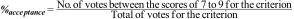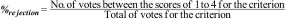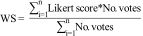Inflammatory bowel disease (IBD) is a chronic and incurable entity. The aim of the Pan American Crohn's and Colitis Organisation (PANCCO) is to create awareness of IBD, with special emphasis on Latin America, and the primary objective of the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU, the Spanish acronym) is to obtain the accreditation of the clinical and therapeutic criteria for the diagnosis and treatment of IBD.
AimTo carry out a consensus for evaluating the approval criteria that a Comprehensive Care Clinic for Latin American IBD patients must meet, to be considered a center of excellence.
Materials and methodsFourteen clinical experts participated in the consensus. They were made up of specialists in gastroenterology, with broad clinical experience, spanning several years, in managing the care of a large number of patients with IBD, as well as advanced specialists in IBD. Thirteen of the participants came from 11 Latin American countries (Argentina, Brazil, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Peru, Puerto Rico, Uruguay, and Venezuela) that have IBD clinics. An expert from Spain, representing the GETECCU, provided the methodologic support.
The consensus consisted of 52 statements divided into three sections: 1) Structure indicators, 2) Process indicators, and 3) Result indicators. The Delphi panel method was applied.
ResultsThe present Latin American consensus describes the quality indicators that a Comprehensive Care Clinic for IBD patients must meet, to be considered a center of excellence, taking into account the needs of our region.
ConclusionsThis is the first Latin American consensus, jointly carried out by the PANCCO and GETECCU, to present accreditation standards for centers of excellence in the care of patients with IBD.
La enfermedad inflamatoria intestinal (EII) es una entidad crónica e incurable. La Pan American Crohn's and Colitis Organisation (PANCCO) tiene como objetivo generar concientización sobre la EII, con especial énfasis en Latinoamérica, y el Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) tiene por objetivo primordial procurar la homologación de criterios clínico-terapéuticos para el diagnóstico y tratamiento de la EII.
ObjetivoRealizar un consenso para la evaluación de los criterios de aprobación que debe cumplir una Clínica de Atención Integral de pacientes con EII a nivel latinoamericano para ser considerada un centro de excelencia.
Material y métodosSe contó con la participación de 14 expertos clínicos especialistas en gastroenterología, con amplia experiencia clínica de varios años en el manejo de la atención a numerosos pacientes con EII, así como también aquellos que tuvieran la formación de alta especialidad en EII. Todos pertenecientes a 11 países latinoamericanos: Argentina, Brasil, Colombia, Ecuador, Guatemala, México, Perú, Puerto Rico, República Dominicana, Uruguay y Venezuela, en donde tienen clínicas de EII; así mismo, también participó un experto de España como apoyo metodológico, representante de GETECCU.
El consenso consistió de 52 enunciados divididos en tres secciones: 1) Indicadores de estructura; 2) Indicadores de proceso y 3) Indicadores de resultados. Se aplicó el método de panel Delphi.
ResultadosEl consenso latinoamericano muestra los indicadores de calidad que debe cumplir una Clínica de Atención Integral de pacientes con EII para ser considerada un centro de excelencia teniendo en cuenta las necesidades de nuestra región.
ConclusionesEs el primer consenso latinoamericano realizado de manera conjunta por la PANCCO y GETECCU para presentar los estándares para aprobar centros de excelencia en la atención de pacientes con EII.
Ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis (IC) are forms of inflammatory bowel disease (IBD), a chronic, incurable disease of multifactorial and complex etiology. It presents with periods of remission and relapse, with extraintestinal involvement in up to 50% of patients, mainly affecting the joints, skin, and eyes, and has a significant impact on patient quality of life.1,2 The aim of the Pan American Crohn’s and Colitis Organisation (PANCCO) is to create awareness about IBD in all countries on the American continents, with special emphasis on Latin America, to provide optimum care for the patients with the disease. The primary objectives of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU, the Spanish acronym) are the study of and research on IBD, as well as obtaining the accreditation of the clinical and therapeutic criteria for its diagnosis and treatment. In 2014, the GETECCU published the first quality standards in the management of IBD, which have been the basis for the comprehensive care unit approval program in Spain.3
Based on the GETECCU standards, and utilizing the modified Delphi method, the PANCCO and GETECCU jointly developed a consensus whose aim was to provide the best agreement possible in relation to the approval criteria that must be met by a Comprehensive Care Clinic for patients with IBD, to be considered a center of excellence in Latin America, thus improving standards and quality of care offered to those patients. The two associations also worked in conjunction with the Colombian Invalue Health Solutions consulting agency, an organization that creates real-life, precise, and applicable management and consulting support solutions.
The aim of the present consensus was to provide the approval criteria that must be met by a Latin American Comprehensive Care Clinic for patients with IBD, to be considered a center of excellence.
MethodsThe development of the present work encompassed 2 important phases: the preparation phase and the consultation phase. The latter phase was carried out in 3 stages: a first round of asynchronous consultation, a synchronous deliberation session, and a second round of asynchronous consultation. Clinical experts from different Latin American countries, all specialists in gastroenterology, were present at the three stages. The second phase followed the modified Delphi panel deliberation method employed by different international organizations, such as the National Institute for Health and Care Excellence (NICE) of the United Kingdom,4 the Haute Autorité de Santé (HAS) of France,5 and the Instituto de Evaluación Tecnológica en Salud (IETS) of Colombia.6 Previous studies have evaluated quality indicators on the care of patients with IBD.7–10
Preparation phaseFirst, a list the approval criteria to be included in the project was obtained from a joint review carried out by the PANCCO and GETECCU, based on the GETECCU standards, to guarantee their adjustment to the Latin American context. Once the preliminary adaptations of the criteria were carried out, they were categorized into three groups, according to their scope: Criteria of Structure, Criteria of Process, and Criteria of Results.11 Fifty-two criteria that would then go on to the subsequent phase were produced and the steps were defined for evaluating their level of importance and the level of agreement reached by the clinical experts. The criteria were also graded as a primary component of a Center of Excellence, based on a Likert scale:
to 9-point Likert scale: Any number between 1 and 9 could be chosen, resulting in the following categories:
Score of 1-3 = In complete disagreement with the approval criterion
Score of 4-6 = Not in agreement or disagreement with the approval criterion
Score of 7-9 = In complete agreement with the approval criterion
Once the scores of the clinical experts were obtained for each of the criteria posited, the corresponding statistical analysis was performed that enabled the classification to be made, according to the importance, acceptance, rejection, and priority of each of the criteria. The measures proposed included:
- 1
Median and interquartile range: Those measures of central tendency were determined, identifying the dispersion of the opinions on each criterion.
- 2
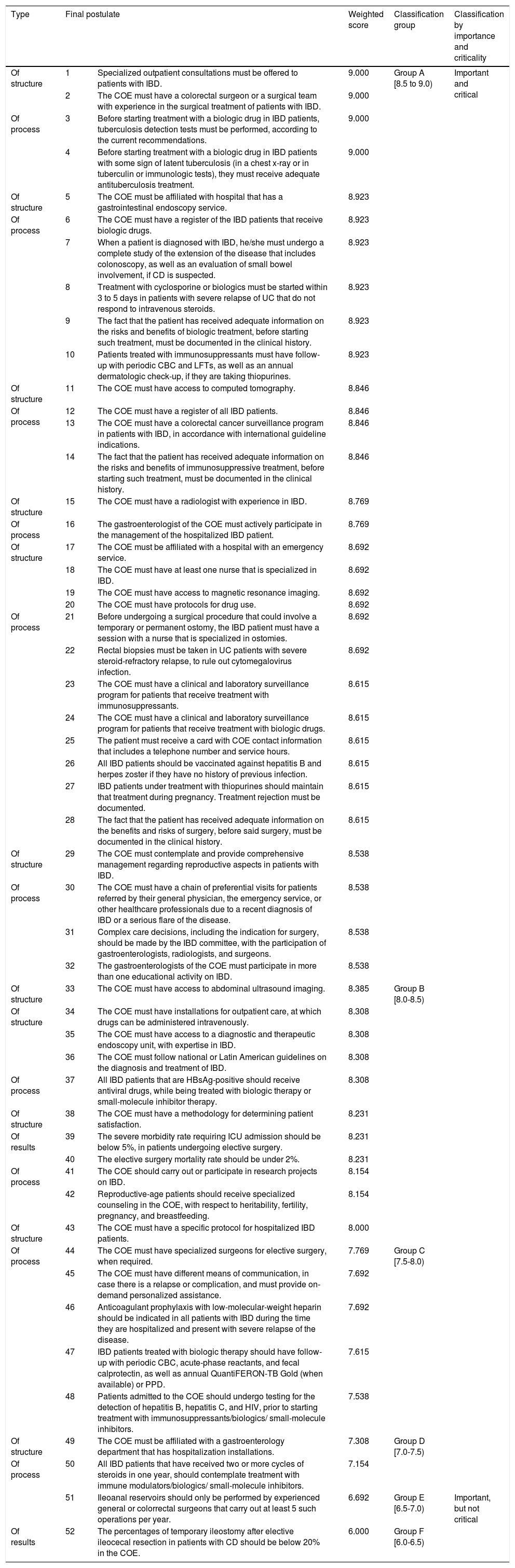
Acceptance percentage: Considering that the scores between 7 and 9 indicated a very high level of agreement for the criterion, as part of the requirements for a Center of Excellence, the acceptance percentage of each criterion was determined by the experts, with the following indicator in mind:
- 3
Rejection percentage: Considering that the score between 1 and 3 indicated a high level of disagreement for the criterion as part of the requirements for a Center of Excellence, the rejection percentage for each criterion was determined by the experts, as follows:
- 4
Weighted score (WS): The weighted Likert score was determined for each criterion, showing the criteria with the highest votes, in the order from greater to lesser usefulness, according to the weight obtained, as follows:
This phase included the use of a modified Delphi panel, consisting of 14 clinical experts, all specialists in gastroenterology. Thirteen of them came from 11 Latin American countries: Argentina, Brazil, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Peru, Puerto Rico, Uruguay, and Venezuela and methodological support was provided by a clinical expert, specialist in gastroenterology, from Spain, representing the GETECCU.
First round of the panelThe first consultation round was asynchronous and carried out on August 16, 2020. A questionnaire was sent to each expert for grading the proposed criteria in the preparation phase, utilizing the defined Likert scale, to be filled out no later than August 26, 2020.
Once the clinical experts provided their level of agreement, utilizing the proposed Likert scale, the votes were managed, according to the methodology described for the preparation phase, preserving the anonymity of the respondents. The criteria were categorized into groups, according to their weighted scores, starting from 9, which was the highest possible score. The following 3 classes were produced:
- •
Class A: Postulates that had a weighted score of [8.5-9.0)
- •
Class B: Postulates that had a weighted score of [8.0-8.5)
- •
Class C: Postulates that had a weighted score of [7.5-8.0)
The synchronous session took place virtually, on August 27, 2020. The following objectives were put forward: to present the results of the first round, to consolidate the interventions of the experts regarding the proposed criteria, and finally, to define the quality criteria to be newly evaluated in a later second consultation round. That session consisted of three steps:
- 1
Introduction: The participants were welcomed, clarifying the goal of the meeting and the methodology to be employed.
- 2
Presentation and discussion of the results: The participants greeted one another and the results of the first round were discussed. The interventions were carried out in turns, assigned by the Invalue Health Solutions facilitators.
- 3
Comment consolidation: The criteria rejected by at least one of the participants, and that had high dispersion, together with a low Likert score, were defined, to carry out the corresponding discussion and determine whether they remained as quality criteria for a Center of Excellence in the care of IBD patients. Adjustments in form and content were made for the final criteria, to proceed with the new grading in the second consultation round.
The second questionnaire with the criteria selected for the final consultation was sent on August 31, 2020, utilizing the question, “Do you agree with the postulate….?”. The deadline for responding to the questionnaire was September 9, 2020.
From the information collected in the second round, the individual processing of all the registers was carried out, utilizing the methodology presented in the preparation phase, preserving the anonymity of each expert, with respect to the responses.
Finally, the approval criteria for Centers of Excellence in the care of IBD patients was consolidated, resulting in the present consensus document and illustrated in Table 1, reviewed and approved by the members of the PANCCO and GETECCU.
Statements of quality required for COE in IBD care.
| Type | Final postulate | Weighted score | Classification group | Classification by importance and criticality | |
|---|---|---|---|---|---|
| Of structure | 1 | Specialized outpatient consultations must be offered to patients with IBD. | 9.000 | Group A [8.5 to 9.0) | Important and critical |
| 2 | The COE must have a colorectal surgeon or a surgical team with experience in the surgical treatment of patients with IBD. | 9.000 | |||
| Of process | 3 | Before starting treatment with a biologic drug in IBD patients, tuberculosis detection tests must be performed, according to the current recommendations. | 9.000 | ||
| 4 | Before starting treatment with a biologic drug in IBD patients with some sign of latent tuberculosis (in a chest x-ray or in tuberculin or immunologic tests), they must receive adequate antituberculosis treatment. | 9.000 | |||
| Of structure | 5 | The COE must be affiliated with hospital that has a gastrointestinal endoscopy service. | 8.923 | ||
| Of process | 6 | The COE must have a register of the IBD patients that receive biologic drugs. | 8.923 | ||
| 7 | When a patient is diagnosed with IBD, he/she must undergo a complete study of the extension of the disease that includes colonoscopy, as well as an evaluation of small bowel involvement, if CD is suspected. | 8.923 | |||
| 8 | Treatment with cyclosporine or biologics must be started within 3 to 5 days in patients with severe relapse of UC that do not respond to intravenous steroids. | 8.923 | |||
| 9 | The fact that the patient has received adequate information on the risks and benefits of biologic treatment, before starting such treatment, must be documented in the clinical history. | 8.923 | |||
| 10 | Patients treated with immunosuppressants must have follow-up with periodic CBC and LFTs, as well as an annual dermatologic check-up, if they are taking thiopurines. | 8.923 | |||
| Of structure | 11 | The COE must have access to computed tomography. | 8.846 | ||
| Of process | 12 | The COE must have a register of all IBD patients. | 8.846 | ||
| 13 | The COE must have a colorectal cancer surveillance program in patients with IBD, in accordance with international guideline indications. | 8.846 | |||
| 14 | The fact that the patient has received adequate information on the risks and benefits of immunosuppressive treatment, before starting such treatment, must be documented in the clinical history. | 8.846 | |||
| Of structure | 15 | The COE must have a radiologist with experience in IBD. | 8.769 | ||
| Of process | 16 | The gastroenterologist of the COE must actively participate in the management of the hospitalized IBD patient. | 8.769 | ||
| Of structure | 17 | The COE must be affiliated with a hospital with an emergency service. | 8.692 | ||
| 18 | The COE must have at least one nurse that is specialized in IBD. | 8.692 | |||
| 19 | The COE must have access to magnetic resonance imaging. | 8.692 | |||
| 20 | The COE must have protocols for drug use. | 8.692 | |||
| Of process | 21 | Before undergoing a surgical procedure that could involve a temporary or permanent ostomy, the IBD patient must have a session with a nurse that is specialized in ostomies. | 8.692 | ||
| 22 | Rectal biopsies must be taken in UC patients with severe steroid-refractory relapse, to rule out cytomegalovirus infection. | 8.692 | |||
| 23 | The COE must have a clinical and laboratory surveillance program for patients that receive treatment with immunosuppressants. | 8.615 | |||
| 24 | The COE must have a clinical and laboratory surveillance program for patients that receive treatment with biologic drugs. | 8.615 | |||
| 25 | The patient must receive a card with COE contact information that includes a telephone number and service hours. | 8.615 | |||
| 26 | All IBD patients should be vaccinated against hepatitis B and herpes zoster if they have no history of previous infection. | 8.615 | |||
| 27 | IBD patients under treatment with thiopurines should maintain that treatment during pregnancy. Treatment rejection must be documented. | 8.615 | |||
| 28 | The fact that the patient has received adequate information on the benefits and risks of surgery, before said surgery, must be documented in the clinical history. | 8.615 | |||
| Of structure | 29 | The COE must contemplate and provide comprehensive management regarding reproductive aspects in patients with IBD. | 8.538 | ||
| Of process | 30 | The COE must have a chain of preferential visits for patients referred by their general physician, the emergency service, or other healthcare professionals due to a recent diagnosis of IBD or a serious flare of the disease. | 8.538 | ||
| 31 | Complex care decisions, including the indication for surgery, should be made by the IBD committee, with the participation of gastroenterologists, radiologists, and surgeons. | 8.538 | |||
| 32 | The gastroenterologists of the COE must participate in more than one educational activity on IBD. | 8.538 | |||
| Of structure | 33 | The COE must have access to abdominal ultrasound imaging. | 8.385 | Group B [8.0-8.5) | |
| Of structure | 34 | The COE must have installations for outpatient care, at which drugs can be administered intravenously. | 8.308 | ||
| 35 | The COE must have access to a diagnostic and therapeutic endoscopy unit, with expertise in IBD. | 8.308 | |||
| 36 | The COE must follow national or Latin American guidelines on the diagnosis and treatment of IBD. | 8.308 | |||
| Of process | 37 | All IBD patients that are HBsAg-positive should receive antiviral drugs, while being treated with biologic therapy or small-molecule inhibitor therapy. | 8.308 | ||
| Of structure | 38 | The COE must have a methodology for determining patient satisfaction. | 8.231 | ||
| Of results | 39 | The severe morbidity rate requiring ICU admission should be below 5%, in patients undergoing elective surgery. | 8.231 | ||
| 40 | The elective surgery mortality rate should be under 2%. | 8.231 | |||
| Of process | 41 | The COE should carry out or participate in research projects on IBD. | 8.154 | ||
| 42 | Reproductive-age patients should receive specialized counseling in the COE, with respect to heritability, fertility, pregnancy, and breastfeeding. | 8.154 | |||
| Of structure | 43 | The COE must have a specific protocol for hospitalized IBD patients. | 8.000 | ||
| Of process | 44 | The COE must have specialized surgeons for elective surgery, when required. | 7.769 | Group C [7.5-8.0) | |
| 45 | The COE must have different means of communication, in case there is a relapse or complication, and must provide on-demand personalized assistance. | 7.692 | |||
| 46 | Anticoagulant prophylaxis with low-molecular-weight heparin should be indicated in all patients with IBD during the time they are hospitalized and present with severe relapse of the disease. | 7.692 | |||
| 47 | IBD patients treated with biologic therapy should have follow-up with periodic CBC, acute-phase reactants, and fecal calprotectin, as well as annual QuantiFERON-TB Gold (when available) or PPD. | 7.615 | |||
| 48 | Patients admitted to the COE should undergo testing for the detection of hepatitis B, hepatitis C, and HIV, prior to starting treatment with immunosuppressants/biologics/ small-molecule inhibitors. | 7.538 | |||
| Of structure | 49 | The COE must be affiliated with a gastroenterology department that has hospitalization installations. | 7.308 | Group D [7.0-7.5) | |
| Of process | 50 | All IBD patients that have received two or more cycles of steroids in one year, should contemplate treatment with immune modulators/biologics/ small-molecule inhibitors. | 7.154 | ||
| 51 | Ileoanal reservoirs should only be performed by experienced general or colorrectal surgeons that carry out at least 5 such operations per year. | 6.692 | Group E [6.5-7.0) | Important, but not critical | |
| Of results | 52 | The percentages of temporary ileostomy after elective ileocecal resection in patients with CD should be below 20% in the COE. | 6.000 | Group F [6.0-6.5) | |
CBC: complete blood count; COE: center of excellence; CD: Crohn’s disease; HIV: human immunodeficiency virus; IBD: inflammatory bowel disease; ICU: intensive care unit; LFTs: liver function tests; PPD: purified protein derivative; UC: ulcerative colitis.
Statement 1. The Center of Excellence (COE) must have installations for outpatient care, at which drugs can be intravenously administered. Percentage of agreement: 92%.
It is essential for the COE to have an infusion area within the hospital for the intravenous administration of medications, including the different types of biologics, such as infliximab, vedolizumab, and ustekinumab, utilized for remission induction and maintenance in patients with IBD.7,8,11
Statement 2. The COE must be affiliated with a hospital that has an emergency service. Percentage of agreement: 100%.
Having an emergency service is indispensable for providing continuous care to patients presenting with severe relapse, a complication of IBD, or a severe treatment-associated adverse event.7,8,11
Statement 3. The COE must be affiliated with a gastroenterology department that has hospitalization installations. Percentage of agreement: 77%.
Having a gastroenterology service or department is important for the comprehensive care of hospitalized patients, especially patients that present with some other type of intestinal disease or extraintestinal manifestation. Hospital beds can be designated to gastroenterology patients at an internal medicine service.7,8,11
Statement 4. The COE must be affiliated with a hospital with a gastrointestinal endoscopy service. Percentage of agreement: 100%.
Having a gastrointestinal endoscopy unit or department, with high-definition endoscopic equipment, is essential for the care of the patient with IBD, with respect to diagnosis, follow-up in identifying dysplasia, and evaluation of treatment response, based on mucosal healing.7,8,11
Statement 5. The COE must provide specialized outpatient consultation for patients with IBD. Percentage of agreement: 100%.
Having specialized outpatient consultation, exclusively for IBD, guarantees adequate patient follow-up, as well as the opportune identification of complications and the optimization of treatment, based on different biochemical markers, such as C-reactive protein and fecal calprotectin, and on endoscopic, radiologic, and histopathologic evaluation.7,8,11 Said consultation can be provided by gastroenterologists and/or specialists in IBD, as well as gastroenterology residents supervised by those medical specialists.
Statement 6. The COE must have at least one nurse that is specialized in IBD.
Percentage of agreement: 100%.
Nursing is an important aspect of the care and follow-up of IBD patients, improving the quality of care, with respect to complying with different programs, such as having an up-to-date vaccine record, treatment adherence, and instruction in the parenteral and subcutaneous application of medications.12
Statement 7. The COE must have a colorectal surgeon or a surgical team with experience in the surgical treatment of patients with IBD. Percentage of agreement: 100%.
Management and follow-up, in conjunction with the colorectal surgeon or the surgeon with experience in the area, is imperative for the success of the surgery and the opportune identification of complications following IBD-related surgical treatment, such as stoma creation, intestinal resection, or colectomy, and the treatment of perianal disease.7,8,11
Statement 8. The COE must include a radiologist with experience in IBD. Percentage of agreement: 100%.
The presence of a general and/or interventional radiologist with experience in the area of IBD is vital for making therapeutic decisions and interpreting specialized studies, such as computed tomography enterography, magnetic resonance enterography (MRE), or Doppler intestinal ultrasound, to evaluate the presence of extramural complications, including the development of intra-abdominal abscesses and internal fistulas, identifying the location, length, and type of fistula (inflammatory vs. fibrotic).13
Statement 9. The COE must have access to computed tomography (CT) imaging. Percentage of agreement: 100%.
The performance of CT is important for the rapid evaluation of acute complications, such as abscesses, in patients that come to the emergency service, enabling decisions to be made regarding medical or surgical treatment.13
Statement 10. The COE must have access to MRE and pelvic magnetic resonance imaging (MRI). Percentage of agreement: 100%.
Having access to MRI is indispensable, given that it is a fundamental tool in the diagnosis of CD, as well as for assessing its location, primarily at the level of the small bowel. It is extremely useful in the long-term follow-up of CD because it does not produce radiation and provides data on the presence of disease activity and the detection of complications, such as stricture, abscesses, and fistulas.13
Statement 11. The COE must have access to a diagnostic and therapeutic endoscopy unit with expertise in IBD. Percentage of agreement: 92%.
Having high-definition diagnostic and therapeutic endoscopy is important because it is a fundamental tool for diagnosing IBD and evaluating mucosal healing as a therapeutic goal, according to the type of treatment utilized; ileocolonoscopy, panendoscopy, capsule endoscopy, and anterograde and retrograde enteroscopy stand out. Enteroscopy is useful in dilating single intraluminal strictures smaller than 4 cm, to avoid intestinal resection.14 In addition, trained personnel are required if flat or raised lesions are found in the follow-up of patients with UC or colonic CD.
Statement 12. The COE must follow national or Latin American guidelines on the diagnosis and treatment of IBD. Percentage of agreement: 92%.
Having standardized protocols according to national or Latin American guidelines is ideal for providing comprehensive, homogeneous, and up-to-date care, according to the available scientific evidence, to achieve treatment goals and prevent the development of complications associated with IBD.15,16
Statement 13. The COE must have protocols for the treatment of IBD. Percentage of agreement: 100%.
Having therapeutic algorithms, according to the risk stratification of each patient is ideal for providing the most adequate treatment, taking into account the efficacy and safety of each treatment, whether conventional, biologic, or with small-molecule inhibitors.7,8,11
Statement 14. The COE must have a specific protocol for hospitalized IBD patients.
Percentage of agreement: 92%.
It is important to have an established protocol for the treatment and follow-up of patients with IBD that become hospitalized due to severe disease activity or the development of an associated complication, to have a homogeneous plan and multidisciplinary focus, and to provide specialized care, according to each case.7,8,11
Statement 15. The COE must have a methodology for determining patient satisfaction. Percentage of agreement: 92%.
A satisfaction survey applied to patients is suggested for the purpose of having feedback on the quality of outpatient and inpatient care, enabling improvement of care-related aspects.17
B. Process indicatorsStatement 16. The COE must have a register of all patients with IBD. Percentage of agreement: 100%.
A database of all IBD patients seen at the clinic or unit is fundamental, for their rapid identification and for having their demographic and clinical characteristics at hand, to optimize their care, as well as their inclusion in research studies.7,8,11
Statement 17. The COE must have a register of all IBD patients that receive biologic drugs. Percentage of agreement: 100%.
Having a register of IBD patients treated with biologic therapy enables optimized follow-up of those patients, in case of loss of response or the need to switch to another biologic with a different mechanism of action, as well as to report adverse events associated with the different types of biologics.7,8,11
Statement 18. The COE must have different means of communication, in case personalized on-demand assistance is needed due to the presentation of a relapse or complication. Percentage of agreement: 92%.
Having close communication with patients by telephone or telemedicine, enables a relapse or complication associated with IBD to be detected and the subsequent personalized assistance to be provided by a specialist Monday through Friday.7,8,11
Statement 19. The COE must have a chain of preferential visits for patients that are referred by their general physician, the emergency service, or other healthcare professionals due to a recent diagnosis of IBD or a severe relapse. Percentage of agreement: 92%.
Having an established chain for the transfer of the patient from primary care, the emergency service, or from the care of other professionals improves communication between them and facilitates access to the adequate treatment of IBD patients, reducing delay in the diagnosis and treatment of relapses or the development of a complication of IBD, preventing time gaps in the care of patients with IBD.7,8,11
Statement 20. The COE must have a clinical and laboratory surveillance program for patients receiving immunosuppressive therapy. Percentage of agreement: 92%.
IBD patients receiving treatment with steroids or immunosuppressants, such as thiopurines or methotrexate, should be under a surveillance protocol related to potential adverse events that can present at the symptomatic level or due to laboratory findings.7,8,11
Statement 21. The COE must have a clinical and laboratory surveillance program for patients receiving treatment with biologics and small-molecule inhibitors. Percentage of agreement: 92%.
Having a surveillance program for monitoring the response to biologic agents is necessary to opportunely prevent, detect, and treat their side effects, so that infectious or neoplastic complications that can present in the long term do not develop.7,8,11
Statement 22. The patient should receive a physical or virtual card with the contact information of the COE that includes the telephone number and service hours. Percentage of agreement: 92%.
Patients should know the names of the personnel treating them, as well as the means of access and service hours, so they can receive answers to any doubts or questions that arise during their follow-up.7,8,11
Statement 23. The COE must have a colorectal cancer surveillance program for IBD patients, in accordance with international guideline indications. Percentage of agreement: 100%.
A standardized follow-up program is indispensable for patients with UC and those with colonic CD, to opportunely detect dysplasia or colorectal cancer, and thus provide early treatment and prevent the progression of colon cancer that negatively impacts quality of life and survival in IBD patients.15,18,19
Statement 24. The complex care decisions, including indications for surgery, should be made by IBD committees, with the participation of gastroenterologists, radiologists, and surgeons. Percentage of agreement: 100%.
Having committees made up of specialists in IBD, gastroenterologists, radiologists, pathologists, nutritionists, colorectal surgeons, and other specialists is ideal for favoring decision-making in complex clinical situations in a multidisciplinary context. Minutes of the meetings should also be available.7,8,11
Statement 25. The COE must have specialized surgeons for elective surgery, when necessary. Percentage of agreement: 92%.
Having specialized surgeons for the surgical treatment of IBD patients is essential. Their specialization is reflected in the number of surgical procedures performed per year.7,8,11
Statement 26. Before undergoing a surgical intervention that can result in a temporary or permanent ostomy, the IBD patient must have a session with a nurse that specializes in ostomies. Percentage of agreement: 100%.
Having a stoma can have a strong impact on the body image of the patient and interfere considerably with his/her quality of life. Therefore, it is important for the patient to have a previous session with a nurse, regarding ostomy care, to provide the patient with verbal and written information on ostomies and clarify any doubts as to hygiene, diet, types of devices, and other special features of the stoma.7,8,11
Statement 27. The gastroenterologist of the COE must actively participate in the management of the hospitalized IBD patient. Percentage of agreement: 100%.
Care in the treatment of IBD patients, as well as supervision of the procedures performed on hospitalized IBD patients must be provided.7,8,11
Statement 28. The gastroenterologists of the COE must participate in educational activities regarding IBD. Percentage of agreement: 92%.
The gastroenterologists or IBD specialists must attend at least one academic activity (congress or course) for keeping abreast of the advances in the knowledge of IBD, to treat patients, based on the best scientific evidence available.20
Statement 29. The COE should conduct or participate in research projects on IBD. Percentage of agreement: 85%.
At least one research project a year should be conducted that includes IBD patients and is reported at the national or international level.7,8,11
Statement 30. When a patient is diagnosed with IBD, a complete study of disease extension should be carried out that includes colonoscopy and an evaluation of small bowel involvement, if CD is suspected. Percentage of agreement: 100%.
Ileocolonoscopy and the radiologic study of the small bowel (if CD is suspected) should be carried out. If stricture has been ruled out, capsule endoscopy can be performed. Those studies are essential in the initial diagnosis of IBD, to know its extension and phenotype.15,19
Statement 31. Before starting treatment with a biologic agent and small-molecule inhibitors, tuberculosis detection tests should be conducted on patients with IBD, in accordance with the current recommendations. Percentage of agreement: 100%.
Chest x-ray and the tuberculin skin test (purified protein derivative [PPD] test) or the QuantiFERON-TB Gold assay must be performed before starting biologic therapy, to detect active or latent tuberculosis and give antituberculosis treatment or chemoprophylaxis, respectively, due to the fact that an increase in tuberculosis reactivation with anti-TNF-alpha therapy has been observed.16,21,22
Statement 32. IBD patients admitted to the COE should undergo tests to detect hepatitis B, hepatitis C, and human immunodeficiency virus (HIV), before starting treatment with immunosuppressants/biologics and small-molecule inhibitors. Percentage of agreement: 85%.
IBD patients undergoing treatment with immunosuppressants are at greater risk for chronic hepatitis B reactivation that can be life-threatening. Therefore, knowing the serologic status of the patients, with respect to hepatitis B, is necessary. Patients with negative serology should have prophylactic vaccination and patients with active infection should receive the adequate treatment, if needed, reducing the risk for reactivation. In addition, tests for detecting hepatitis C and HIV infection should be performed.16,21,22
Statement 33. All patients with IBD should be vaccinated against hepatitis B and herpes zoster if they have no history of prior infection. Percentage of agreement: 92%.
Reactivations of hepatitis B virus (HBV), and some that have been fatal, have been described in patients with positive HBV markers. Therefore, the patients that show no signs of previous contact with HBV should be vaccinated. In addition, serologic testing for varicella zoster is indispensable, especially in patients with UC that will start treatment with small-molecule inhibitors, such as tofacitinib, which has displayed an increased risk for herpes zoster infection.16,21,22
Statement 34. In patients with UC and severe steroid-refractory relapse, rectal biopsies should be taken, to rule out cytomegalovirus infection (CMV). Percentage of agreement: 92%.
The reactivation of CMV infection could be a cause of refractoriness to steroids in patients with severe UC, and so rectal biopsies need to be performed to investigate the presence of CMV, and if positive, start antiviral treatment with ganciclovir.15,18
Statement 35. Before starting treatment with a biologic drug or small-molecule inhibitor, IBD patients with any sign of latent tuberculosis (in chest x-ray or tuberculin or immunologic tests) should receive adequate antituberculosis treatment. Percentage of agreement: 100%.
Patients with IBD and tuberculosis should receive adequate antituberculosis treatment with isoniazid, prior to starting treatment with a biologic drug due to the danger of tuberculosis reactivation.21,22
Statement 36. All HBsAg-positive IBD patients should receive antiviral drugs, while they are being treated with biologic therapy or a small-molecule inhibitor. Percentage of agreement: 85%.
HBV reactivation is due to an increase in the replication of the virus in patients that are inactive carriers or that have had past infections. Said reactivation can be produced spontaneously or be secondary to immunosuppressants. Reactivation in patients undergoing immunosuppressive treatment has been associated with the development of severe acute hepatitis that can be fatal. Prophylactic antiviral treatment has been shown to have a protective effect against reactivation in patients that are inactive carriers and in some patients with past HBV infections that are undergoing immunosuppressive treatments.16,21,22
Statement 37. All patients with IBD that have received two or more cycles of steroids in a year, should contemplate treatment with an immunomodulator/biologic/small-molecule inhibitor. Percentage of agreement: 85%.
Steroids are associated with a large number of side effects and are not efficacious for remission maintenance in patients with IBD. Immunomodulators, such as thiopurines, as well as biologic therapy or small-molecule inhibitors, are indicated in steroid-dependent patients.15,22
Statement 38. In patients with severe relapse of UC that do not respond to intravenous steroids, treatment with cyclosporine or infliximab should be started within 3 to 5 days. Percentage of agreement: 100%.
In patients with severe intravenous steroid-refractory UC, rescue therapy, based on cyclosporine or infliximab, should be started. Both treatments are efficacious in patients with severe UC that do not respond to intravenous steroids.15,19
Statement 39. Anticoagulant prophylaxis with low-molecular-weight heparin should be indicated in all patients with IBD, during the time they are hospitalized and present with severe disease relapse. Percentage of agreement: 85%.
The risk for thromboembolic complications is increased in patients with IBD, primarily during hospitalizations. In fact, it is one of the main causes of death in those patients. Therefore, anticoagulant prophylaxis with low-molecular-weight heparin is recommended in hospitalized IBD patients.18
Statement 40. The fact that the patient has received adequate information with respect to the benefits and risks of immunosuppressive treatment, before starting said therapy, must be recorded in the clinical history. Percentage of agreement: 100%.
This indicator attempts to measure the actions taken toward reinforcing patient autonomy, providing him/her with adequate information, thus enabling him/her to make his/her own informed decisions.7,8,11
Statement 41. The fact that the patient has received adequate information with respect to the benefits and risks of biologic treatment, before starting said therapy, must be recorded in the clinical history. Percentage of agreement: 100%.
It is important to involve the patient in deciding on the type of biologic agent to be started, based on the efficacy and safety profiles of each drug, so that he/she has absolute knowledge of the potential adverse events that can present in the short term and the long term.7,8,11
Statement 42. Patients treated with immunosuppressants should have follow-up with periodic complete blood counts and liver function tests, as well as a yearly dermatologic check-up, if they are taking thiopurines. Percentage of agreement: 100%.
Periodic and systemic monitoring should be carried out through complete blood counts in patients treated with immunomodulators, such as thiopurines. There is great variability in the periodicity of the monitorization reported in the studies that recommend such testing. In general, the long-term recommendation is every 3 to 6 months.18
Statement 43. The patients treated with biologic therapy should have periodic follow-up that includes complete blood count, acute-phase reactants, and fecal calprotectin, as well as yearly QuantiFERON-TB Gold (depending on availability) or PPD. Percentage of agreement: 85%.
Biologic agents are associated with high cost, and the anti-TNF-alpha agents are especially associated with a variety of potential adverse effects. Therefore, periodic treatment monitoring is recommended through complete blood count, serologic acute-phase reactants, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as periodic fecal calprotectin determination (every 3-6 months), to detect asymptomatic IBD relapse and provide its opportune treatment. Yearly QuantiFERON-TB Gold (depending on availability) or PPD are vital for detecting tuberculosis reactivations, especially when anti-TNF-alpha therapy is being used.18
Statement 44. Ileoanal reservoirs should only be created by experienced general surgeons or colorectal surgeons that perform a minimum of 5 such procedures per year. Percentage of agreement: 92%.
Ileoanal reservoir surgery should be exclusively performed by surgeons that carry out more than 5 such procedures yearly, given that surgeons with said experience have been shown to have better success rates and fewer complications. 23–25
Statement 45. The fact that the patient has received adequate information, with respect to the benefits and risks of surgery, before the procedure, must be recorded in the clinical history. Percentage of agreement: 92%.
This indicator attempts to evaluate the actions for reinforcing patient autonomy, providing him/her with adequate information that enables him/her to make his/her own informed decisions.23–25
Statement 46. The COE must contemplate and offer comprehensive management concerning the reproductive aspects of patients with IBD. Percentage of agreement: 92%.
All aspects of reproduction must be explained to the patients with IBD to provide them with comprehensive care and management, in conjunction with their gynecologist/obstetrician.16,19
Statement 47. Reproductive-age patients must receive specialized counselling in the COE, with respect to heritability, fertility, pregnancy, and breastfeeding. Percentage of agreement: 92%.
The patient should be advised that disease heritability is very low, and that conventional or biologic treatment does not affect fertility. Those treatments do not affect the progression of pregnancy or breastfeeding, with the exception of methotrexate, which must be suspended at least 3 months prior to conception.16,19
Statement 48. Patients with IBD undergoing treatment with thiopurines, should maintain that treatment during pregnancy. Treatment rejection must be documented.
Percentage of agreement: 92%.
Given that the maintenance of IBD remission is essential during gestation, the primary goal during pregnancy of an IBD patient is to optimize disease control through medical treatment. Thiopurines are utilized for IBD remission maintenance. The vast majority of published data suggest that thiopurines are safe for the fetus and neonate, and so said treatment should be maintained during pregnancy.16,19
Statement 49. IBD patients undergoing treatment with biologics should maintain said treatment during pregnancy. Treatment rejection must be documented. Percentage of agreement: 92%.
Pregnant IBD patients should maintain biologic therapy, given that it has been documented as a safe therapy that does not increase the risk for miscarriages or congenital malformations. IBD relapse during pregnancy has been shown to result in an increase in complications in the product and the mother, therefore the suspension of biologic treatment by the patient must be documented.16,19
C. Result indicatorsStatement 50. In patients with IBD that undergo elective surgery, severe morbidity rates that require admission to an intensive care unit (ICU) should be below 5%. Percentage of agreement: 85%.
The severe morbidity rate that requires admission to the ICU, after an elective surgical intervention, should be below 5%, as a quality result indicator.23–25
Statement 51. The mortality rate for elective surgery should be below 2%. Percentage of agreement: 85%.
The mortality rate for elective surgery should be below 2%, as a quality result indicator, according to international guidelines. Said percentage is described as acceptable for considering the experience of surgeons involved in specialized procedures at the colorectal level to be adequate.23–25
Statement 52. The percentages of temporary ileostomy after elective ileocecal resection, in patients with IBD, should be below 20%, within the COE. Percentage of agreement: 85%.
The percentages of temporary ileostomy, following elective ileocecal resection, should be below 20%, according to international guidelines. Higher rates are considered inadequate results in that context, given that in hospitals that have expert surgeons, there is no need to keep the ileostomy, in the majority of patients that undergo primary anastomosis, resulting in an adequate experience.23–25
ConclusionsThis is the first Latin American consensus that posits the quality indicators necessary for comprehensive care clinics for patients with IBD to be considered a center of excellence. In the present document, we describe standards for evaluating the quality of care in inflammatory bowel disease. However, we have not established or quantified the percentage of meeting those standards for achieving excellence. The GETECCU regards meeting 90% as Excellence and 80% as Advanced. We hope the present description of standards is a tool that will be a starting point for Latin American countries, but the establishment of the percentages and their application will be left to the consideration of the PANCCO and the individual local gastroenterology societies of each of the Latin American countries. The advantage of having centers of excellence in IBD is the resulting positive impact on opportune diagnosis, appropriate follow-up, and specialized treatment, with homogeneous criteria for improving the quality of care in patients with IBD, in Mexico and other Latin American countries. Such has been the experience in Spain, in which the approval of centers of excellence in IBD by the GETECCU has had the impact of a higher number of patients being treated in those centers, with improved clinical outcomes and quality of life. The disadvantage is that some Latin American countries cannot apply the indicators described herein because they do not have clinics specializing in IBD. Nevertheless, they do have gastroenterology services, in which they can meet the requirements necessary for a center of excellence. Finally, a likely limitation is that the majority of patients cannot be treated at centers of excellence, given that most of the centers are located in the biggest cities of each country, with very few in the smaller cities.
Ethical considerationsNo patients participated in the present study nor were patient data utilized, thus informed consent was not required. Likewise, given that no intervention, maneuver, or management of information was carried out, the study was considered low-risk and needed no review or approval by a local ethics committee. Even so, the study meets the current research and personal and identification data confidentiality regulations and guarantees the anonymity of the participants (all voluntarily participating healthcare workers). The present article contains no information that could identify the participants.
Financial disclosureThe regional Janssen laboratory provided the financial support for the InValue agency that organized the logistics for the virtual development and analysis of the consensus results, without intervening in the content or discussion of the consensus or its results. No participant received honoraria. Importantly, neither the Janssen laboratory nor the InValue agency participated in the content of the present consensus or its results.
Conflict of interestJesús Kazuo Yamamoto Furusho is a member of the Advisory Board, opinion leader, and speaker for AbbVie Laboratories de México, AbbVie International, Takeda International, Takeda México, Pfizer International and regional, Janssen Cilag international and México. He is an opinion leader and speaker for Farmasa, Ferring, and Farmasa Schwabe and a research consultant for UCB México. He has received funds for research studies from the Shire, Bristol Myers Squib, Pfizer, Takeda, and Celgene laboratories.
David Andrade: has no conflict of interest.
Josué Barahona-Garrido: has been a speaker for Janssen, Eurofarma, Asofarma, MD Pharma, and AB Biotics.
Sócrates Bautista: has no conflict of interest.
Francisco Bosques-Padilla: is a speaker for the AbbVie, Takeda, Janssen, and Celltrion laboratories.
Juan de Paula: is a speaker for the AbbVie and Takeda laboratories.
María T. Galiano: is a speaker for the AbbVie, Takeda, and Janssen laboratories.
Beatriz Iade: is a speaker for the AbbVie and Takeda laboratories and has received funds for research studies from AbbVie.
Fabián Juliao-Baños: is a speaker for the AbbVie, Takeda, Pfizer, and Celltrion laboratories.
Guillermo Otoya: has no conflict of interest.
Flavio Steinwurz: is a speaker for the AbbVie, Takeda, Janssen, and Pfizer laboratories.
Esther Torres: has received educational and research donations for the University of Puerto Rico from AbbVie, Takeda, Tigenix, and Bristol Myers Squibb.
Guillermo Veitia: is a speaker for AbbVie, Ferring, Janssen, and Takeda.
Manuel Barreiro-de Acosta: is a member of the Advisory Board, opinion leader and/or speaker for MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte, and Vifor Pharma.
Please cite this article as: Yamamoto-Furusho JK, Andrade D, Barahona J, Bautista S, Bosques-Padilla F, de Paula J, et al. Consenso latinoamericano acerca de indicadores de calidad para Clínicas de Atención Integral para pacientes con enfermedad inflamatoria intestinal: PANCCO-GETECCU. Revista de Gastroenterología de México. 2021. https://doi.org/10.1016/j.rgmx.2021.05.003