The critically ill patient can develop gastric erosions and, on occasion, stress ulcers with severe gastrointestinal bleeding that can be fatal.
AimsThe purpose of this review was to provide current information on the pathophysiology, risk factors, and prophylaxis of digestive tract bleeding from stress ulcers in the intensive care unit.
MethodsWe identified articles through a PubMed search, covering the years 1970 to 2013. The most relevant articles were selected using the search phrases “stress ulcer”, “stress ulcer bleeding prophylaxis”, and “stress-related mucosal bleeding” in combination with “intensive care unit”.
ResultsThe incidence of clinically significant bleeding has decreased dramatically since 1980. The most important risk factors are respiratory failure and coagulopathy. Proton pump inhibitors (PPIs) or H2 receptor antagonists (H2RAs) are used in stress ulcer bleeding prophylaxis. Both drugs have been shown to be superior to placebo in reducing the risk for gastrointestinal bleeding and PPIs are at least as effective as H2RAs. Early enteral feeding has been shown to reduce the risk for stress ulcer bleeding, albeit in retrospective studies.
ConclusionsAdmittance to the intensive care unit in itself does not justify prophylaxis. PPIs are at least as effective as H2RAs. We should individualize the treatment of each patient in the intensive care unit, determining risk and evaluating the need to begin prophylaxis.
El paciente críticamente enfermo puede desarrollar erosiones gástricas y, en ocasiones, úlceras por estrés con sangrado gastrointestinal grave que puede ser fatal.
ObjetivosEl propósito de esta revisión fue proporcionar información actualizada acerca de la fisiopatología, factores de riesgo y profilaxis por sangrado del tubo digestivo causado por ulceras de estrés en la unidad de cuidados intensivos.
MétodosIdentificamos artículos a través de una búsqueda en PubMed, que abarcaba de los años 1970 a 2013. Los artículos más relevantes fueron seleccionados utilizando las frases de búsqueda «úlceras de estrés», «profilaxis de sangrado por úlceras de estrés» y «sangrado de la mucosa relacionado con el estrés» en combinación con «unidad de cuidados intensivos».
ResultadosLa incidencia de sangrado clínicamente significativo ha disminuido drásticamente desde 1980. Los factores de riesgo más importantes son el fallo respiratorio y las coagulopatías. Los inhibidores de la bomba de protones (IBP) o los antagonistas de receptores H2 (H2RA) se utilizan en la profilaxis de sangrado por úlceras de estrés. Ambos medicamentos han mostrado ser superiores al placebo en la reducción del riesgo de sangrado gastrointestinal, y los IBP son por lo menos tan efectivos como los H2RA. Se ha mostrado que la alimentación enteral temprana reduce el riesgo de sangrado por úlceras de estrés, aunque solo en estudios retrospectivos.
ConclusionesLa hospitalización en la unidad de cuidados intensivos por sí sola no justifica la profilaxis. Los IBP son por lo menos tan efectivos como los H2RA. Debemos individualizar el tratamiento de cada paciente en la unidad de cuidados intensivos, determinando el riesgo y evaluando la necesidad de comenzar la profilaxis.
The critically ill patient may develop gastric erosions and, at times, stress ulcers with severe gastrointestinal bleeding that can be fatal. Stress ulcers are gastric mucosa injuries, associated with stressful events such as extensive burns, mechanical ventilation, major surgery, sepsis, coagulopathy, and severe trauma.1 The purpose of this review is to present an update of the incidence, risk factors, pathophysiology and prevention of stress ulcer bleeding in the intensive care unit.
MethodsRelevant articles in the English language literature were identified through a PubMed search (1970-March/2013) using the search terms «stress ulcer», «stress ulcer prophylaxis», and «stress related mucosal bleeding» in combination with «intensive care unit». We selected clinical reviews, randomized clinical trials, meta-analyses, and therapeutic guidelines. We extracted data on pathophysiology, epidemiology, risk factors, and prophylaxis. It was not our objective to perform a systematic review.
PathophysiologyThe pathogenesis of stress-related mucosal disease and stress ulcers is multifactorial.2 One important factor is splanchnic hypoperfusion due to stress-related effects associated with critical illness. These effects include sympathetic nervous system activation, increased catecholamine release and vasoconstriction, hypovolemia, decreased cardiac output, and release of proinflammatory cytokines. The stress-related responses damage the integrity of the gastric mucosa by reducing gastrointestinal blood flow, oxygen delivery, and bicarbonate secretion. As the mucosal barrier permeability is compromised, back-diffusion of hydrogen ions and pepsin further damages the mucosal epithelial layer. Slowed mucosal blood flow impairs mucosal healing. Decreased gastric motility prolongs acid contact time with the gastric mucosa, increasing the risk of ulceration. Another factor is reperfusion injury. When blood flow is restored after long periods of hypoperfusion, elevated nitric oxide synthase levels lead to hyperemia, cell death, and enhanced inflammatory response. This results in further GI epithelial damage and ulceration. Stress-related mucosal lesions are typically located in the acid-producing areas of the stomach (i.e. upper body and fundus). Common endoscopic findings range from superficial erosions to deep focal ulcers that penetrate the submucosa, generally occurring between the third and seventh day after intensive care unit (ICU) admission.3
EpidemiologyImportant bleeding from stress ulcers is not a frequent event. Endoscopic evidence of mucosal damage is seen in most patients, 74 to 100%, within hours of admission to the ICU.4 Occult bleeding (guaiac positive stools) prevalence ranges from 15 to 50%.5 Overt bleeding is seen between 5 and 25% of critically ill patients.5–8 The incidence of clinically important bleeding (i.e. tachycardia, hypotension, and need for blood transfusion) has declined since 1980 to ranges between 3.7% in patients with risk factors to 0.1% in patients with no risk factors.9–12.
Decreased incidence of major bleeding can be explained by technological advances that have improved patient reanimation in the intensive care unit such as optimization of hemodynamic status, better tissue oxygenation, successful treatment of sepsis, and early enteral feeding instead of PPI and H2RA use.13,14
Nosocomial gastrointestinal bleeding due to stress ulcers is associated with higher in-hospital mortality rates, commonly in the intensive care unit.13,15 This has encouraged the prophylactic administration of several drugs, such as antacids, sucralfate, H2RAs, and PPIs.
Antacids and sucralfate are no longer used in most intensive care units. H2RAs reduce gastric acid secretion through a reversible, competitive inhibition of histamine-stimulated acid secretion and is frequently associated with tachyphylaxis (reduced effect after 48h of use). PPIs irreversibly suppress gastric acid production at the hydrogen/potassium-adenosine triphosphate level, providing long-lasting inhibition, and are the most potent antisecretory agents currently available. Unlike H2RAs, PPIs inhibit both histamine-induced and vagally-mediated gastric acid secretion.
In recent years, PPIs have become the most frequently used drugs, in relation to H2RAs, for preventing stress ulcers and gastrointestinal bleeding. However, it has been shown that overuse of these drugs in patients with no risk factors in the intensive care unit, as well as in non-ICU hospitalized patients, is a very frequent event. This inappropriate prescription has increased both the adverse effect rate and hospitalization costs.16–19 Likewise, prescription of these drugs is usually continued once the patient is out of the hospital, with the same consequences in relation to adverse effects and costs.20 In 2006, gastric secretion inhibitor sales in England rose to £ 425 m (€ 527 m; 701 m USD). Approximately 25 to 70% of those prescriptions had no clear indication.19
The administration of these drugs over a long period of time is not harmless. The main complications, albeit uncommon are: increased risk of nosocomial and community acquired pneumonia21, low calcium absorption with osteoporosis and risk of hip fracture22,23, and increased risk of Clostridium difficile infection.24–26
Risk factors for stress related mucosal bleedingSurprisingly, there are no therapeutic guidelines on stress ulcer prophylaxis published by either the American Gastroenterological Association (AGA) or the American College of Gastroenterology (ACG). In 1999 the American Society of Health-System Pharmacists (ASHP) issued guides for the use of these drugs.27
These evidence-based guides have only identified 2 main risk factors in ICU patients as predictors of stress-induced bleeding in whom prophylaxis is strongly suggested (strength of evidence A):
- A.
Respiratory Failure (mechanical ventilation for at least 48h)
- B.
Coagulopathy (ICU-hospitalized patients with platelet count<50,000; INR>1.5; abnormal PTT).
Other risk factors with lower degree of evidence are:
- 1.
Head injury<10 Glasgow scale (B).
- 2.
Thermal injury over 35% BSA28 (B).
- 3.
Partial hepatectomy (C).
- 4.
Gastrointestinal bleeding or ulcer in the previous year (D).
- 5.
Multiple trauma (Injury Severity Score ≥ 16) (D).
- 6.
Hepatic failure (D).
- 7.
Spinal cord injuries27 (D).
- 8.
Hepatic or renal transplantation (D)
- 9.
More than 2 of the following: sepsis, ICU stay>1 week, high corticosteroid dose (> 250mg of hydrocortisone [D]), occult or overt bleeding for 6 days or more [D])
Because overt gastrointestinal bleeding occurs in a minority of ICU patients and clinically important bleeding in only 1-3%, some authors have argued that prophylaxis is now overused. Therefore, the question is: which ICU patients should receive prophylaxis? Certainly, patients with respiratory failure requiring mechanical ventilation for >48h are at risk, as well as those with severe coagulopathy. Patients presenting with closed head injury and a low Glasgow score or severe burns should also receive prophylactic therapy. Patients who do not fall into any of these groups have less than 0.5% risk of clinically significant bleeding and probably do not require prophylactic therapy.
ProphylaxisThe efficacy of gastric acid inhibition to prevent gastrointestinal bleeding is conflicting. Previously, H2RAs have been used successfully. With higher acid suppression offered by PPIs, intensive care physicians expected better results. However, the evidence is not solid.
Several clinical trials have evaluated the efficacy of H2RAs for the prevention of stress-related gastrointestinal bleeding. A meta-analysis published in 1998 showed that H2RAs were significantly better than placebo in reducing the incidence of overt and clinically important bleeding. This study also reported that H2RAs were significantly better than sucralfate in reducing clinically important bleeding in mechanically ventilated patients.29
A recent meta-analysis published by Marik in 201030 assessed whether prophylactic administration of H2RAs for stress ulcers reduced the incidence of bleeding compared with placebo. This meta-analysis analyzed 17 trials with a total of 1,836 patients. The main result was a decrease in the risk of bleeding in patients with acid inhibitors compared with placebo (odds ratio OR=0.47; 95% CI, 0.29-0.76; p<0.002; I2=44%). Conversely, several studies have shown no significant reduction in clinically important bleeding using H2RAs, compared with either placebo or sucralfate.11,12,31,32
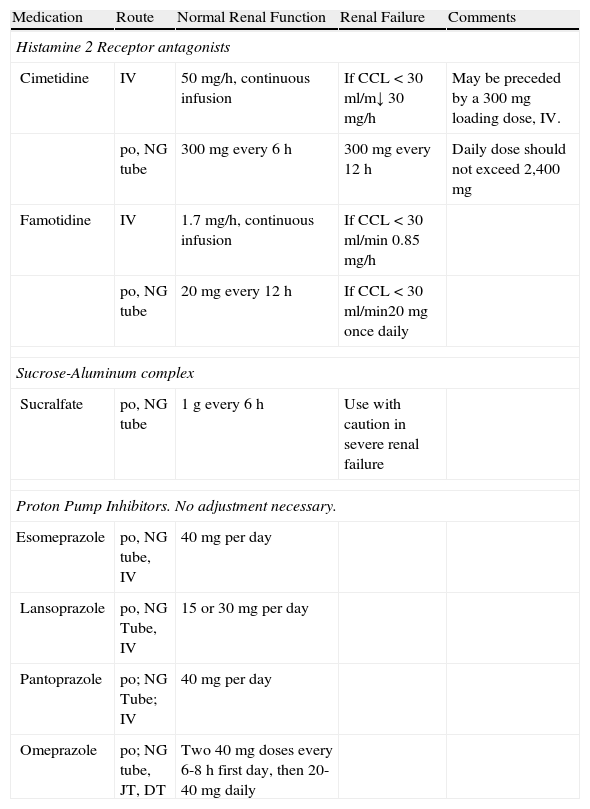
The use of PPIs as stress ulcer prophylaxis has increased more recently. It seems that PPIs are at least as effective as H2RAs. The first meta-analysis by Pongrasobchai in 2009, found that prophylaxis with PPIs was associated with fewer bleeding events, compared with H2RAs (OR=0.42, 95% CI, 0.20 to 0.91, number needed to treat [NNT]=22). There were no differences in the frequency of adverse events, such as nosocomial pneumonia.33 A second meta-analysis by Lin PC et al. compared PPIs and H2RAs as prophylactic agents in critically ill patients. This meta-analysis included 7 trials and 936 patients. There was no difference between the two groups for this main endpoint. They also failed to demonstrate any difference in the incidence of pneumonia and mortality between the 2 groups.34 Another important meta-analysis was published in 2012 by Barkun et al. and included a total of 13 studies involving 1,587 patients. They found that prophylactic PPI administration significantly decreased the incidence of bleeding compared with H2RAs (OR=0.30; 95% IC 0.17- 0.54, NNT=39, without statistical heterogeneity p=0.93, I2=0.0%). There were no differences for the development of nosocomial pneumonia, duration of ICU stay, and mortality. As they noted, definitions of bleeding varied among the studies, treatment included different drugs (omeprazole, pantoprazole, rabeprazole, lansoprazole, ranitidine, famotidine, and cimetidine), dosage and route of administration, analyses included abstracts, the study quality of many trials was poor, and some trials were performed on Asian patients known as slow metabolizers.35 The most recent meta-analysis evaluating efficacy and safety of PPIs vs H2RAs was published in 2013 by Alhazzani and Cook from McMaster University in Canada. They included fourteen trials for a total of 1,720 patients. PPIs were more effective than H2RAs at reducing both clinically important upper gastrointestinal bleeding (RR=0.36; 95% CI 0.19-0.68; p=0.002; I2=0%; NNT with prophylaxis=78) and overt upper gastrointestinal bleeding (RR=0.35; 95% CI 0.21-0.59; p<0.001; I2=15%; NNT with prophylaxis=30). There were no differences between PPIs and H2RAs in the risk of nosocomial pneumonia (RR=1.06; 95% CI 0.73-1.52; p=0.76; I2=0%), ICU mortality (RR=1.01; 95% CI 0.83-1.24; p=0.91; I2=0%), or ICU length of stay (mean difference – 0.54 days; 95% CI 2.20 to 1.13; p=0.53; I2=39%). No trials reported on C. difficile infection. Nevertheless, several factors suggest caution on the interpretation of these results. The subgroup analysis based on trial quality suggested that there were no differences in the treatment effect among trials of higher quality. It is possible that suboptimal trial design, such as the lack of blinding, has enhanced the observed benefits of PPIs.36 Currently, most ICUs are using PPIs instead of H2RAs as stress ulcer prophylactic agents. In a limited survey of pattern use, the standard prophylactic regimen rarely included intravenous ranitidine.37 Most U.S. institutions appear to be using a variety of proton pump inhibitor regimens, including intravenous pantoprazole 40mg/12h, 80mg/12h, or an 80mg bolus followed by 8mg/h continuous infusion. Other regimens being used include lansoprazole 60mg/12h and esomeprazole 40mg/12h. However, there are few published data to support the use of intravenous proton pump inhibitors for the prevention of upper gastrointestinal bleeding in critically ill patients. Furthermore, pH data for intravenous pantoprazole in ICU patients was obtained in patients on enteral diet, and whether this pharmacological effect could be similar in fasted patients is unknown and requires further study.38Table 1
Common medications used for prophylaxis of stress-related bleeding.
| Medication | Route | Normal Renal Function | Renal Failure | Comments |
| Histamine 2 Receptor antagonists | ||||
| Cimetidine | IV | 50 mg/h, continuous infusion | If CCL<30ml/m↓ 30mg/h | May be preceded by a 300mg loading dose, IV. |
| po, NG tube | 300mg every 6 h | 300mg every 12 h | Daily dose should not exceed 2,400 mg | |
| Famotidine | IV | 1.7 mg/h, continuous infusion | If CCL<30ml/min 0.85 mg/h | |
| po, NG tube | 20mg every 12 h | If CCL<30ml/min20mg once daily | ||
| Sucrose-Aluminum complex | ||||
| Sucralfate | po, NG tube | 1g every 6 h | Use with caution in severe renal failure | |
| Proton Pump Inhibitors. No adjustment necessary. | ||||
| Esomeprazole | po, NG tube, IV | 40mg per day | ||
| Lansoprazole | po, NG Tube, IV | 15 or 30mg per day | ||
| Pantoprazole | po; NG Tube; IV | 40mg per day | ||
| Omeprazole | po; NG tube, JT, DT | Two 40mg doses every 6-8 h first day, then 20-40mg daily | ||
po: orally; IV: intravenous; CCL: creatinine clearance; NG: nasogastric; JT: jejunal tube; DT: duodenal tube.
Finally, early enteral feeding had been postulated as a useful tool in preventing stress-related gastrointestinal bleeding. Enteral nutrients buffer acid and may act as a direct source of mucosal energy, induce secretion of cytoprotective prostaglandins and mucus, and improve mucosal blood flow.39,40 In addition, enteral feeding has been shown to be more effective in raising gastric pH>3.5 when compared with either PPIs or H2RAs41. Therefore, it has been suggested that early enteral feeding would be beneficial in preventing UGI bleeding secondary to stress-related mucosal disease. Several trials, mainly in burn patients, have concluded that early enteral nutrition is effective in preventing stress-related gastrointestinal bleeding.42–44 However, the evidence is not solid enough because no clinical randomized controlled trial has prospectively tested the influence of enteral nutrition on the risk of stress ulcer prophylaxis. In a former meta-analysis by Marik, that included 17 trials and 1,836 patients, they showed that patients who received enteral feeding (3 studies with a total patient population of 262) did not benefit from the stress ulcer prophylaxis with H2RAs (OR=1.26; 95% CI 0.43-3.7). On the whole, H2RAs did not increase the risk of hospital-acquired pneumonia (OR=1.53; 95% CI 0.89-2.61; p=0.12; I2=41%). However, this complication was increased in the subgroup of patients who received prophylaxis and were fed enterally (OR=2.81; 95%CI 1.20-6.56; p=0.02; I2=0%). Surprisingly, hospital mortality was higher in those studies (n=2) including patients fed enterally that received H2RAs (OR 1.89; 95% CI 1.04-3.44; p=0.04, I2=0%). The results of this meta-analysis suggest that in patients receiving enteral tube feeding, stress ulcer prophylaxis may not be required, and indeed, it could increase the risk of complications.30
ConclusionsThe incidence of clinically important bleeding related to stress ulcers has declined. The pathogenesis of stress-related mucosal disease and stress ulcers is multifactorial. Admission to the hospital or the ICU alone is not reason enough to provide prophylaxis. Only patients with respiratory failure requiring mechanical ventilation for more than 48h and those with coagulopathy, head injury, and severe burns are at significant risk for such bleeding and are likely to benefit from prophylaxis. However, other risk factors must be considered. The most appropriate prophylactic agent to prevent stress-related bleeding remains to be determined. A few years ago, H2RAs were the most widely used drug for prophylaxis. However, PPIs are increasing in acceptance. PPIs are at least as effective as H2RAs, but they are more expensive, and there is still limited evidence. We must individualize every patient in the ICU, evaluate risk, and assess the need to start stress- related bleeding prophylaxis.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Avendaño-Reyes J, Jaramillo-Ramírez H. Profilaxis para sangrado por ulceras de estrés en la unidad de cuidados intensivos. Revista de Gastroenterología de México. 2014;79:50–55.