therapy with clarithromycin of 7-day duration and the triple therapy with metronidazole.29 Likewise, no significant difference was found between the doses of levofloxacin of 500mg once a day versus 500mg twice a day. Effectiveness was unacceptable in the two cases, whereas in relation to the 10-day sequential therapy, greater eradication rates in the PP and ITT analyses were obtained, excellent versus acceptable, respectively.30,56 The above results are similar to those described by Kuo et al.,13 Molina-Infante et al.,79 and Cheng et al.80 As second-line rescue therapy, the eradication rates varied from acceptable to excellent with 10-day therapies,28,56,59,60 whereas they ranged from poor to unacceptable with 7-day treatments.40,54 The least successful eradication rate was obtained in a clinical trial conducted in Turkey, and might be related to the fact that this therapy could present success failures in Asian and European populations with a high resistance to fluoroquinolones, reducing their effectiveness to rates of approximately 33%.81,82 The abovementioned studies suggest that the triple therapy with a dose of 400mg/day of levofloxacin is less effective compared with a therapy using 800mg per day, whereas better results could be obtained with more prolonged therapies.28 Finally, it is concluded that the triple therapy with levofloxacin is an effective first and second-line treatment that offers better eradication rates in relation to the standard, quadruple, and sequential therapies.
Other second-line rescue antibiotics such as rifabutin and furazolidone have been employed. In this review, we evaluated a 7-day therapy with rifabutin associated with omeprazole and amoxicillin and it had a quite unacceptable effectiveness, much lower in relation to the bismuth-containing quadruple therapy that obtained poor effectiveness in the ITT analysis.36 However, these results can most likely be explained by the fact that a 7-day duration is not sufficient, given that other studies have published more effective results with longer treatments; therapies of 10 and 12 days have offered good eradication rates and those of 7-day duration have shown poor rates.83 The latter result could be related to the doses of amoxicillin and PPI employed, taking into account the study conducted by Borody et al.,84 in which higher 12-day doses of these antibiotics were used, showing effective results as second-line rescue therapy. Nevertheless, currently there is no clear optimal therapeutic regimen with rifabutin.13 In regard to furazolidone, an additional antibiotic that has been used in rescue treatments in quadruple combination (bismuth, doxycycline, PPI, and furazolidone), had excellent success in the ITT analysis and good success in the triple combination (furazolidone, levofloxacin, and PPI).49 Upon increasing the dose of furazolidone, we observed a poor eradication rate with a dose of 200mg per day and a good rate with a dose of 400mg.48 The promising eradication rates of the therapy with furazolidone could be explained by the absence of primary resistance to this antibiotic and the absence of crossed resistance with metronidazole.85 Therefore, in the underdeveloped countries with a high resistance to metronidazole, therapies with furazolidone could be effective rescue therapies.86
The eradication of H. pylori is also a challenge in patients that are allergic to penicillin, in both first-line treatments and in cases of previous therapeutic failure. In our review, therapy with omeprazole, clarithromycin, and metronidazole of 7-day duration obtained quite unacceptable effectiveness of under 70% success in the ITT and PP analyses. On the other hand, triple therapy with levofloxacin, omeprazole, and clarithromycin of 10-day duration was a good second-line strategy, with good cure rates in the ITT and PP analyses.55 Nevertheless, these results differ from previous studies in which eradication success was poor with the use of PPI, clarithromycin, and metronidazole. Once again, these results can be correlated with the area in which the clinical trial was conducted, given that in Spain there is very high resistance to metronidazole and clarithromycin, of approximately 32.8% and 49.2%.87 In addition, different authors that have evaluated the combination of levofloxacin as second-line rescue therapy reported similar eradication rates, with excellent effectiveness in the ITT analysis.29,88 With this in mind, our suggestion for patients with a history of allergy to penicillin is the triple therapy with PPI, clarithromycin, and metronidazole; it is an adequate first-line strategy in countries where there is low resistance to these antibiotics. In other words, the regimens with levofloxacin achieve good eradication rates as a second-line alternative in this type of patient.29,58,89
In a systematic review carried out by Gisbert et al.90 that included 18 randomized clinical trials in their analysis there was a difference that was not statistically significant between the eradication rates of the triple regimens based on nitroimidazoles and amoxicillin; these results were similar to ours. According to the findings of 4 systematic reviews91–93 that compared triple regimens with different PPIs as first-line treatment, 3 of them did not show a significant difference in their eradication rates, concurring with our review. However, in contrast, an additional systematic review favored the regimens based on esomeprazole.91 Our results indicate that treatment duration above 7 days does not improve the effectiveness of the standard triple therapy, but this differs with the systematic review of Calvet et al.;94 they included 7 randomized clinical trials in their analysis and justify the use of 14-day therapies versus 7-day therapies. Nevertheless, in these 7 studies different therapeutic combinations were employed: PPI plus amoxicillin and clarithromycin, clarithromycin plus metronidazole or amoxicillin and clarithromycin and metronidazole. Because of the above, it is difficult to compare our findings with those of that study. Even so, these results would indicate that the high rates of antibiotic resistance in different geographic regions are the main cause of eradication failure, more so than the length of time of treatment.
Adamek et al., 71 support our results with a similar effectiveness between the triple and quadruple therapies as first-line regimens. This is also validated by the results obtained in the systematic review by Calvet et al., 94 analyzing 5 randomized clinical trials without finding significant differences in the eradication rates of these therapies. Likewise, the latter work indicates that the triple therapies based on nitroimidazoles and amoxicillin show similar eradication rates for H. pylori, while the triple therapies using different PPIs show equal eradication effectiveness.71 Like ours, a systematic review by Huang and Hunt95 reports similar eradication rates between clarithromycin at single and double doses. Two systematic reviews72 that include 12 clinical trials in their analysis support our results with respect to the use of the 10-day sequential regimen, compared with the 7 and 10-day triple regimens as first-line treatments. However, the Italian authors indicate that these results must be reliably corroborated by large-scale studies.
For reaching more effective eradication rates in regard to the second-line therapies, in their review Megraud and Marshall65 recommend not using clarithromycin in the case of therapeutic failures of first-line regimens based on this antibiotic, and suggest doubling the dose of metronidazole in the case of a previous eradication failure with that drug; they also state that second-line treatment should be given for a longer period of time than first-line treatment. Even though we did not find systematic reviews that compared the quadruple versus the triple regimens as second-line regimens, there is a current controversy as to which regimen is more effective, according to the results of 3 randomized clinical trials by Lee et al.,96 Magaret et al.,97 and Peitz et al.98 The results of 2 large meta-analyses 99,100 similarly corroborate that the triple therapy with levofloxacin is capable of reaching eradication rates even higher than the quadruple therapy in those patients that have had first-line standard triple therapy failure.
A limitation of the present systemic review, now proposed for a future study, was not having incorporated the antimicrobial susceptibility of the different therapeutic regimens into the search parameters; doing so would have addressed the important problem of resistance that does not allow for eradication rates above 95% to be obtained. Likewise, given today's complex regimens, treatment adherence, safety, and adverse effects, as well as the cost-benefit evaluation of therapeutic interventions should all be taken into consideration, enabling an overall approximation in the evaluation of the ideal H. pylori treatment. On the other hand, because of the high clinical heterogeneity (populations, interventions, study durations and designs) of the clinical trials evaluating the effectiveness of both first and second-line therapies, future systematic reviews should be carried out. This type of epidemiologic design is thought to be the most appropriate today for elaborating the solid knowledge that can provide the medical community with the most adequate competencies for the ideal management of this infection. The inclusion of clinical trials at the worldwide level makes this a comprehensive work in which it is possible to have representative results in different patient populations.
ConclusionsInfection from Helicobacter pylori should be treated as any other infectious disease, which is why a therapy must be capable of curing more than 95% of the cases in the first therapeutic attempt in order to be regarded as excellent.6 The effectiveness of the different available treatment strategies varies significantly within different geographic regions. Such is the case with the standard triple therapy, which continues to be ineffective in countries with strains that are resistant, mainly to agents such as clarithromycin and metronidazole, making this treatment unacceptable as empirical therapy. Among the first-line treatment options is the bismuth-based quadruple therapy, which did not show significant differences in its eradication rates versus those of the standard triple therapy. However, the sequential and concomitant therapies have superior eradication results, with the potential to improve their effectiveness through increasing treatment duration, whereas the triple therapy with levofloxacin offers the best results as first and second-line treatment. With this in mind, a 10-day sequential treatment could be used as first-line treatment and a 10-day levofloxacin-based regimen as retreatment. As a second-line regimen it should be administered for a longer period of time than the first-line treatment; if there is initial failure with the clarithromycin-based regimen, this antibiotic should not be used again. On the contrary, if there is initial failure with metronidazole, increased dose and treatment duration with this same drug is recommended as rescue treatment.
The order of choice of the different H. pylori eradicating regimens will depend on local studies in which the rates of resistance to the different antibiotics are determined, in order to give the appropriate initial treatment, ruling out the less effective therapies according to the experiences of each country.70 However, we consider that antimicrobial sensitivity should not be systematically evaluated after a first therapeutic failure so that effective eradication results can be achieved. 65,101
It is imperative to carry out a greater number of large-scale studies and of double-blind, randomized clinical trials with excellent methodological quality that compare current standard treatments with new therapies, so that the ideal therapeutic regimen can be established. The success rate of such beneficial treatment should be as close to 100% as possible.102
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare there was no conflict of interest.
Please cite this article as: Sierra F, Forero JD, Rey M. Tratamiento ideal del Helicobacter pylori: una revisión sistemática. Revista de Gastroenterología de México. 2014;79:28–49.