Introduction
It was in 1963, in the city of Denver, CO, USA, that Dr. Thomas E. Starzl performed the first worldwide liver transplantation (LT),3 and, in 1967, he performed the first transplant with a survival of >1 year.4 Time has elapsed and, after nearly four decades, the evolution and advances in the transplantation field have allowed considering LT, in its diverse modalities, as the therapeutic option-of-choice for end-stage liver disease.5 Regarding liver function failures, these have led to the search new alternatives for organ viability and donor availability, because the waiting list in all countries with well-established transplantation programs has been increasing, surpassing the demands of the population.6 Other aspects to take into account include the following: the study of mechanisms to improve liver preservation;7 decreasing the effects of the ischemia-reperfusion injury;8, 9 liver preconditioning,10 new and better drug schemes to decrease acute or chronic rejection; management of postoperative complications, and a fundamental point deals with the health education of the population, as well as the establishment of specialized centers and a defined legal setting.6
A clear example of the afore mentioned is the immunosuppression scheme, which has permitted to make a difference in medical treatment. During the 1980s, inclusion of cyclosporine in clinical protocols allowed for remarkable advances in the multidisciplinary management of transplanted patients; the drug was initially used in patients with renal transplants and then in liver transplantation.11 There is no doubt that this drug favorably modified the treatment of transplanted patients, constituting a remarkable advance in the treatment of patients with end-stage hepatic disease.5
Another important topic in LT is the model for end-stage liver disease (MELD) score. MELD is a numerical scale that ranged from 6 (less ill) to 40 (gravely ill) that is employed for liver transplant candidates aged 12 years and older. MELD provides each person with a "score" (number) that is based on how urgently he/she requires a liver transplant within the next 3 months.12,13 The MELD score number is calculated by a formula that utilizes the results of three routine laboratory tests: bilirubin, which measures how effectively the liver excretes bile; INR (prothrombin time), which measures the liver's ability to manufacture blood clotting factors, and creatinine, which measures kidney function. (Impaired kidney function is often associated with severe liver disease.) The MELD is used for candidates aged 12 and older, while the PELD (Pediatric end-stage liver disease model) is used for patients aged 11 years and younger.14 A patient's score may rise or fall over time depending on the status of his/her liver disease. The majority of candidates will have their MELD score assessed a number of times while they remain on the transplantation waiting list. This will aid in ensuring that donated livers are received by the patients in greatest need at that moment. The only priority exception to MELD is a category known as Status 1. Status 1 patients have acute (sudden and severe onset) liver failure and a life expectancy of hours to a few days without a transplant. Fewer than 1% of liver-transplant candidates are in this category. All other liver candidates aged 12 years and older are prioritized by the MELD system.13,15
In Mexico, the transplantation program was initiated at the beginning of the 1980s;16 the first successful LT case was reported in 1991.17 The number of organ transplant centers, especially kidney transplantation, are increasing in Mexico,18 and with regard to LT programs, these have achieved notable advances in diverse hospitals.19-21 Hence, in Mexico it is necessary to strive for a better medical infrastructure, improvement of organ viability, training of specialized personnel, and adjustments in the high costs involved in transplant programs.21,22
The multidisciplinary work in transplant medicine and surgery has offered a better insight of this field of knowledge. The present review is aimed at providing the interested reader with a clear and concise panorama of the relevant advances in LT, covering diverse topics, such as surgical aspects that deal with the procurement of segment(s) of the organ from a living related donor, indications for patients with hepatic cancer (hepatocellular carcinoma and cholangiocarcinoma), up to the expectations in cellular transplantation of hepatocytes and temporal artificial support for patients with end-stage liver failure, implying a close relationship with molecular, genomic, and proteomic biology.
Other aspects that continue to be of potential clinical interest, but which remain in the primary experimental stage, are xenotransplantations;23 the concept of this type of transplantation, using, for example, the pig as a donor species, may provide a potential solution to the lack of human organs available for transplantation. Initial clinical experiences have been gained, but have not yet been established as a long-term viable element, mainly because of immunological limitations that could be overcome through potential modifications in the genetics of donor animals.24 The most studied animal is the pig, due to its similarity in terms of its hepatic morphology to that of humans; this option for obtaining organs for transplantation is at present the subject of extensive studies. Xenotransplantation employing pig organs could solve the significant increasing shortage of donor organs for allotransplantation.24-26 Survival of pig-organ xenografts in primates is initially limited by humoral rejection, which can be either hyperacute (defined as occurring within 24 h) or delayed for days or even weeks (variously termed acute humoral xenograft rejection, acute vascular rejection, or delayed xenograft rejection).25 Pre-formed and/or elicited cytotoxic antibodies against Gala1,3Gal (Gal) epitopes in the pig vascular endothelium are major causative components of the primate anti-pig immune response, and for many years proved to be a major barrier for achieving the prolonged survival of pig grafts in non-human primates.26
Hepatic transplant with related living donor organ (HTRLD)
Potential donations and viable organs have been and will continue to be one of the greatest limitations in the transplantation of whole or partial livers. Other factors comprise the ideological tendencies of certain regions of the world, such as, for example, those of Japan and other Eastern countries, where transplant from a cadaver donor has not been feasible until now. Thus, new alternatives are sought for these patients awaiting a hepatic transplant.27 A good surgical option is provided by living related donors, as in renal transplantation; but in this case, we have only one organ capable of regenerating. Therefore, procuring a hepatic segment for the recipient and leaving the remnant liver in the donor in adequate proportions to allow both individuals to survive and lead healthy active lives are essential aspects.
In the U.S., the clinical stage of HTRLD began at the end of the 1990s. Currently, ca. 5-7% of transplants carried out in adults correspond to this modality.28 Complications frequently associated with HTRLD include size of the donated organ,29 use of this type of transplant for patients with hepatocellular cancer,30 viral neo- and re-infection with hepatitis C with long-term follow-up,31 need for immunosuppression, histocompatibility problems, and even the death of either the donor or the recipient; thus, their use in transplant centers was limited in its beginnings.27 However, recently, successful case series have been published by both Eastern and Western research teams. In Canada, a study with 101 cases reported 0% mortality in patients subjected to right lobe hepatectomy, with a 4-year follow-up and good clinical evolution.32 In a multicenter study performed by nine specialized centers, HTRLD was performed in 385 patients, who presented 87% of organ survival at 90 days and 81% at 1 year, respectively. In this study, it was observed that geriatric donors and a prolonged cold-ischemia time were the most frequent adverse prognostic factors.33
In pediatric population, this HTRLD modality and reduced-size hepatic transplantation (RSHT) exert a great impact because mortality among patients awaiting transplantation is higher than in adults; these data increase each year, and it must be considered that approximately 7-10% of HT are performed in children.34,35 With this surgical technique, in general, two liver lobes are obtained: one for a child, and the second for an adult. The technique requires careful and expert dissection based on good surgical training; time of ischemia is longer than with conventional HT, and viable donors are also a limiting factor for this group of patients. HTRLD and RSHT are options that have spurred an increase in transplanted patients in both adult and pediatric populations.36 In Mexico, the team from the Federico Gómez Hospital Infantil de México published a series of 35 HT patients, including the first case of HTRLD in a child, with 77.1% survival in the first year and 74.2% at 5 years.20
Liver transplant and cancer
HT has been indicated in cases of malignant liver neoplasm since its beginning; in the U.S.; ca. 400 patients are treated yearly with this pathology, and LT is a first-choice line of treatment.30 In hepatic cancer, we must differentiate the pathology according to which cellular groups of the liver are primarily affected, for example, hepatocytes (hepatocellular cancer)37 and cells of the bile ducts (cholagiocarcinoma); the latter has had a greater increase in number of patients subjected to LT,38 but LT is not recommended in all cases. In patient selection for LT with hepatocellular cancer, it is considered either a <5-cm tumor, or no more than three lesions <3 cm, without vascular invasion.37
When adjusting inclusion criteria for LT in patients with a diagnosis of hepatocellular cancer, survival has been similar to organ viability in transplanted patients with non-malignant pathologies.39 Currently according to these guidelines (Milan Criteria and later adjustments), in patients with tumor size £ 5 cm or 2-3 lesions £ 3 cm, not >3 cm in circumference, without vascular invasion, and subjected to LT, results have been more encouraging than at its beginnings.40 One research team has proposed increasing the dimensions in the criteria, allowing up to 6.5 cm as maximal tumor size, or three tumors not >4.5 cm, obtaining similar good results to those achieved with the previously mentioned criteria.41 Liver transplantation (LT) is the treatment-of-choice for many patients with unresectable hepatocellular carcinoma (HCC), but the long waiting time due to the shortage of donor organs can result in tumor progression and drop-out from LT candidacy. Furthermore, even in candidates who meet the restrictive Milan criteria, there is risk of HCC recurrence; this risk rises significantly when patients with more advanced HCC are included. Chemoembolization, radiofrequency ablation, and ethanol injection all have well-documented antitumor activity; however, there is no high-level evidence that waiting-list HCC treatment with these modalities is effective in achieving any of the three above mentioned aims. Nevertheless, particularly in the U.S. where continued waiting-list priority depends on maintaining HCC within Milan criteria, use of non-surgical HCC treatment will likely continue in an effort to forestall tumor progression and waiting-list drop-out.42
In selected patients with HCC, LT is indicated for small, early-stage hepatocellular carcinoma (HCC) in patients with cirrhosis and requires, in the majority of cases, a long waiting period. Tumor development during the waiting period may be associated with vascular invasion, which is a strong factor for post-operative recurrence. Therefore, local treatment of the tumor including trans-arterial chemoembolization (TACE), percutaneous radiofrequency (RF), or partial liver resection can be used prior to transplantation.43
A study from the University of Toronto in Canada showed the critical analysis in 347 patients in the controversial issue of waiting time prior to LT. Reported survival after liver transplantation (OLT) for early hepatocellular carcinoma (HCC) is superior to the results of liver resection (LR), but few analyses have considered long waiting times and patient drop-outs due to tumor progression. This group concluded in their report that unless waiting time is short (<4 months), survival of patients with early HCC is similar between LR and LT.44
However, in cases of cholangiocarcinoma in advanced stages, prognosis remains poor as compared with that for hepatocellular carcinoma.45 Patients with combined hepatocellular carcinoma and cholangiocarcinoma had poor post-operative survival rates.46
In order to understand the anatomical localization of this type of tumor on the biliary tree, the Bismuth-Corlette classification of hilar cholangio-carcinoma is described as follows:
- Type I involves the common hepatic duct, distal to the bifurcation of the biliary tree.
- Type II affects bifurcation.
- Type IIIa affects the right hepatic duct, in addition to the bifurcation.
- Type IIIb affects the left hepatic duct, in addition to the bifurcation.
- Type IV involves the bifurcation and both right and left hepatic ducts, or indicates multifocal cholangiocarcinoma.47
Cholangiocellular carcinoma is a biliary malignancy that frequently presents in advanced unresectable stages. Liver transplantation for cholangiocarcinoma is unclear; because of a high recurrence rate and poor patient survival, the disease has been viewed as an absolute contra-indication to transplantation. Based on good results using neoadjuvant and palliative radiation, a protocol for liver transplantation in selected patients with unresectable hilar cholangiocarcinoma at the Mayo Clinic, neoadjuvant radiation is followed by operative staging to rule out patients with lymph node metastases prior to liver transplantation. This approach has achieved results superior to those of standard surgical therapy, with a 72% 5-year survival for patients with unresectable disease.48 With data from the University of Nebraska on 17 patients, this study evaluates the effect of neoadjuvant chemoradiation therapy combined with orthotopic liver transplantation in a carefully selected group of patients with hilar cholangiocarcinoma.49
The neoadjuvant protocol included 6 000 cgy biliary brachytherapy delivered through percutaneous transhepatic catheters and intravenous infusion of 5-fluorouracil (300 mg/m2/day) until transplantation. Five of the 17 patients demonstrated tumor progression precluding transplantation. Eleven patients underwent liver transplantation, a median of 3.4 months (range = 1-26 months) after diagnosis. Five of the 11 (45%) patients are alive without evidence of tumor recurrence, with a median follow-up of 7.5 years (range = 2.8-14.5 years). Six deaths occurred in the trasplanted patients. Tumor recurrence was responsible for two deaths at 10 and 18 months, respectively, after transplantation. Hence, cholangiocarcinoma should not be considered an absolute exclusion criterion for orthotopic liver transplantation. Long-term, tumor-free survival was achieved in 45% of the transplanted patients. Complications of biliary catheter placement for brachytherapy were associated with poor outcome.49
In other results from the University of Dokkyo in Japan, 25 patients were identified: nine patients with extrahepatic cholangiocarcinoma (five patients, Klatskin-type; two patients, the middle third, and two patients, the distal third) and 16 patients with intra-hepatic cholangiocarcinoma.50
Tumor stage was local (stages I and II; n = 9) or advanced (stages III and IV; n = 16). Overall and disease-free survival rates were 71 and 67% at 1 year and 35 and 32% at 3 years, respectively. Analysis of variables showed statistically significant improved outcomes (p<0.05) for absence of contiguous organ invasion at LT, small tumor size, and single tumor foci. This study indicates that early survival after LT for cholangiocarcinoma is acceptable. Three-year disease-free survival is achieved in approximately 30% of patients. These outcomes can be improved by applying strict selection criteria based on the prognostic variables identified in this study.50
In patients with stage I or II cholangiocarcinoma without hilar-positive ganglia, a prospective comparison was conducted of results between patients subjected to HT and neoadjuvant therapy (NT) and patients subjected to conventional resection, revealing that HT plus NT is the best option for this group of patients.38
In other types of less aggressive neoplasias, such as epithelioid hemangioendothelioma and hepatoblastoma, LT yields even better results when patients are treated in early disease stages.30
Cellular hepatocytes transplant (CHT)
Studies have been performed in humans and in other species, including autologous and xeno-transplants with increasingly encouraging results, achieving advances in mean life and functionality of hepatocytes.51,52
Within CHT, one modality consists of hepatocytes injection through portal route or at ectopic sites, such as splenic pulp; in experimental animals, it has been found that hepatic function values tend toward normality for a given time, depending on the model employed.51-53 The small number of studied patients and the short viability of hepatocytes have been one of the causes limiting the viability of livers available for LT, because of their high fat content; thus, livers with these tissular characteristics are not yet recommended to be used for CHT.54 With these characteristics, the potential group of donors is reduced, considering that fatty liver is a pathology frequently associated with and diagnosed in individuals with Western diets having high carbohydrate and fat contents. This clinical entity has raised the interest of Gastroenterologists, Hepatologists, and Internists, as well as its relationship with obesity, dyslipidemia, or metabolic syndrome.55
Regarding hepatic cells, these can derive from the same individual based on stem cell technology, from other individuals, or from xenografts.56 Another viable option is stem cells proceeding from bone marrow-derived mesenchymal cells, thereby providing a novel option for treatment of hepatic diseases.57
However, the largest numbers of studies performed in this area have focused on xenotrans-plantation, arising from the cooperation between basic and clinical research, both indispensable for progress in LT. This type of transplant has been studied as a viable option in humans; organ procurement has been researched in several species, among which pigs and primates are the main species considered, although some initial experiments have been carried out in rodents. The limiting factor in using the whole organ for transplantation or hepatic cells for implantation comprises the transmission of pathogens, particularly viruses.58,59 Therefore, gene therapy prior to cellular transplant becomes an attractive field for modifying this immunological and genetic potential.60 Another research line entertaining great potential is the study of knockout (KO) or Gal KO (alpha1,3-galactosyltransferase knockout) animals. Encouraging data and survival times have been published with this technique in heart transplantation.61 However, an acute rejection effect due to antibodies to the kidney antibodies when using Gal-KO animals has been published,62 giving rise to controversy in the utilization of this type of animal as potential sources of organs for transplantation.
Genetic and immunological manipulation is feasible for the use of hepatic cells, which can be stored under cryopreservation in a cell bank or stocked for their later use. However, controversy is remains regarding definite authorization for scientific and non-restricted handling of stem cells for specific pathologies; as in all areas, clinical hepatology and transplants are no exceptions.
Artificial support in patients with fulminant or end-stage liver failure and candidates for LT consists of diverse initial procedures to purify the blood, such as hemofiltration and plasmapheresis, as well as bio-artificial liver support. The latter constitutes an option when no organ is available immediately; its use is aimed at improving hepatic function, as well as ammonia levels in these patients while they await an organ.63,64
At the clinical level, this external hepatic support has been based on the use of pig hepatocytes. In a multicenter comparative study in 171 patients, its relative efficacy was demonstrated in patients with fulminant liver failure.65
Given the initial promising results, several research teams are currently studying the feasibility of its clinical use.
Conclusion
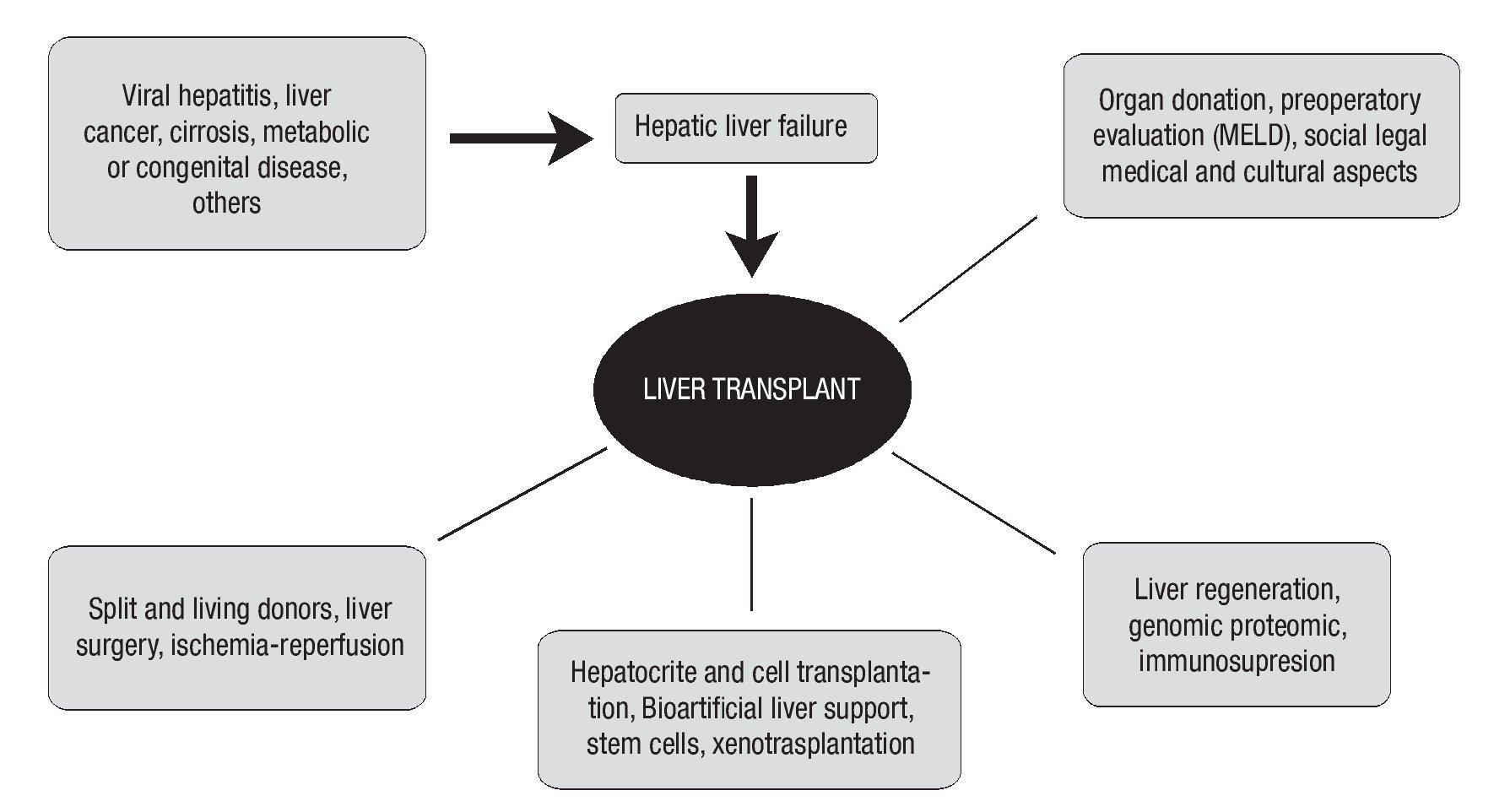
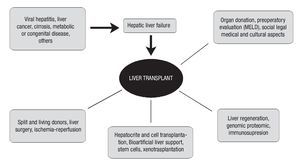
The range of factors involved in liver transplantation is very broad; we must take into account the etiology of hepatic disease, its treatment or maintenance options prior to surgery, type of surgical procedure, including cellular transplant of hepatocytes, and post-operative follow-up. We consider follow-up and survival at the short, medium, and long term as fundamental issues due to the potential risk for acute and/or chronic rejection and therefore, the need for an appropriate immunosuppressant therapy, among other measures. Long-term survival of kidney-transplanted patients without immunotherapy and of bone marrow-transplanted patients has been published recently.66 Thus, it is possible that we might soon observe the use of this modified therapy in LT. We have attempted to include the main factors associated with LT in Figure 1, to express our viewpoint regarding a disease that affects the worldwide population, with Mexico not devoid of this reality.
Figure 1.
Correspondence author: Eduardo E. Montalvo-Javé, MD, FACS.
Departamento de Cirugía, Facultad de Medicina, UNAM; Av. Universidad 3000,
Circuito Escolar, Ciudad Universitaria, Delegación Coyoacán, C.P. 04510, México, D. F.
E-mail:montalvoeduardo@hotmail.com