The management of abdominal pain in patients with inoperable pancreatic cancer is a clinical challenge. This symptom is significantly associated with an impaired quality of life,1 herein the necessity for an effective treatment. There are different modalities of treatment for this problem: non-steroidal anti-inflammatory drugs (NSAID), narcotics, fluoroscopy and computed tomography (CT)-guided celiac plexus neurolysis (CPN) and surgery. The success and complications in these different modalities are variable. Narcotics are the most commonly used treatment, however some problems related to their chronic use are difficult to tolerate, e.g. dependency, constipation, delirium, nausea, and vomiting. All these secondary effects can adversely affect quality of life. The injecting of alcohol into the celiac plexus to destroy the nervous fibers is an effective method for treatment of pain in patients with pancreatic cancer. There are reports of better overall health-related quality of life in patients with CPN than those patients with only pharmacological management.2 The percutaneous route to inject absolute alcohol into the celiac plexus under either fluoroscopic or CT guidance are both effective techniques but previous reports show that CPN endoscopic ultrasound-guided (EUS CPN) is safer, effective and less expensive than the percutaneous route.3-5 The aim of the present study was to evaluate the safety and efficacy of EUS CPN for the palliation of patients with pain related to inoperable pancreatic cancer.
Materials and methodsIn a retrospective analysis of data obtained prospectively the records of patients with pain caused by pancreatic cancer who underwent EUS CPN at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” from March 2005 to May 2007 were evaluated. All patients have unresectable pancreatic cancer confirmed by computed tomography (CT), magnetic resonance imaging (MRI) and/or EUS; EUS criteria and fine-needle aspiration (FNA) were used when a tissue diagnosis was not available before EUS. They underwent to EUS CPN during the same EUS procedure for diagnosis. Before the procedure all patients had laboratory tests including prothrombin time and full blood count. The patients were placed in left decubitus position and sedated by using a combination of midazolam, propofol and phentanyl by anesthetist. Patients were continually monitored with an automated noninvasive blood pressure device, electrocardiogram, and pulse oximetry throughout the procedure. EUS CPN was performed with a linear array echoendoscope GFUCT-140 (Olympus) by an experienced echoendoscopist. The angle formed by the aorta and celiac trunk was identi- fied through the posterior gastric wall. Under direct EUS visualization, a 22 gauge, 8 cm aspiration needle (Wilson-Cook Medical, Inc. Winston-Salem, N.C.) primed with normal saline solution was placed immediately adjacent and anterior to the aorta at the level of the celiac trunk (figure 1). After injecting 2 mL of saline solution to clear the needle, an aspiration test was performed, if no blood was obtained, 10 mL of 1% lidocaine was injected (figure 2). The aspiration test was repeated, and if no blood, 20 mL of dehydrated 98% absolute alcohol was injected. The needle was then flushed with 3 mL of saline solution and withdrawn from the patient. After the procedure a Doppler ultrasound of the celiac trunk and aorta was made to evaluate permeability. The average estimated time for the EUS CPN portion of the procedure was 10 minutes. After the procedure all patients remained under observation for at least 2 hours to rule out any complications. All patients were reevaluated for complications 7 days after the procedure. Dosage and class of pharmacologic treatment were evaluated before the procedure as well as 15 days and 30 days after procedure. Measurement of intensity of pain was made with a validated visual analog pain scale (0-10) in all patients6 by a pain specialist. Measurements two and four weeks after the procedure were made. The complications related to the EUS CPN were determined in agreement with the medical records. Medians, ranges and proportions were used to summarize the demographics and clinical variables. EUS CPN pain scores paired before and after (15 and 30 days) were compared with the Friedman test.
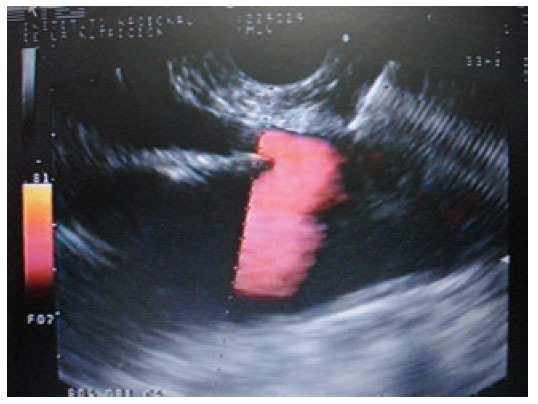
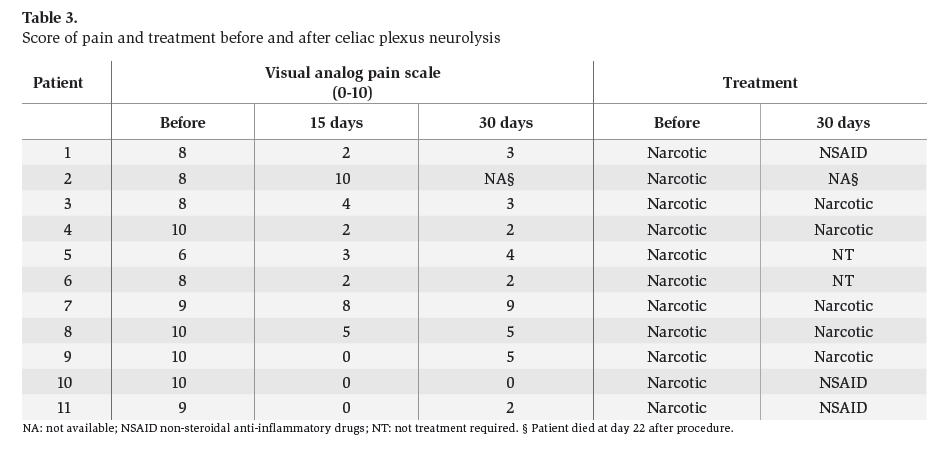
Figure 1. Needle adjacent and anterior to the aorta at the level of the celiac trunk.
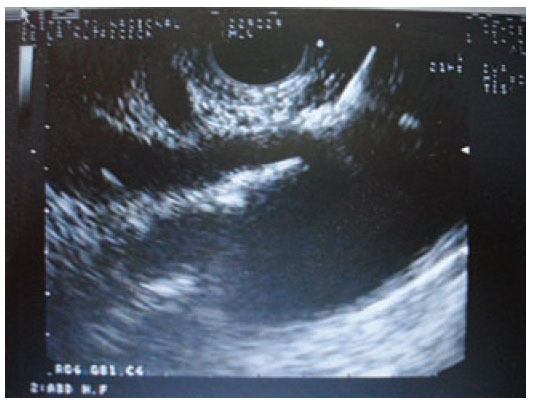
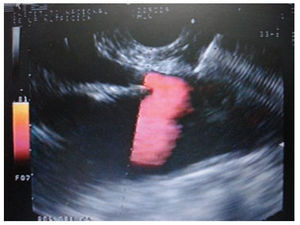
Figure 2. Injection of 1% lidocaine.
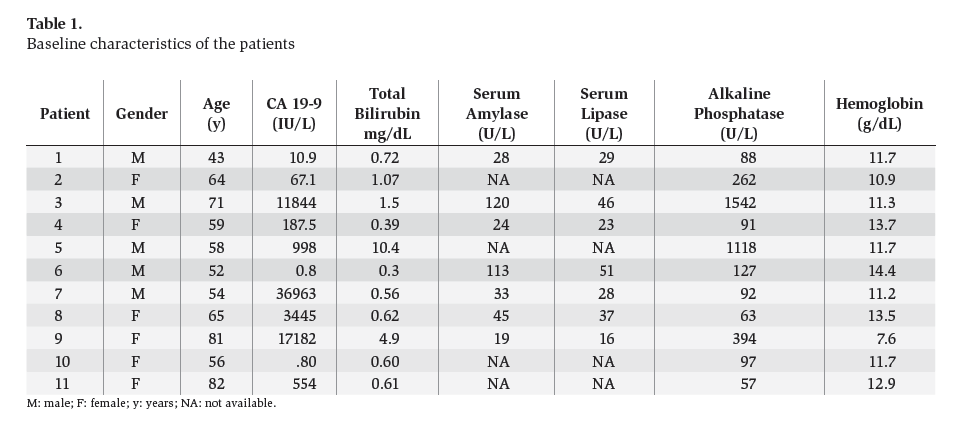
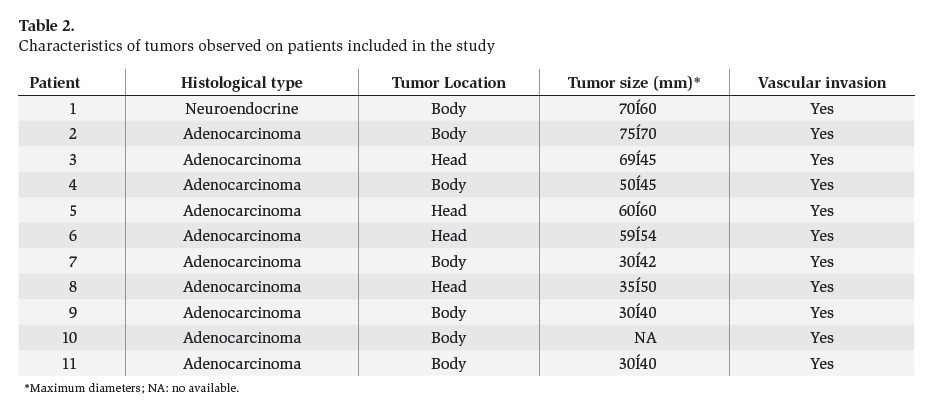
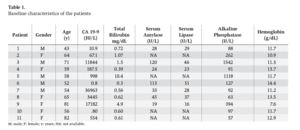
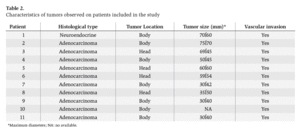
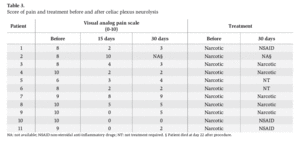
ResultsEleven patients underwent EUS CPN. The characteristics of the patients are in table 1. Five men (45.5%) and six women (54.5%) were included (median age was 59 years; range 43 to 82 years). The tumor was located in the head of the pancreas in 4 patients (36.4%), and 7 (63.6%) in the body. Adenocarcinoma was the most common histological type (10/11 cases). 100% of the patients had major arterial or mesenteric venous invasion based in EUS and/or CT findings. The characteristic of the tumor are in table 2. Overall, pain scores decreased significantly from median of 9 (range 6-10) before EUS CPN to a median of 2 (range 0-10) at 2 weeks after EUS CPN. The differences persisted 4 weeks after EUS CPN (median 3; range 0-9) (p < 0.001). Two weeks after the procedure, compared with baseline, eight patients had a reduction of ≥ 4 points in visual analog pain scale. At 4 weeks these 8 patients continued to show this improvement. All patients were treated with narcotics before EUS CPN. After the procedure, two patients went without any medical treatment and another three patients only received NSAID. Morphine usage was not significantly different over time in 5 (45.4%) patients; however their score on the visual analog pain scale had reduced (table 3).
Table 1. Baseline characteristics of the patients
Table 2. Characteristics of tumors observed on patients included in the study
Table 3. Score of pain and treatment before and after celiac plexus neurolysis
ComplicationsThere were no major complications. No patient was hospitalized after an outpatient procedure. Among patients already hospitalized, none had prolongation of their hospital stay because of the procedure. Five (45.4%) patients had transient abdominal pain after the procedure. More frequent stools for a period ≤ 72 hours were noted in six (54.5%) patients.
DiscussionThis work represents the first report in full-text from a Mexican center in relationship with EUS CPN. Our results show that EUS CPN reduced pain scores in 81.8% of the patients. Pain scores decreased in at least 50% from baseline within 2 and 4 weeks after the procedure. These results are similar to previous reports.5 The CPN with different methods has been described with good results. Eisenberg et al7 and Ischia et al,8 both concluded that CPN was effective in reducing pain in 70 to 90% and 60 to 75% of patients, respectively. They used different percutaneous techniques including fluoroscopy and CT guidance. Both EUS CPN and CT-guidance are effective and safe methods. According with our results and those obtained by other authors, advantages of EUS CPN are the ability to obtain a tissue sample, apply therapeutic measures like prosthesis and obtain better sensibility and speci- ficity for tumor staging all in the same procedure.9-10 EUS CPN is performed with the patient under conscious sedation and may therefore be better tolerated than CPN performed by percutaneous techniques with the patient under local anesthesia. Laparoscopic CPN has been recently reported,11 one study with nine patients shows good initial results of the procedure performed surgically. When, hypothetically, we compare with the surgical approach, proposed advantages of EUS CPN are less time, cost and minimal invasion. A systematic review that included five RCTs (no one included EUS CPN) involving 302 patients concluded that in patients with inoperable pancreatic cancer, neurolytic celiac plexus blockade (NCPB) is associated with improved pain control and reduced narcotic usage.12 All this studies8,11,12 show that the CPN or NCPB are the key to the procedure irrespective of the technique. Regarding “blockade” vs. “neurolysis”, the first is a temporary method using steroids and the second is a permanent treatment using alcohol to destroy the neural fibers of the celiac trunk. In our study neurolysis was the only procedure applied. The limitations of this study include the inherent difficulties in retrospective studies, the small number of patients, lack of quality-of-life evaluation, and difficulties in measuring pain, which is a variable and subjective experience. It is important to mention that pain specialists obtained pain scores and not endoscopic team members which eliminate a potential bias. In conclusion, the EUS CPN is an efficient and safe method for pain management in patients with inoperable pancreatic cancer.
Correspondence: Miguel A. Ramírez, M.D. Department of Endoscopy. Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”. Vasco
de Quiroga #15. Col. Sección XVI. Del. Tlalpan. C.P. 14000. Mexico City, Mexico. (+525) 554870900, ext: 2715. E-mail: mangelramirez@yahoo.com