Antecedentes: Helicobacter pylori (Hp) es reconocido como carcinógeno tipo 1 para cáncer gástrico, ya que se asocia a lesiones preneoplásicas (atrofia y metaplasia intestinal (MI)), y su relación con el p53, quien interviene en el ciclo celular, ha tenido resultados contradictorios. Objetivos: Analizar la expresión del p53 en la mucosa gástrica y su relación con la infección por Hp.
Métodos: Estudio prospectivo, transversal y observacional, de 3 meses de duración. A pacientes sin evidencia de afección aguda o clínicamente significativa del Hospital Juárez de México se les tomó una biopsia según el sistema de Sydney y se realizó el estudio histopatológico en Hospital Español de México.
Resultados: La prevalencia del Hp fue del 32,7% en 104 pacientes. Ningún caso de atrofia ni displasia. El 91% de los infectados tuvieron p53+. De los no infectados, el 14% presenta p53+; el 60% de estos tienen MI. De los pacientes con MI, el 75% presentó p53+. De los pacientes sin MI, el 31% presentó p53+; en el 85% se encontró Hp+. Se encontró asociación entre Hp y p53 (p < 0.0001) y entre p53 y MI (p < 0.0006).
Conclusiones: Se demuestra asociación significativa entre Hp y la expresión de p53, incluso en pacientes con lesiones preneoplásicas y ya sin la presencia del Hp. Ya que la identificación de lesiones preneoplásicas es importante para la prevención del cáncer; la toma de biopsias rutinarias durante la endoscopia para la detección de Hp podría verse beneficiada de la inmunohistoquímica, identificando a pacientes con expresión de p53, un importante oncogén regulador.
Background: Helicobacter pylori (Hp) is recognized as a type 1 carcinogen for gastric cancer associated with pre-neoplastic lesions (atrophy and intestinal metaplasia [IM]). Its relation with p53, which intervenes in the cell cycle, has had contradictory results.
Aims: To analyze p53 expression in gastric mucosa and its relation with Hp infection.
Methods: A 3-month prospective, observational, cross-sectional study was conducted. Patients that had no evidence of acute or clinically significant gastric pathology had biopsies taken according to the Sydney system at the Hospital Juárez de México and the histopathologic studies were done at the Hospital Español de México.
Results: Hp prevalence was 32.7% in 104 patients. There were no cases of atrophy or dysplasia. A total of 91% of the infected patients were positive for p53. Of the non-infected patients, 14% were positive for p53 and 60% of them had IM. Of the IM patients, 75% presented with positive p53. Of the patients without IM, 31 presented with positive p53, and Hp was positive in 85% of them. There was association between Hp and p53 and between p53 and IM (P<.0001 and P<.0006, respectively).
Conclusions: Significant association was shown between Hp and p53 expression, even in patients with pre¿neoplastic lesions that no longer presented with Hp. Given that the identification of pre-neoplastic lesions is important for the prevention of cancer, immunohistochemistry could benefit routine biopsy carried out during endoscopy for the detection of Hp, by identifying patients with expression of the important oncogene regulator, p53.
Introduction
Gastritis is defined as an inflammatory response of the gastric mucosa to a harmful stimulus and is a problem of considerable magnitude worldwide. Its global prevalence has increased through the use of endoscopy and the possibility of taking directed biopsies. Infection from Helicobacter pylori (Hp) is a major cause of chronic inflammation of the human gastric antral mucosa.
The development of atrophy is due to the disappearance of glands, and its final stage is atrophic chronic gastritis (CG).1 Antral CG with or without atrophy is clearly related to the presence of Hp. In contrast, if CG presents exclusively in the corpus, it is unlikely that it is due to Hp,1 given that the presence of the microorganism diminishes parallel to the development of mucosal atrophy.1 Populations with elevated CG prevalence show an earlier age of Hp infection.1,2 Some areas of the stomach, such as the lesser curvature and the incisura angularis are more prone to develop CG1,2 and intestinal metaplasia (IM), and the pre-pyloric zone is the best site for Hp detection.
There are multiple gastritis classifications, each one with its own terminology, which produces difficulty and confusion for referring to the same type of gastritis. Therefore, in 1990 the Sydney system (SS) was created in order to have a single classification. In the present study, the section on biopsy specifications is particularly important. It states that a minimum of 2 biopsies should be taken at each site, as well as from all identified suspicious lesions, taking biopsies from the anterior and posterior wall, from the antrum between 2 and 5 cm from the pylorus, and from the gastric corpus at approximately 10 cm from the cardia. The SS was re-evaluated in Houston in 1994, and among other things, it was concluded that biopsies from the corpus are especially useful, because that is where Hp is located after proton pump inhibitor use. However, the maximum grade of atrophy and IM is found in the incisura angularis, which is also where the greatest prevalence of dysplasia is found, and so it has been proposed as the fifth biopsy site to be included in the protocol.2
Infection occurs in the antrum in at least 85% of the cases, but up to 15% will only infect the corpus, which explains the reasoning behind the SS biopsy proposals.1,2
Routine staining should be carried out with the hematoxylin and eosin (H&E), Giemsa, and periodic acid-Schiff (PAS)/alcian blue (AB) methods. Each one of these staining methods is necessary to assess the SS as it is described.2
To determine the presence of Hp when IM is found, other samples where there is no IM have to be evaluated, because Hp does not colonize this mucosa.2 IM is recognized morphologically and it has been subclassified as complete or incomplete.2 The risk for progression to gastric cancer (GC) in the complete type is low; it is higher in the incomplete type.
Hp is a Gram-negative bacterium that colonizes the human stomach, causing antral gastritis. It is the main cause of peptic ulcer, gastric lymphoma, and adenocarcinoma.3 Hp colonizes the stomach at infancy and persists throughout life if left untreated. Its spiral shape and its flagella enable it to cross the mucus barrier of the mucosa and attach itself to the cells.3 Despite evading the immune response of the host, it manages to trigger a reaction that produces inflammation in the gastric mucosa, thus inducing gastritis.3
Hp possesses genetic factors whose functions and interactions with the host are important to point out:
— One of them is CagA, which can be present, absent, or non-functioning; most commonly it is present and functioning.3,4 In the United States, at least 60% of the isolated strains are of this type, and the figure goes up to 90% in other countries.4 This strain is also found more often in patients that develop GC,4 and it causes greater inflammation of the gastric mucosa and higher proliferation levels. Some studies have shown that it functions as an oncoprotein, promoting gastric epithelial cell growth.5 It also induces cytokine production.3 The induced transcription factors produce gene p53 mutation.
— Vacuolating protein, or VacA, is found in practically all bacilli. It is an exotoxin that is resistant to degradation by pepsin. It inhibits the activation of T lymphocyte transcription, inducing cell cycle arrest,3 and thus is regarded as being associated with precancerous lesions.
— At times it modifies and repairs its genetic composition itself, creating mutant CagA and VacA strains.
Hp infection is associated with structural alterations,1 such as the loss of intercellular union between gastric mucosal cells, loss of form and uniformity of the mucosal cells, loss of the cytoskeleton, loss of mucus content, strong adherence of Hp to epithelial cells, and intracellular edema. After treatment, all these alterations disappear and the mucosa returns to normality.1
Gastric carcinogenesis is thought to be a multistep and multifactorial process that affects the expression of oncogenes, suppressor genes, intercellular adhesion molecules, and microsatellite instability, producing a chain going from gastritis of the corpus or pangastritis,1-3 to atrophic CG, to IM, to dysplasia, and finally to GC;3 this chain can take decades to complete. In the majority of cases, tumor presents in the stomach with severe atrophy and IM. The crucial step is atrophy; Hp appears to lead to its induction. One hypothesis as to how atrophy progresses to GC is that hypochlorhydria allows for an increase in Hp colonization, which results in greater damage to DNA;3 another theory is that the free radicals produced by the inflammatory response may act as carcinogens;3 and finally, it has been suggested that hypergastrinemia produces such an increase in cellular proliferation that control over it is lost.3
Hp is the main causal factor that has been identified for the development of gastropathy, including CG, ulcer disease, intestinal GC, and MALT lymphoma. Only a small number of infected patients will develop clinical disease. Approximately 1 in every 5 or 6 cases will develop ulcer disease in an entire lifetime and fewer than 1% will develop GC.6 A 50 to 60% risk for GC7 has been attributed to Hp and the International Agency for Research on Cancer defined it as a definitive carcinogen in GC, listing it in group 1 of the carcinogens recognized by the World Health Organization.7,8
Reports in the literature have been inconsistent in relation to the effects of Hp on gastric cell proliferation, and to a certain point, the findings have been contradictory, and have shown that many signals and signaling cascades can be affected by the presence of Hp.
It has been demonstrated that Hp eradication implies a reduction in GC incidence, as long as there are no existing premalignant lesions.8
The results of studies indicate that Hp inhibits the G1 cell cycle, preventing it from entering into the S phase. This inhibition is associated with genetic alterations, such as that of p53, and finally with apoptosis.9
Since 1979, p53 has been called a tumor antigen, an oncogene, or a tumor suppressor gene. Certain immunohistochemistry studies have shown an increase in p53 levels in patients infected with Hp. However, other studies have reported that there is no difference in the levels or in associated p53 activity. Currently, p53 is regarded as a transcription factor whose increase in expression or whose mutation is associated with cell cycle inhibition, predominantly with the arrested G0 or G1 phase of the cell cycle,7 which does not let it enter into the S phase, and in a secondary manner, does not allow for the repair of the damaged genome. Cycle arrest and consequent apoptosis induction may be a result of the same p53 activation and stabilization that inhibits cellular proliferation.4
Many studies have shown that p53 expression is altered in precancerous gastric lesions such as atrophy or metaplasia, which can be produced in an attempt to stop the cycle of the damaged cell,10 and its accumulation indicates suppressor function loss. Similar changes have been reported in Barrett's esophagus.
The increase in p53 expression may be a protective mechanism on the part of the host that results in cell cycle arrest in order to enable genome repair. The lack of this protective mechanism would increase the risk for GC in infected subjects. The absence of p53 activation may reduce gastric epithelial cell sensitivity to Hp-induced apoptosis.
In the study by Unger et al.,11 they found that there was p53 overexpression in the cases of gastritis that were related to Hp with no IM. There was less overexpression in the cases with IM. However, there was an elevated apoptosis rate in the cases with no IM, whereas in the patients with IM this rate was not different from that of the control group. It was confirmed that Hp infection increased p53 expression and the apoptosis rate when there was no IM. This is due to the fact that Hp cannot colonize the IM, and so its effects on apoptosis disappear, as would happen if it were eradicated.
A study by Ozturk et al.12 on a pediatric population revealed p53 alterations in 20% of the children with CG, and Hp infection was found in 91% of the patients with altered p53. Even though IM was more common in patients with Hp, there was no difference in p53 expression in relation to its presence. The study concurs with the hypothesis that the Hp associated with the inflammatory response it produces, damages DNA in a sequence of multiple steps that includes the addition of p53 mutations, thus initiating gastric carcinogenesis.
Due to the fact that association of Hp with p53 alterations is not yet clear, various authors studying the theme have concluded that there is no relation between them, such as in the study by Craanen et al.13 in which no p53 alterations were found in atrophic CG or in IM.
Wei et al.14 found that p53 levels before Hp infection were low or undetectable. However, when the cells were exposed to Hp infection a 2¿peak pattern of p53 levels was observed, one at 4-8 hours from Hp inoculation that later diminished; but at 12 weeks the levels were again elevated and accompanied with intense inflammation. When Hp was experimentally eradicated, these 2 p53 peaks were not produced, suggesting that Hp must be alive in order to induce changes. The ability of Hp to affect p53 was considerably compromised with the loss of CagA or CagE, given that p53 levels were seen to be higher in the CagA strains. The regulation of the demonstrated reduction of p53 may also aid carcinogenesis because it would allow the genetic changes in the cells to accumulate. The study results also show a balance between p53 degradation and activation in the p53 levels of the cells infected with Hp, as part of the cellular protection response to the stress it is being exposed to. The interaction of these processes can explain the dynamic changes in p53 levels during Hp infection. The authors believe that the first p53 peak is potentially related to failure on the part of the cell to protect itself. The reduction of the levels would be part of the adaptation of the cells to the infection, and finally a second peak could be due to premature DNA damage associated with the same inflammation, triggering apoptosis and the changes in the cell cycle.
Shibata et al.15 studied the prevalence of p53 mutations in different locations of GC. Tumors associated with the CagA strain presented with greater p53 mutation prevalence. These mutations were more frequent in men and the intestinal adenocarcinoma subtype was predominant.
According to the study by Ahmed et al.,7 cells cultivated with Hp were found to be in phase G1 of the cell cycle and p53 overexpression was observed, whereas the opposite occurred in cells that were not cultivated with Hp. The results suggest that cell cycle arrest in G1 is associated with a reduction in cyclin E levels and an increase in p53 and p21 expression. The presence of the CagA strain did not modify the results.
As has been seen, Hp is an agent that can induce cell stress, reduce the ability to repair damaged cells, and can increase the number of changes in the genome, leading to genetic instability and finally to GC. It is not clear how bacterial factors induce apoptosis. Bacterial products have been related to the production of gastritis and apoptosis; it is thought that their combination with the immune response of the host is responsible for the consequences.
Hp can alter the cycle at various steps and in various manners. Apoptosis is a normal component of the cell cycle, but the effects of Hp are not uniform in all of the gastric mucosa. Specific Hp strains may contribute to the heterogeneity of the results.
It is particularly important to recognize and define the characteristics of benign lesions and premalignant ones. It is not possible at this time to conclude whether p53 expression is produced by an inflammatory reaction or if it will persist through time as part of the carcinogenesis chain.
Aim
To analyze p53 gene expression in a gastric mucosa sample and its relation to the presence of Hp.
Methods
A 13¿week observational, prospective, comparative, cross-sectional study was conducted.
Patients sent to the Endoscopy Service of the Hospital Juárez for upper endoscopy were fit to the inclusion criteria (above 18 years of age, both sexes undergoing upper endoscopy and signing letters of informed consent, patients whose study indications included gastroesophageal reflux disease, acid peptic disease, dyspepsia, dysphagia, or GC screening) and for the exclusion criteria (patients with a history of GC, active hemorrhage, portal hypertension, patients that did not cooperate during biopsy, that did not authorize biopsy, or that presented with gastropathy of another etiology that merited routine biopsy). Clinical and demographic data were gathered and biopsies were taken according to the guidelines established by the SS. The biopsies were labeled and transported to the Pathology and Immunohistochemistry Service of the Hospital Español, where they were processed and stained using the following methods: H&E, PAS/AB, Giemsa, and p53 immunohistochemistry. The 2 participating pathologists did not have access to the clinical and demographic data.
Definition of the variables
p53 expression: the brown coloring in the cell nucleus was immunohistochemically measured and expressed as positive (present) or negative (absent).
Presence of Hp: measured with the Giemsa stain and expressed as positive or negative.
Presence of IM: measured with H&E and PAS/AB staining, expressed as positive or negative, and specified as complete or incomplete.
Statistical analysis
Sample size calculation: This is the first study in Mexico showing the relation of p53 expression in patients infected by Hp. Therefore no previous information was available and due to the contradictory results of the studies reviewed in the literature, a difference in proportion with a Δ of 0.4 was taken into account to obtain an α error of 0.5 and a β error of 0.2. The calculated total was 50 patients.
The Fisher exact test was used for nominal variables with a statistical significance of p<0.05.
Results
Within the study time frame, 104 patients were enrolled that fit the protocol inclusion criteria.
Of that total, 70 patients (67.3%) were women and 34 patients (32.7%) were men, with a 2:1 ratio. The mean age ± standard deviation was 47.6 years ± 13 years, with a range of 18 to 80 years. The general results are summarized in Table 1.
No cases of atrophy of the gastric mucosa or evidence of dysplasia were found and all the cases of IM were classified as complete.
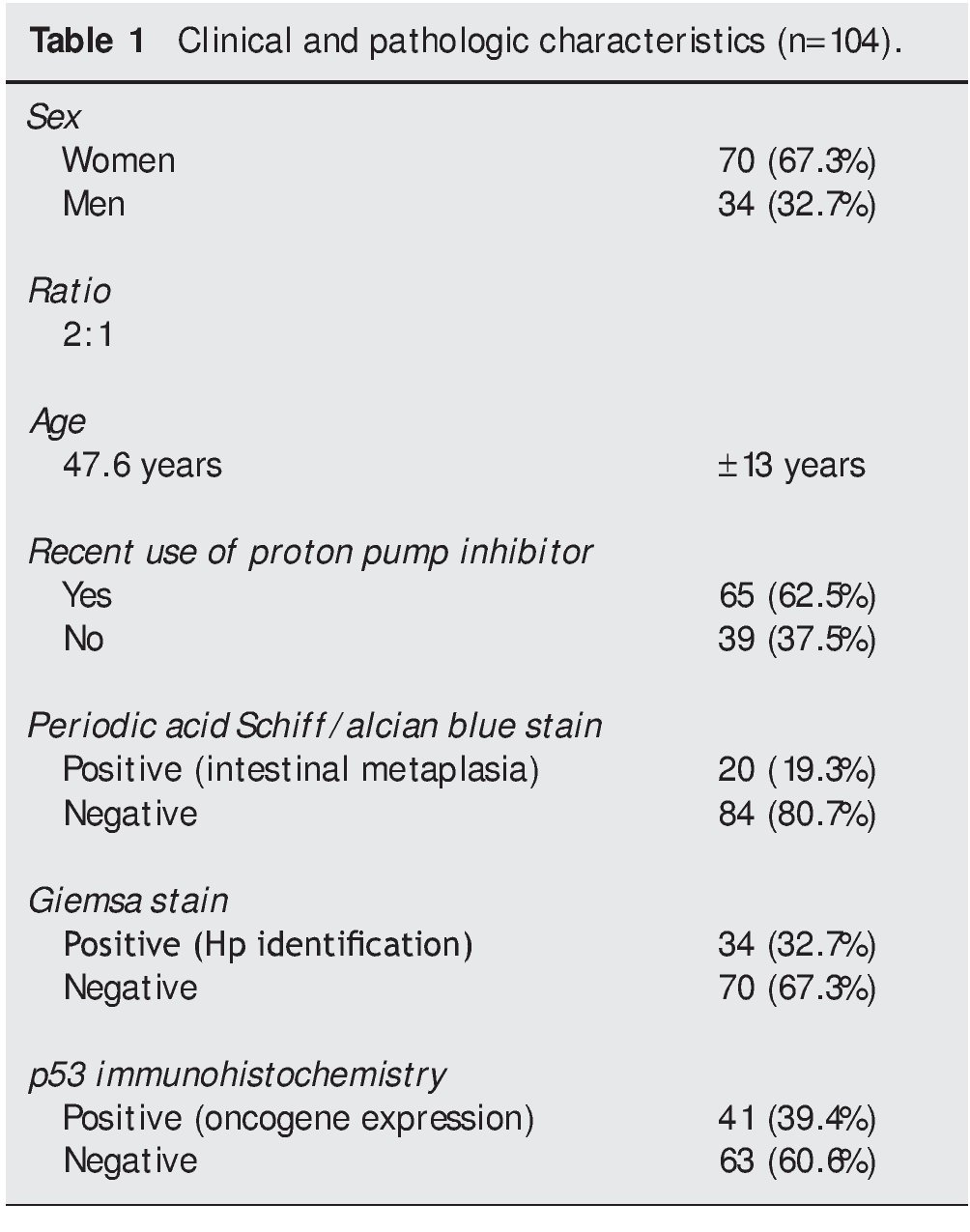
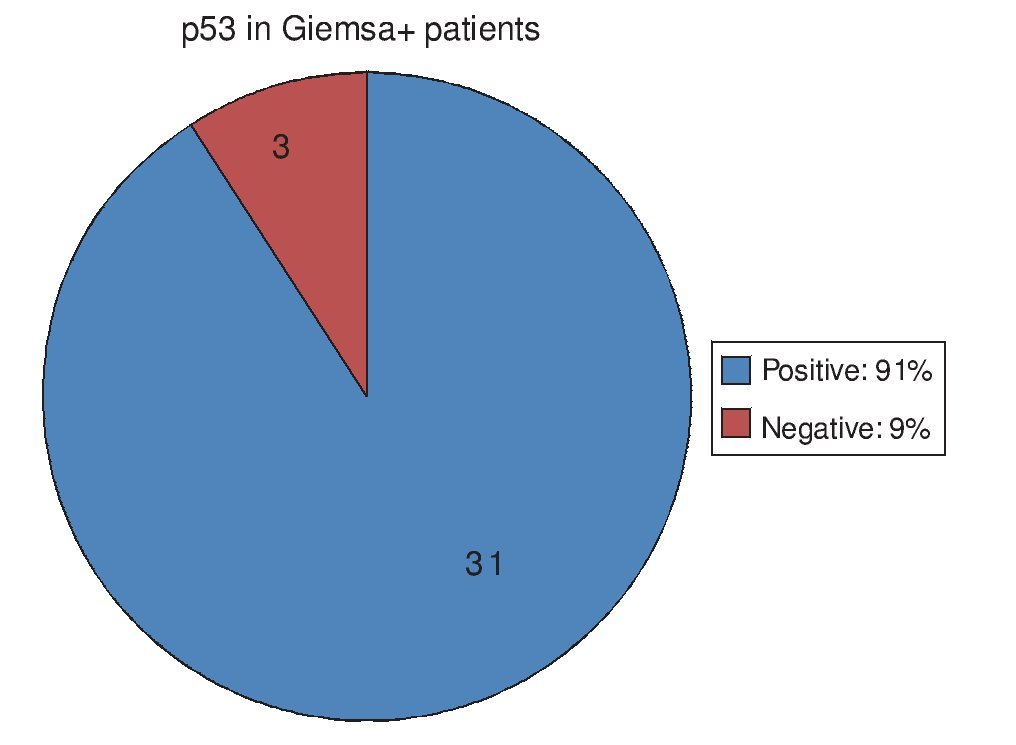
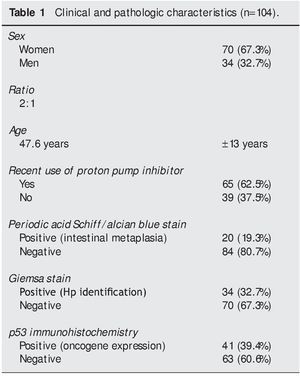
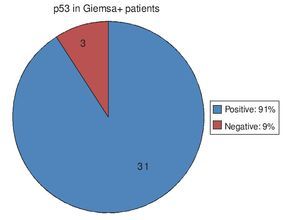
Of the 34 patients with Giemsa stain that was posi tive for Hp infection, 31 (91%) also had positive p53 immunohistochemistry. Only 3 patients (9%) had negative p53 results (Fig. 1). Of the 70 patients with negative Giemsa stain, 10 (14%) had positive p53 and 60 (86%) had negative p53 (Fig. 2).
Figure 1. p53 expression in patients with Hp infection.
Figure 2. p53 expression in patients with no Hp infection.
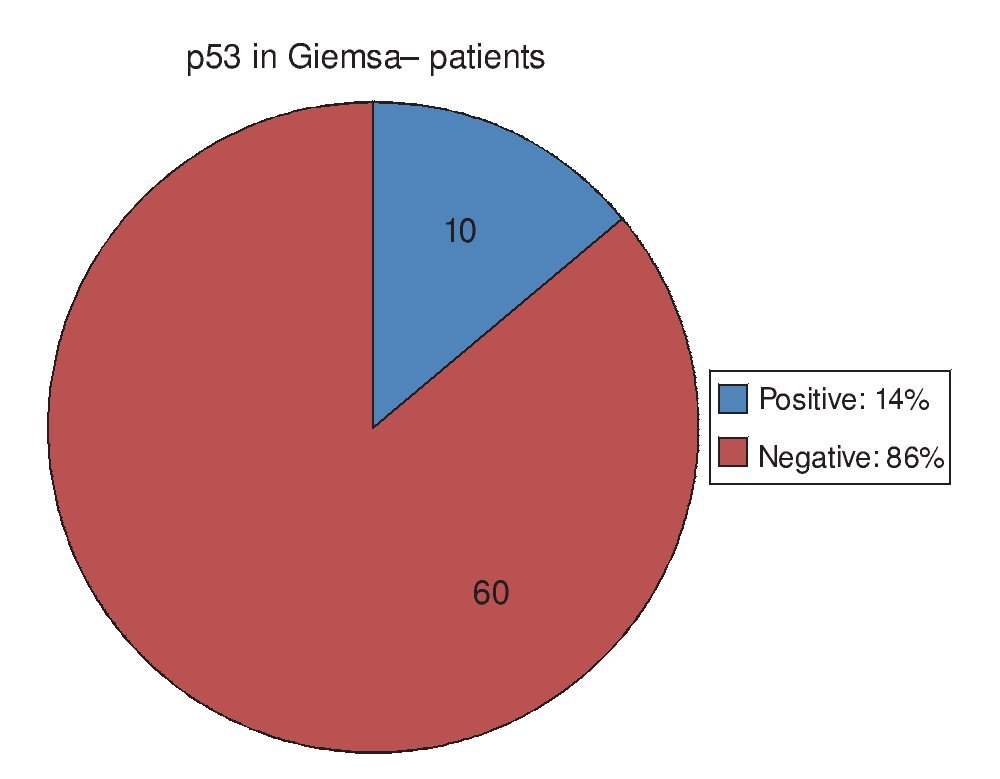
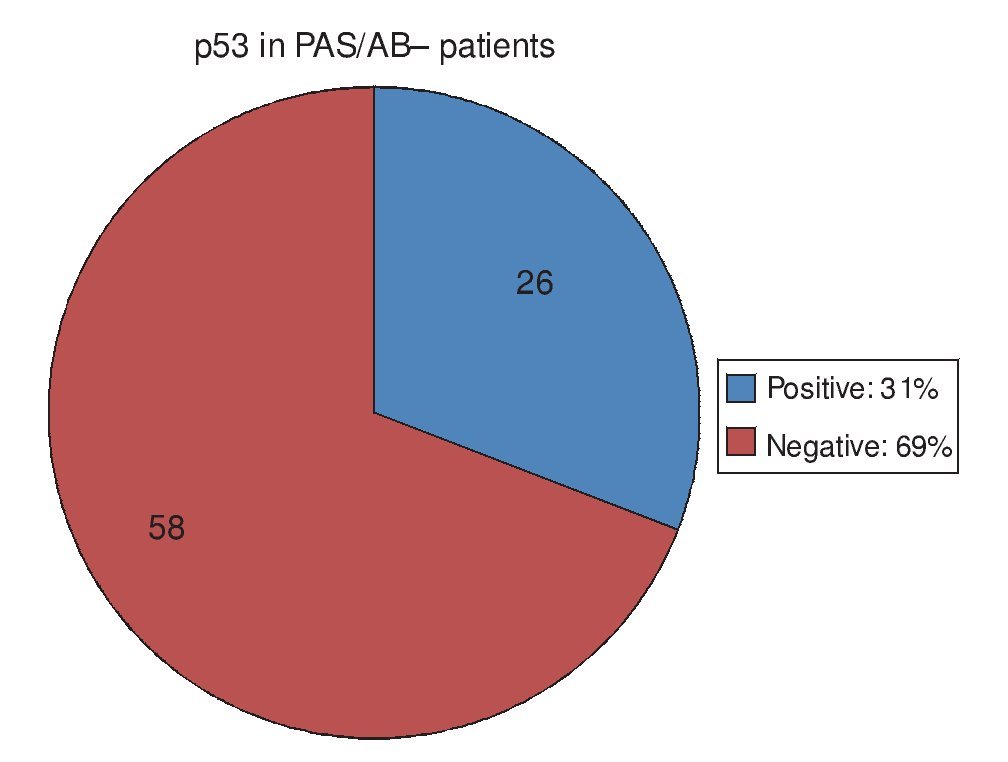
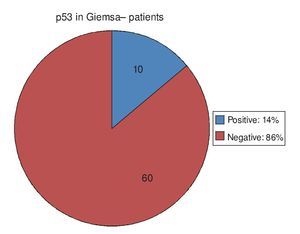
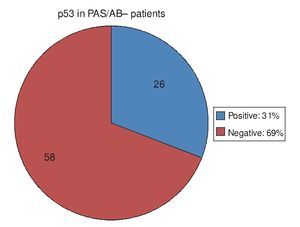
Regarding the 20 PAS/AB+ patients, in other words, those with IM, 15 of them (75%) had positive p53 and 5 (25%) had negative p53 (Fig. 3). Of the 84 PAS/AB- patients, 26 (31%) had positive p53 and 58 (69%) had negative p53 (Fig. 4).
Figure 3. p53 expression in patients with intestinal metaplasia intestinal. PAS/AB: Periodic acid-Schiff/Alcian blue stain.
Figure 4. p53 expression in patients without intestinal meta-plasia intestinal.
The three stains were positive in 9 of the 20 PAS/AB+ patients, resulting in Hp infection, complete IM, and p53 nuclear expression. Of the remaining 11 PAS/AB+ patients, 5 were not infected with Hp and did not have p53 expression, and the other 6 patients had p53 expression but Hp presence was not confirmed through positive Giemsa stain.
The Fisher exact test was used when the Giemsa stain was positive for Hp and the immunohistochemistry stain showed nuclear p53 expression, and it produced a statistically significant result of p<0.0001 (OR= 62, 95% CI, 15.8-241.8). The test was also used for analyzing the association between p53 expression and PAS positivity, that is to say, patients with IM, and produced the statistically significant results of p<0.0006 (OR= 6.69, 95% CI, 2.1-20.3) (Table 2).
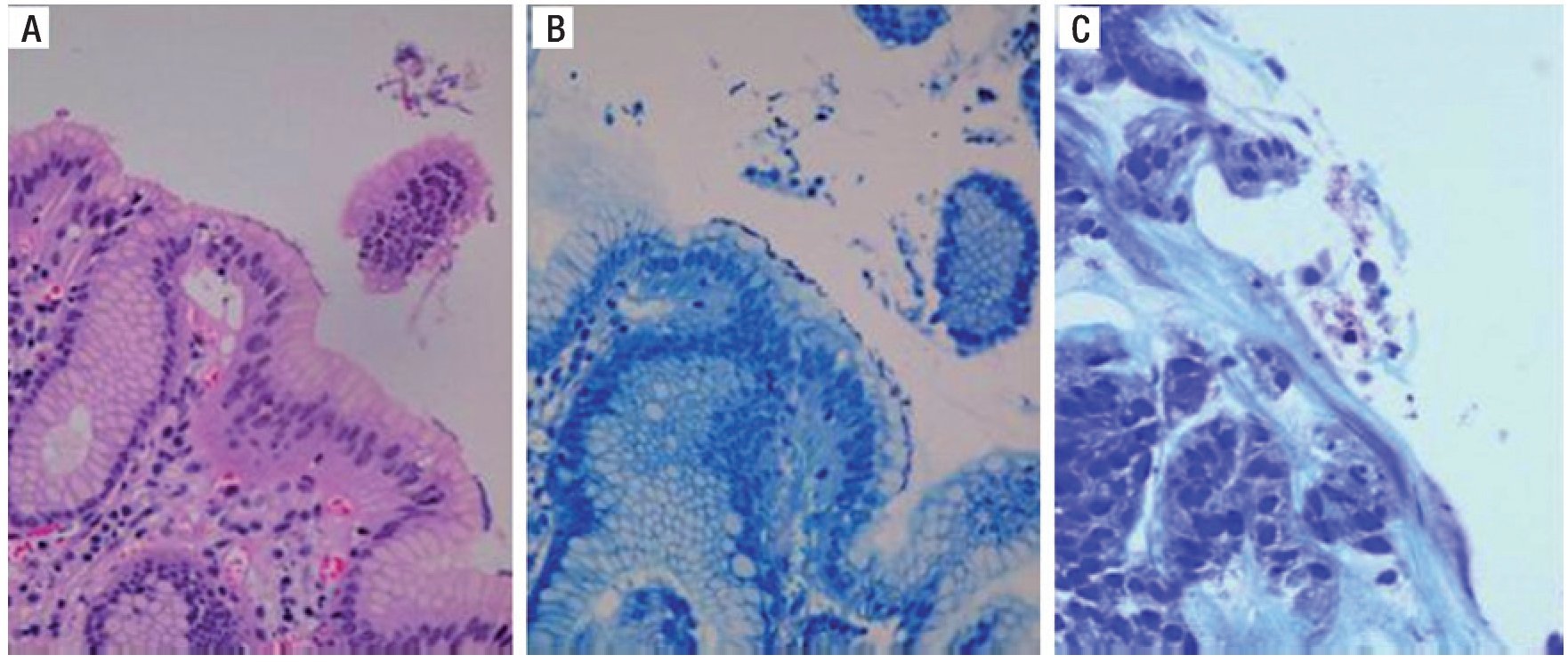
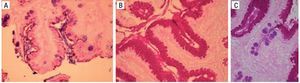
Figure 5 shows the PAS/AB stain in which the goblet cells with acid mucins are stained blue/purple with the AB, indicating the presence of IM. The magenta-colored stain indicates the presence of pseudo-goblet cells that are characteristic of the gastric epithelium and are base mucin producers.
Figure 5. Staining with PAS/AB. A) Alcian blue positive intestinal metaplasia B) PAS/AB positive. C) PAS/AB positive with evidence of intestinal metaplasia. PAS/AB: Periodic acid-Schiff/Alcian blue stain.
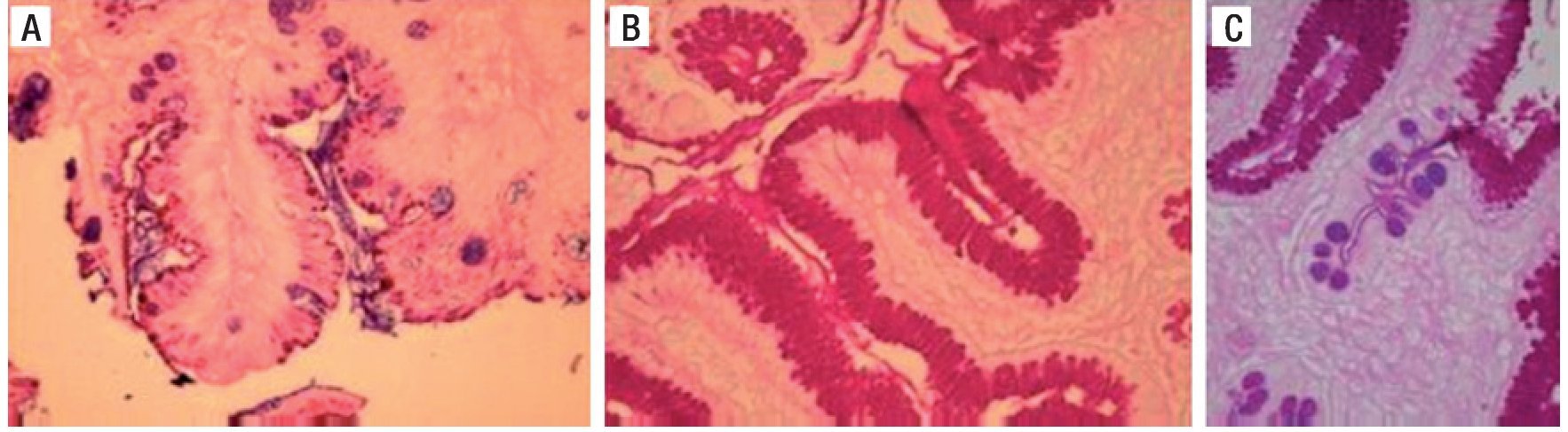
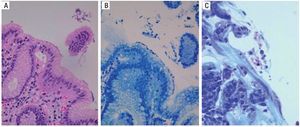
Figure 6 is a 40× microscopic photo of an H&E stain that shows bacilli suggestive of Hp on the epithelium of the gastric glandular surface that was confirmed by the Giemsa stain.
Figure 6. A) H&E stain with suspicion of Hp on the surface epithelium. B) Giemsa stain highlighting the Hp on the surface epithelium. C) Another example of positive Giemsa stain for Hp bacilli. H&E: Hematoxylin/Eosin stain, Hp: Helicobacter pylori.
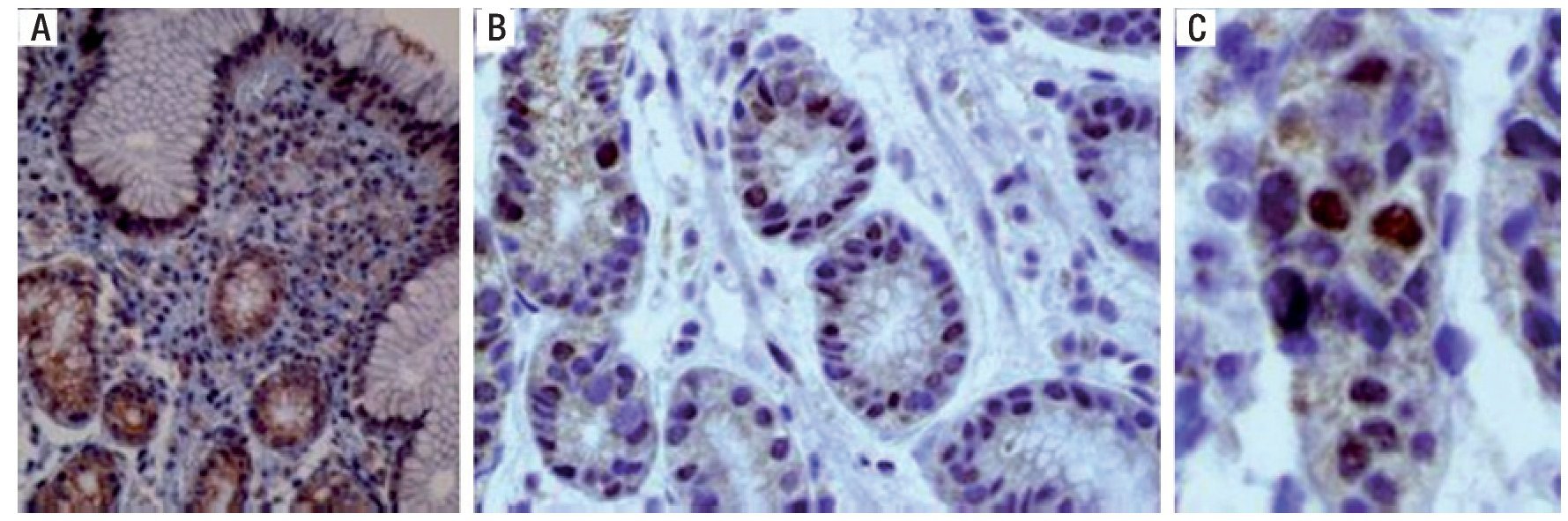
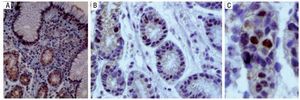
Figure 7 is a 40× microscopic photo of an immuno histo-chemistry stain for the p53 protein in which positivity is manifested through the staining of the nucleus, indicating gene expression. The presence of the stain in the cell's cytoplasm is what is known as "deep staining" and it does not have diagnostic value.
Figure 7. A) Immunohistochemistry stain with positive p53 in the nucleus and cytoplasm, making it inadequate for evaluation. B) Positive and negative for p53 in the nucleus. C) Nuclear positivity for p53.
Discussion
The patients making up the greater portion of our population were women; 62% of them were taking, or had recently taken, proton pump inhibitors either of their own accord or prescribed medically.
The endoscopic findings in the order of frequency were CG in 44%, acute gastritis in 17%, reactive gastritis in 12%, and nodular gastritis in 12%. Other findings, such as superficial gastritis, atrophic gastritis, and normal study presented less frequently. We observed that CG was found in almost half of our patients that did not present with the specific characteristics proposed by the SS. The finding of nodular gastritis is noteworthy because it implicates a high endoscopic suspicion of Hp in the patient.
Histopathologic diagnoses were made according to the SS proposals and moderate CG was the most predominant, representing 31% of the cases. It was followed by histologically normal mucosa, with 13% of the cases, superficial CG with 12%, active CG with 10%, and active follicular CG with 10%. The remaining diagnoses included superficial CG with a reactive component, moderate CG with a reactive component, reactive CG, mild CG, active follicular CG with a reactive component, and moderate follicular CG. CG was found in 87% of the cases and its subdivisions were dependent on the grade of activity and penetration into the mucosa, with the characteristic that in some patients, reactive gastritis data were found. The presence of lymphoid follicles, or follicular CG, was highly suggestive of Hp and was not a predominating diagnosis. Not one case of mucosal atrophy or dysplasia was found.
It should be kept in mind that the biopsies were taken from mucosa that had no macroscopic lesions that were apparent using conventional endoscopy.
In regard to the use of staining methods, PAS/AB was positive in 19% of the patients, all of them presenting with complete metaplasia. Within the SS criteria, biopsy is taken from the incisura angularis, which is where the greatest prevalence of this pre-neoplastic lesion is found, and it is highly unlikely to be identified endoscopically with conventional endoscopy.
Hp infection was confirmed by Giemsa stain in 32.7% of the patients, which is below that reported in the Mexican medical literature. It is noteworthy that the majority of patients were taking, or had recently been taking, a proton pump inhibitor, which produces Hp migration to the corpus and gastric fundus from its habitual location in the antrum and pre¿pyloric region, which is why biopsy was not taken at those sites.
In relation to p53 immunohistochemistry, it was positive in 39.4% of the study patients. As mentioned before, p53 is an oncogene that is expressed once it has been mutated. Also called the «wild gene», it alters the cell cycle, in turn producing altered cell proliferation and apoptosis rates that condition the accumulation of mutations and DNA damage, initiating a chain of events that leads to GC. In some in vivo studies, p53 expression has been related to Hp infection, and in vitro studies have confirmed that acute Hp infection produces p53 expression.
Concerning the relation between variables, it was demonstrated that in 91% of the patients with Giemsa+, meaning Hp infection, there was mutated p53 expression. This was not the case in the remaining 9% of Giemsa+ patients, because p53 expression was negative.
However, of the Giemsa- patients, that is to say, patients in whom Hp infection could not be confirmed, 14% (10 patients) presented with p53 expression; 60% of those patients (6 patients) had positive PAS/AB, that is to say, complete IM diagnosis. And as was mentioned before, Hp does not colonize IM. Other elements that could influence this result would be the possibility of genetic or environmental factors causing p53 mutation in the patient. Among the latter, the reactive gastritis that was found could be a factor worth investigating in this area in later studies.
There was a statistically significant relation between the Giemsa stain and the p53 immunohistochemistry.
Of the patients with confirmed IM, 75% had positive p53, which according to the rest of the results, suggests that Hp infection could be a predisposing factor for p53 mutation. By changing the epithelium toward IM, Hp was no longer able to colonize it, but the induced genetic damage would remain and continue in the oncogene chain leading to GC, given that IM is regarded as a pre-neoplastic lesion. However, 25% of the patients with IM did not present with p53 reactivity.
Finally, of the PAS/AB- patients, 31% (26 patients) had p53 expression, and Giemsa was positive in 85% (22 patients) of them, meaning that the p53 expression could be explained by the presence of Hp; in the remaining 15% p53 expression could again be due to other genetic or external factors. Of the PAS/AB- patients, 69% (58 patients) did not present with p53 reactivity, and of those patients, 95% (55 patients) were regarded as having no findings, due to the fact that in the remaining 5% (3 patients) Hp was found in the Giemsa stain and p53 expression was absent.
The relation between the PAS/AB stain and p53 immunohistochemistry also has a statistically significant association, and taking into account the abovementioned, that relation is confirmed by the Giemsa stain.
It should be underlined that the majority of the analyses that have been conducted are in vitro or animal model studies in which both the amount of time of Hp infection and its specific strain have been controlled. Until now, no epidemiologic studies have been carried out that indicate the true incidence of p53 mutations in asymptomatic patients or in those that do not present with macroscopic lesions during the endoscopy. The study was conducted, for the first time, on a Mexican population presenting with high Hp prevalence rates that are dependent on the geographic zone, providing a good opportunity for studying the chain of origin of gastric cancer. And to the best of our knowledge, this study is a first step in such an analysis. Our study design was a limitation because it did not allow for clinical follow-up in relation to the presence or absence of p53 expression and its progression to cancer; another limitation was the size of the study sample. Genetic studies for the confirmed detection of p53 mutations were not conducted in this study, only its expression was considered positive.
Conclusions
The study presents a strong association between the presence of Hp and the immunohistochemically visible expression of the p53 gene. The expression of this gene was even detected in patients that already presented with pre-neoplastic infections as a consequence of Hp infection, as occurs in IM, with the bacillus no longer present.
As with any other oncogene, p53 overexpression may be due to genetic factors or to external ones, such as infectious factors. The latter was found in this study in rela tion to Hp infection. Reactive gastritis as a possible cause of p53 alteration is an interesting finding; in patients with gastric surgery, biliary reflux in anastomosis has been related to a greater probability of cancer, but the possible role played by the p53 gene in this gastric cancer chain has not been studied.
The early identification of GC is of vital importance for its adequate treatment and cure. Gastric atrophy and IM have currently been identified as pre¿neoplastic lesions and should be under sur veillance for their potential to become malignant. In addition to routine biopsy taken during upper endoscopy for Hp detection by means of Giemsa staining, the complementary use of p53 immunohistochemistry can identify the population presenting its expression, which in its role as oncogene signifies cell cycle alteration.
Follow-up of the patients that present with p53 is neces sary in order to fully understand the effect of the expression or mutation and its actual predisposition for GC. It is a known fact that all stomach cancers present genetic alterations, but it is not known which of them is the first to occur, the time that elapses between each event, and how they are produced by Hp. Due to the high prevalence of p53 expression in these patients, and taking into account that in Mexico Hp prevalence is variable but constantly elevated, there would be many detected patients with this test. Therefore it is necessary to know which other factors lead to the development of cancer, possibly starting from a first step, such as p53 expression.
For now, p53 expression must be thought of as a marker for cell cycle alteration in patients with active or past Hp infection, the same as in patients with histologic consequences of that infection.
Financial disclosure
Internal: the Endoscopy Service of the Hospital Juárez de México provided the necessary endoscopic equipment.
External: the Pathology Service of the Hospital Español de México covered the costs of the biopsy processing.
Conflict of interests
The authors declare that there is no conflict of interest.
Acknowledgements
The authors wish to thank the Endoscopy Service of the Hospital Juárez for collecting the samples and the Pathology Service of the Hospital Español de México for processing the biopsies.
Received 6 September 2012; accepted 26 November 2012
Available online 29 January 2013
qPlease cite this article as: Morales-Fuentes GA, et al. p53 expresado en la mucosa gástrica de pacientes infectados por Helicobacterpylori. Revista de Gastroenterología de México. 2013;78:12-20.
* Corresponding author:
C/ Enrique González Martínez #18, Circuito Poetas, Colonia Ciudad Satélite, CP 53100, Naucalpan de Juárez, Estado de México, Mexico.
Tel.: 55-62-58-77 and 59-14-52-50.
Email address:gerar3d@yahoo.com (G.A. Morales-Fuentes).