Patients presenting with Barrett's esophagus (BE) should be under life-long surveillance in an attempt to detect cancer in its early stages. Esophageal capsule endoscopy (ECE) is a new technique that enables a noninvasive evaluation of the esophagus.
AimsTo evaluate ECE effectiveness compared with methylene blue (MB) chromoendoscopy for the detection of esophageal lesions in which there was suspicion of cancer, the length and pattern of BE, and the presence of hiatal hernia.
Material and methodsTwenty-one patients with BE who underwent Nissen fundoplication and had a follow-up period of more than five years were prospectively enrolled in the study. The patients underwent ECE and chromoendoscopy with MB performed by different physicians who were blinded to each of the procedures.
ResultsECE sensitivity, negative predictive value, and accuracy were 100%, 100%, and 79%, respectively, for the detection of esophageal lesions suspected of cancer. ECE accuracy in assessing BE length was 89% and in the evaluation of finger-like projections, circumferential BE, and mixed BE was 74%, 79%, and 74%, respectively.
In relation to hiatal hernia detection, ECE sensitivity was 43% and its accuracy was 74%.
ConclusionsECE appears to be a good method for detecting lesions in which there is suspicion of esophageal cancer and it had modest results in regard to the accurate identification of BE length and pattern. ECE is not a good method for detecting hiatal hernia. Further studies are needed in order to define the definitive role of ECE in BE monitoring.
Los pacientes con esófago de Barrett (EB) deben ser seguidos durante toda la vida en un intento de detectar el cáncer en una etapa temprana. La cápsula endoscópica del esófago (CEE) es una nueva tecnología que permite la evaluación no invasiva del esófago.
ObjetivosEvaluar la eficacia de la CEE en comparación con la cromoendoscopia con azul de metileno (AM) para la detección de lesiones sospechosas de cáncer de esófago, longitud y patrón de EB, y la presencia de hernia hiatal.
Materiales y métodosVeintiún pacientes con Barrett sometidos a funduplicatura de Nissen, con más de 5 años de seguimiento, fueron incorporados prospectivamente. Los pacientes fueron sometidos a la CEE y a cromoendoscopia con AM por diferentes medicos, cegados a cada uno de los procedimientos.
ResultadosLa sensibilidad, el valor predictivo negativo y precisión de la CEE fueron del 100, el 100 y el 79%, respectivamente, para la detección de lesiones sospechosas de cáncer de esófago. La precisión de la CEE para la evaluación de la longitud del EB fue del 89%. La precisión de la CEE para la evaluación de las proyecciones digitiformes, circunferencial y EB mixto fue del 74, el 79 y el 74%, respectivamente.
La sensibilidad de la CEE y la precisión para la detección de hernia hiatal fueron del 43 y el 74%, respectivamente.
ConclusionesLa CEE parece ser un buen método para la detección de lesiones sospechosas de cáncer de esófago. Con respecto a la identificación precisa de la longitud y el patrón del EB, la CEE tuvo resultados modestos. La CEE no es un buen método para la detección de una hernia hiatal. Son necesarios más estudios para definir el papel definitivo de la CEE en el seguimiento de EB.
Gastroesophageal reflux disease (GERD) and its complications, including Barrett's esophagus (BE), are common public health issues that have experienced significant growth rates in developed countries. It is estimated that 50% of adults in the United States experience GERD symptoms each month, and 14–20% report that their symptoms occur at least once a week.1,2 GERD is associated with a significant decrease in the quality of life and enormous economic costs, with direct costs exceeding US$10 billion and indirect costs of approximately US$5 billion per year.3
BE occurs in approximately 10% of patients with GERD4 and is characterized by the replacement of the normal stratified squamous epithelium of the esophagus with columnar epithelium containing intestinalized cells in any part of the organ.5,6 The main clinical significance of BE is its status as a predisposing condition for adenocarcinoma of the esophagus; the incidence of which has increased substantially in the United States, Western Europe, Australia, and other developed parts of the world over the last four decades.7,8 Once a person develops BE, neither clinical nor surgical treatments for GERD can prevent the progression of BE to adenocarcinoma.9–11 Thus, endoscopic monitoring of patients with BE is routinely used for early detection of esophageal adenocarcinoma, with the goal of reducing mortality.12,13
Currently, the gold standard for monitoring patients with BE is a series of upper gastrointestinal endoscopic biopsies from four quadrants, obtained from every 1–2cm and, if present, from any areas of mucosal irregularity.14 Techniques such as chromoendoscopy with methylene blue can be used to facilitate the identification of BE and, more importantly, areas of intraepithelial neoplasia and early carcinoma. Several studies have shown that methylene blue chromoendoscopy increases the detection rate of early neoplasia in BE and reduces the number of biopsies needed compared with conventional endoscopy with randomized biopsies.15,16 The cost, invasiveness, discomfort, need for sedation, and absence from work are potential obstacles to the widespread use of upper gastrointestinal endoscopy.17,18 The need for a less invasive, safer, and reliable exam has generated great interest in the study of esophageal capsule endoscopy (ECE).19
ECE, which was approved by the FDA in November 2004, is a new tool that allows visualization of the esophagus and is associated with less discomfort and fewer risks than conventional upper gastrointestinal endoscopy (UGE).18 Although several studies have evaluated the accuracy of ECE for the diagnosis of BE, data on follow-up remain scarce.
The primary aims of this prospective, blind study were to evaluate the accuracy of ECE in the detection of lesions suspected to be esophageal cancer in patients with BE and to evaluate the characteristics of BE according to length and pattern. As secondary objectives, we evaluated the accuracy of ECE for the detection of hiatal hernia and the tolerability and safety of the procedure in general.
MethodsPatientsThis study was conducted at the Gastrointestinal Endoscopy Unit of the University of São Paulo School of Medicine (São Paulo, Brazil) from March to September 2008. Patients who had BE and had undergone Nissen fundoplication were referred for evaluation according to the following inclusion criteria: age 18 years or older, BE that was diagnosed by histology at least five years prior to the study, previous treatment with Nissen fundoplication at least five years prior to the study, and the ability to provide written informed consent. Exclusion criteria were: swallowing disorders, Zenker diverticulum, obstructions of the esophagus, stenosis of the esophagus, gastrointestinal fistulas (known or suspected), previous esophageal surgery, previous diagnosis of neoplasia in BE, prior endoscopic treatment of BE, a cardiac pacemaker, pregnancy, a history of allergies to dyes, the inability to undergo intravenous sedation, and refusal to sign the informed consent.
Esophageal capsule endoscopyThe Given Diagnostic System (PillCam ESO, Given Diagnostic System: Given Imaging, Yoqneam, Israel) used for this study consists of three main components: an esophageal capsule endoscope, a PillCam data recorder and a RAPID workstation. The ECE is a cylindrical capsule that measures 11mm×26mm and weighs 3.7g. It has two convex optical domes where images can be captured at a speed of two frames per second per side for a total of four images per second. The images are transmitted by radio to the data recorder via sensors that were previously attached to the thoracic-abdominal wall of the patient. At the end of the examination, the data recorder is connected to the RAPID workstation, and the captured images are ready to be evaluated.
Patients were instructed to fast for 8h prior to the test. Before each test, the system was prepared, and the sensors were positioned in the abdomen and chest of the patient. Next, the patient swallowed the ECE according to a standardized protocol. Patients were instructed to drink 100ml of water to remove the secretions that cover the esophagus. They were then placed on a gurney in the supine position, and they ingested the endoscopy capsule along with 10ml of water. After 2min in this position, the hospital gurney angle was raised to 30°, and the patient was kept in this position for 1min. The gurney was then tilted to 60° for 1min. Finally, the gurney was raised to 90°, and the patient remained seated in this position for 1min. Patients were asked not to speak or make sudden movements during the 6-min test. The same physician, who did not have access to the test results from the UGE examination, evaluated all the ECE exams. The data parameters analyzed by the researcher were the esophageal transit time, quality of preparation of the esophagus, presence or absence of lesions indicative of esophageal cancer, estimated length of BE, BE pattern and the presence or absence of a hiatal hernia. The esophageal transit time was calculated by subtracting the time of the first esophageal image from the time of the first gastric image. The quality of the esophageal preparation was classified as good, fair, or poor. Lesions suspected of neoplasia included raised, flat, or depressed lesions located in regions of BE. The BE was classified as long (≥3cm) or short (<3cm) based on its estimated length. With regards to the pattern found, the BE was classified as finger-like when it was shaped as one or more columns; as circumferential when it surrounded the organ with similar length, and as mixed when both aspects were present, that is, if the BE had distal circumferential involvement with digitiform projections on its proximal portion.
Upper gastrointestinal endoscopy combined with chromoendoscopy with methylene blueAll patients underwent UGE on the same day that they underwent the ECE examination, with the UGE always performed after the ECE. All examinations were performed by an experienced endoscopist and were supervised by a single physician, neither of whom had access to the results of the ECE examinations. A topical anesthetic spray containing lidocaine and an intravenous solution of midazolam and fentanyl citrate were used for UGE. All examinations were performed with a standard video endoscope (Olympus, GIF V; Olympus Optical Co., São Paulo, Brazil). First, the esophageal mucosa was cleaned with a solution of 0.9% saline and 10% N-acetylcysteine. After 2min, the distal esophagus was flushed with a 0.5% methylene blue solution. Two minutes after the flush, excess methylene blue was flushed away with distilled water. Staining was considered positive when the mucosa remained blue after washing. Non-stained areas within Barrett's epithelium were considered suspicious for dysplasia, as well as mucosal abnormalities such as nodules, plaques, ulcers, erosions, or focally depressed areas. Random 4-quadrant biopsies every 2cm were taken from all patients, and target biopsies were taken from suspicious areas when encountered. All specimens were examined by an experienced pathologist. When high-grade dysplasia or adenocarcinoma was suspected, a second pathologist reviewed the sample to confirm diagnosis.
The length of the BE was defined as the difference between the distance from the incisors to the esophagogastric junction and the distance from the incisors to the proximal margin of the BE. The BE was classified as long (≥3cm) or short (<3cm). In regard to BE pattern, it was classified as finger-like, circumferential or mixed, as previously described. The potential presence of a hiatal hernia was investigated, and the size of the hernia (if present) was measured as the distance between the esophagogastric junction and the diaphragmatic pinch.
Three days after the examination, patients were asked about the degree of discomfort they experienced during the ECE and UGE examinations.
Statistical analysisUGE with esophageal chromoendoscopy was considered to be the gold standard method. To compare the results obtained with ECE to those obtained with UGE, we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of each method. We also calculated the Kappa index of agreement. We used the Fisher exact test to determine whether there was an association between the examination discomfort levels of the ECE and UGE methods. A significance level of 5% (p≤0.05) was used for all tests.
ResultsA total of 21 patients met the criteria for inclusion and were enrolled in the study. Two patients were excluded because evaluation by ECE became impossible: one due to inadequate esophageal preparation, and one due to esophageal stenosis. Of the 19 remaining patients, 11 (57.9%) were male and 8 (42.1%) were female. The average age of the patients was 58.1 years (range, 20–81 years). Each of the patients had undergone fundoplication between 5 and 26 years prior to this study (average, 10.6 years). Of the 19 patients studied, 14 had a good esophageal preparation and 5 had a fair preparation. The mean esophageal transit time of the ECE was 383.8s (SD, 404.6s; range, 2–1211s).
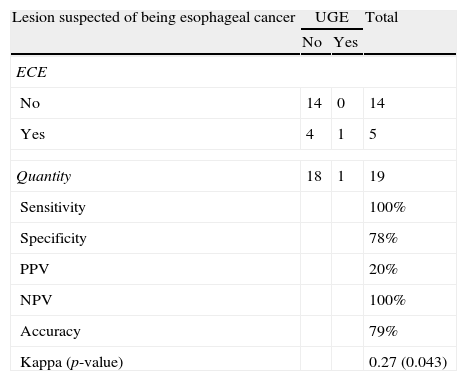
Comparative evaluation of esophageal capsule endoscopy and methylene blue chromoendoscopyLesions suspected of being esophageal cancerThere was only a single case of a lesion suspected of being esophageal cancer. It was identified by UGE and ECE. ECE identified four additional suspected cases, but they were not confirmed by UGE. Thus, ECE had a sensitivity of 100%, a specificity of 78%, a PPV of 20% and an NPV of 100%. According to the Kappa index, the correlation between ECE and UGE was satisfactory and statistically significant (Table 1).
Lesions suspected of being esophageal cancer: the results of ECE vs. UGE.
| Lesion suspected of being esophageal cancer | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 14 | 0 | 14 |
| Yes | 4 | 1 | 5 |
| Quantity | 18 | 1 | 19 |
| Sensitivity | 100% | ||
| Specificity | 78% | ||
| PPV | 20% | ||
| NPV | 100% | ||
| Accuracy | 79% | ||
| Kappa (p-value) | 0.27 (0.043) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
After the analysis of esophageal biopsies, only a single case of early esophageal cancer was identified. So when we considered the outcomes of esophageal biopsies performed during UGE to be the gold standard for the diagnosis of esophageal cancer, we obtained the same results described above.
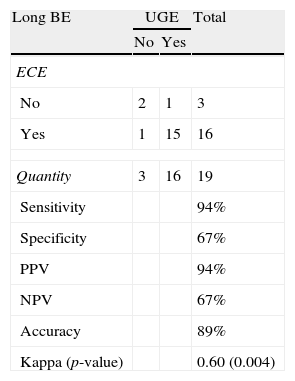
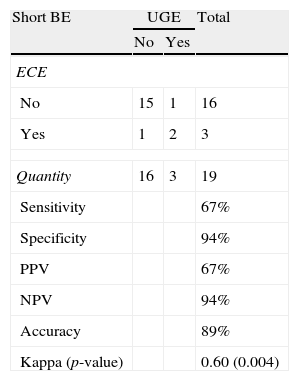
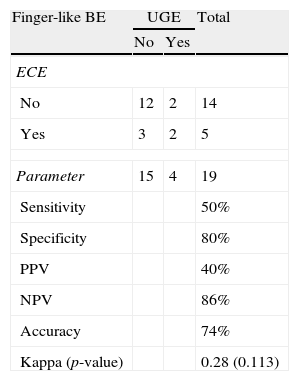
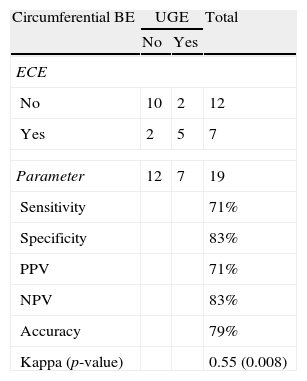
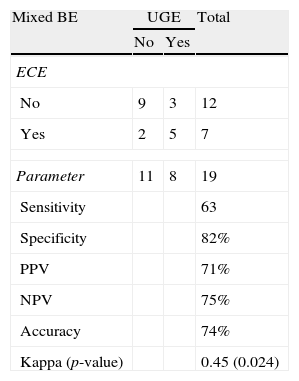
Length and pattern of Barrett's esophagusWe evaluated the ability of ECE to correctly identify the length of the BE and determined that this method had an accuracy level of 89% and a Kappa index of agreement of 0.60 (moderate agreement), which was statistically significant (p=0.004) (Tables 2 and 3). Regarding the ability of ECE to correctly detect the BE pattern, ECE had 50% sensitivity, 80% specificity, 40% PPV and 86% NPV for the finger-like type. In cases of circumferential BE, ECE had 71% sensitivity, 83% specificity, 71% PPV and 83% NPV. For cases of mixed BE, ECE had a sensitivity of 63%, specificity of 82%, PPV of 71% and NPV of 75%. The Kappa index revealed that ECE showed a moderate agreement with UGE in the identification of circumferential and mixed BE, but there was no significant agreement in the identification of finger-like BE (p=0.113) (Tables 4–6).
Detection of long BE: ECE vs. UGE.
| Long BE | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 2 | 1 | 3 |
| Yes | 1 | 15 | 16 |
| Quantity | 3 | 16 | 19 |
| Sensitivity | 94% | ||
| Specificity | 67% | ||
| PPV | 94% | ||
| NPV | 67% | ||
| Accuracy | 89% | ||
| Kappa (p-value) | 0.60 (0.004) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
Detection of short BE: ECE vs. UGE.
| Short BE | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 15 | 1 | 16 |
| Yes | 1 | 2 | 3 |
| Quantity | 16 | 3 | 19 |
| Sensitivity | 67% | ||
| Specificity | 94% | ||
| PPV | 67% | ||
| NPV | 94% | ||
| Accuracy | 89% | ||
| Kappa (p-value) | 0.60 (0.004) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
Detection of finger-like BE: ECE vs. UGE.
| Finger-like BE | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 12 | 2 | 14 |
| Yes | 3 | 2 | 5 |
| Parameter | 15 | 4 | 19 |
| Sensitivity | 50% | ||
| Specificity | 80% | ||
| PPV | 40% | ||
| NPV | 86% | ||
| Accuracy | 74% | ||
| Kappa (p-value) | 0.28 (0.113) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
Detection of circumferential BE: ECE vs. UGE.
| Circumferential BE | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 10 | 2 | 12 |
| Yes | 2 | 5 | 7 |
| Parameter | 12 | 7 | 19 |
| Sensitivity | 71% | ||
| Specificity | 83% | ||
| PPV | 71% | ||
| NPV | 83% | ||
| Accuracy | 79% | ||
| Kappa (p-value) | 0.55 (0.008) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
Detection of mixed BE: ECE vs. UGE.
| Mixed BE | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 9 | 3 | 12 |
| Yes | 2 | 5 | 7 |
| Parameter | 11 | 8 | 19 |
| Sensitivity | 63 | ||
| Specificity | 82% | ||
| PPV | 71% | ||
| NPV | 75% | ||
| Accuracy | 74% | ||
| Kappa (p-value) | 0.45 (0.024) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
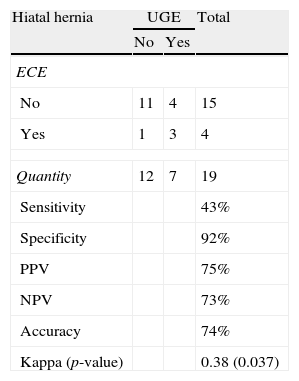
Seven cases of hiatal hernia were identified by UGE, whereas ECE identified only three cases. For the detection of hiatal hernia, ECE had sensitivity of 43%, specificity of 92%, PPV of 75% and NPV of 73%. The Kappa index was 0.38 (fair agreement) (Table 7).
Comparison between ECE and UGE in the detection of hiatal hernia.
| Hiatal hernia | UGE | Total | |
| No | Yes | ||
| ECE | |||
| No | 11 | 4 | 15 |
| Yes | 1 | 3 | 4 |
| Quantity | 12 | 7 | 19 |
| Sensitivity | 43% | ||
| Specificity | 92% | ||
| PPV | 75% | ||
| NPV | 73% | ||
| Accuracy | 74% | ||
| Kappa (p-value) | 0.38 (0.037) | ||
ECE: esophageal capsule endoscopy; UGE: upper gastrointestinal endoscopy; PPV: positive predictive value; NPV: negative predictive value.
Of the 19 patients who completed the study, 3 reported minimal discomfort during the ingestion of the ECE, and 6 and 2 reported minimal and moderate discomfort, respectively, during the UGE. A greater number of patients reported discomfort during the UGE compared with the ECE, but this difference was not statistically significant (p=0.186). With respect to complications, there was one case of retention of the ECE in the esophagus. In this case, the ECE was visualized and then removed without complications during the UGE examination; this patient had stenosis of the distal esophagus and was excluded from the study.
DiscussionOnce a patient has developed BE, he or she will always have an increased risk of developing esophageal adenocarcinoma. Several studies have shown that neither the clinical treatment of BE using proton pump inhibitors nor the surgical treatment of BE with fundoplication prevents the progression of BE to adenocarcinoma.9–11 Several studies have shown that patients who participated in endoscopic monitoring programs had their tumors diagnosed at earlier stages and generally had a significant improvement in their survival rate.20–22 Mainly in cases of intraepithelial neoplasia and early adenocarcinoma, various techniques such as conventional chromoendoscopy and optics can be used to facilitate the identification of BE. In this study, we used chromoendoscopy with methylene blue to identify BE because this method is more compatible with the reality of our country, given that few Brazilian or South American endoscopy providers have access to high-resolution video endoscopes with optical chromoendoscopy.
Although capsule endoscopy is considered by many to be a front-line method for the diagnosis of diseases of the small intestine,23,24 this technique has proven to be ineffective for the assessment of esophageal disorders mainly because of the short esophageal transit time, the low frequency of captured images (two per second) and the presence of only one camera.25 Thus, ECE, which uses two cameras with speeds of 4–14 frames per second, was developed to overcome these limitations. Initial results indicated that ECE was a sensitive, specific method for evaluating esophageal disorders in patients with symptoms of GERD.17,25 However, later studies achieved more modest results for the ECE-based diagnosis of esophageal disorders.19,26,27 No studies have yet compared the efficacy of the ECE and UGE methods in studying the characteristics of BE or in the tracking of lesions suspected of being malignant in BE patients who had previously undergone fundoplication.
In this study, the average esophageal transit time was 383.8s (range, 2–1211s), which is higher than that found by most previous studies.17,26,28 We believe that this discrepancy is due to the prior fundoplication that these patients had undergone, but we cannot be sure that a longer esophageal transit time necessarily contributes to better visualization of the esophageal transition.
The ECE method had a sensitivity and NPV of 100% for the detection of lesions suspected to be esophageal cancer. Thus, despite the lower specificity of ECE compared with UGE (78% vs. 100%, respectively), the use of ECE produced excellent results in terms of sensitivity and NPV, the most important indices for a screening test. When the ability of ECE to classify the length of BE as long or short was evaluated, ECE had an accuracy of 89% with higher sensitivity and PPV for long BE (94% for both parameters) than for short BE. Regarding the accurate identification of the BE pattern, ECE had modest results. There was moderate agreement in cases of circumferential and mixed types of BE, and there was no significant agreement in cases of columnar finger-like BE. As previously reported by Sharma et al., ECE is not a good method for the detection of hiatal hernia.27 Our findings on the degree of discomfort experienced by patients during the examinations, although not statistically significant, were consistent with those reported by most previous studies, including Eliakim et al. and Bhardwaj et al.17,19 Among the 19 patients included in the final study group, there were no cases of complications during the two endoscopic procedures.
Some limitations of this study should be considered. The ECE used in this study is a first generation capsule model (PillCam ESO), which captures only four images per second. It is possible that the use of a more modern capsule endoscope, such as the PillCam ESO 2, which is capable of taking 14 pictures per second and has a greater field of vision, could improve the results. In addition, the traditional ingestion protocol used could have influenced our results. A new ingestion protocol – the simplified ingestion protocol – appears to allow greater visualization of the esophagogastric junction, as demonstrated by De Jonge et al.29 Moreover, the results of the ECE examination were evaluated by a single investigator in the present work; Lai et al. demonstrated that there is a moderate discrepancy between different observers in the interpretation of capsule endoscopy examinations of the small intestine.30 A significant limiting factor of ECE is its inability to obtain tissue for anatomopathological evaluation, which means that a subsequent UGE biopsy is required in cases where there are lesions suspected of malignancy. In the near future, it is likely that the capsule endoscope will be remotely controlled by the examiner and will allow for tissue samples to be taken from potentially malignant sites that can be studied for anatomopathologies.
The advantages of ECE over conventional UGE, such as convenience, tolerability, a minimum of time away from work, and safety, are undeniable. However, despite the advantages that make ECE attractive, this method cannot currently be recommended as a first choice to monitor BE. On the other hand, we believe it could be useful for patients who have contraindications to UGE or who refuse to undergo a conventional UGE. Further studies with larger sample sizes are necessary to define the future role of ECE in the monitoring of BE.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Except for providing the capsules at no charge, given imaging was not involved in either the study design or in the analyses, or in the review of the paper prior to submission.