Abdominal compartment syndrome occurs when 2 or more anatomic compartments have a sustained intra-abdominal pressure >20mmHg, associated with organ failure. Incidence is 2% and prevalence varies from 0% to 36.4%.
A literature search was conducted utilizing different databases. Articles published from 1970 to 2018 were included, in English or Spanish, to provide the concepts, classifications, and comprehensive management in the approach to abdominal compartment syndrome, for its treatment and the prevention of severe complications associated with the entity. Intravesical pressure measurement is the standard diagnostic method. Treatment is based on evacuation of the intraluminal content, identification and treatment of intra-abdominal lesions, improvement of abdominal wall compliance, and optimum administration of fluids and tissue perfusion. Laparotomy is generally followed by temporary abdominal wall closure 5–7 days after surgery. Reconstruction is performed 6–12 months after the last operation.
Abdominal compartment syndrome should be diagnosed and operated on before organic damage from the illness occurs. Kidney injury can frequently progress and is a parameter for considering abdominal decompression. Having a biomarker for early damage would be ideal. Surgical treatment is successful in the majority of cases. A multidisciplinary focus is necessary for the intensive care and reconstructive needs of the patient. Thus, efforts must be made to define and implement strategies for patient quality of life optimization.
El síndrome compartimental abdominal se define cuando 2 o más compartimentos anatómicos tienen una presión intraabdominal con valor sostenido >20mmHg, asociado a una falla orgánica. La incidencia es del 2% y la prevalencia del 0 al 36.4%.
Se realizó la búsqueda bibliográfica en diferentes bases de datos. Se incluyeron artículos publicados desde 1970 al 2018, en inglés o español, con el fin de proporcionar los conceptos, clasificaciones y manejo integral en el abordaje del síndrome compartimental, para tratar y evitar complicaciones severas en los pacientes asociados a dicha entidad.
La medición de la presión intravesical es el método estándar para el diagnóstico. El tratamiento se basa en la evacuación de contenidos intraluminales, identificar y tratar las lesiones intraabdominales, mejorar la complianza de la pared abdominal, y la óptima administración de fluidos y perfusión tisular. La laparotomía es generalmente seguida de un cierre temporal de la pared abdominal de 5 a 7 días después de la cirugía. La reconstrucción es realizada de 6 a 12 meses después de la última operación.
Se debe diagnosticar e intervenir antes del daño orgánico ocasionado por el síndrome compartimental abdominal. Con frecuencia la lesión renal puede progresar, siendo este un parámetro para considerar la descompresión abdominal. Contar con un biomarcador de daño temprano sería ideal. El tratamiento quirúrgico es exitoso en la mayoría. Un enfoque multidisciplinario es necesario para los cuidados intensivos y necesidades reconstructivas del paciente, por lo que es preciso seguir trabajando para definir e implementar estrategias que mitiguen la calidad de vida del paciente.
The World Society of Abdominal Compartment Syndrome (WSACS) defines intra-abdominal pressure (IAP) as the end-expiratory abdominal pressure in the supine position in a setting of fully relaxed abdominal wall musculature.1 IAP measurement is useful for calculating the abdominal perfusion pressure (APP), by subtracting the IAP from the mean arterial pressure (MAP). In that sense, APP can be thought of as the abdominal analog to cerebral perfusion pressure and utilized as a predictor of tissue perfusion.1,2
In adults, IAP levels are considered physiologic up to 5mmHg (normal range is from 2 to 7mmHg), but IAP can vary from 10 to 15mmHg in obese patients.1–3
In the critically ill patient, IAP is frequently found between 5 and 7mmHg, and when added to obesity, contributes to increased baseline IAP measurements.4
The WSACS defines intra-abdominal hypertension (IAH) as IAP sustained above 12mmHg1–3,5 and divides it into 4 grades: grade I IAH (12–15mmHg), grade II IAH (16–20mmHg), grade III IAH (21–25mmHg), and grade IV IAH (> 25mmHg).1
According to the WSACS, abdominal compartment syndrome (ACS) is defined as a syndrome in which 2 or more anatomic compartments have an IAP>20mmHg (grade III and grade IV IAH) in the abdominal cavity, with or without APP<60mmHg, and associated with organ dysfunction or failure.1–3,5–7
The aim of the present updated review was to provide information on the concepts, classifications, and comprehensive management in the approach to abdominal compartment syndrome, for the treatment of patients presenting with that entity and the prevention of severe complications.
Materials and methodsA search of the literature was conducted utilizing the following databases: PubMed, Medline, Index Medicus, the Latin American and Caribbean Health Sciences Literature (LILACS, Portuguese acronym), International Serials Data System, PERIÓDICA (Index of Latin American Scientific Journals)-CICH-UNAM, Bibliomex Salud, and Ulrich's International Directory. Articles published from 1970 to December 2018, in English or Spanish, were included, utilizing the keywords
abdominal compartment syndrome, intra-abdominal hypertension, intra-abdominal pressure, treatment, clinical manifestations.
ResultsEpidemiologyAccording to a 2014 Scandinavian study, there was a 39% incidence of IAH and a 2% incidence of ACS.8 Incidence of ACS in intensive care units (ICUs) was reported to vary from 0.5% to 58.8%2,5,9,10 and to reach 14% in patients with a history of trauma.2
The prevalence of ACS varied from 0% to 36.4% in patients with visceral damage,6 and from 0.9% to 36.4% in patients that underwent laparotomy for abdominal trauma.1,6
Based on the WSACS classification, incidence is reported from 23% to 27% in grade I IAH, from 9% to 14% in grade II, from 2$ to 3% in grade III, and from 1$ to 2% in grade IV.6,7 A higher IAH grade increases the risk for death.8
ACS-associated mortality is high, with 2 recent articles reporting a mortality rate of 47.1% and 53.1%, respectively.2,6
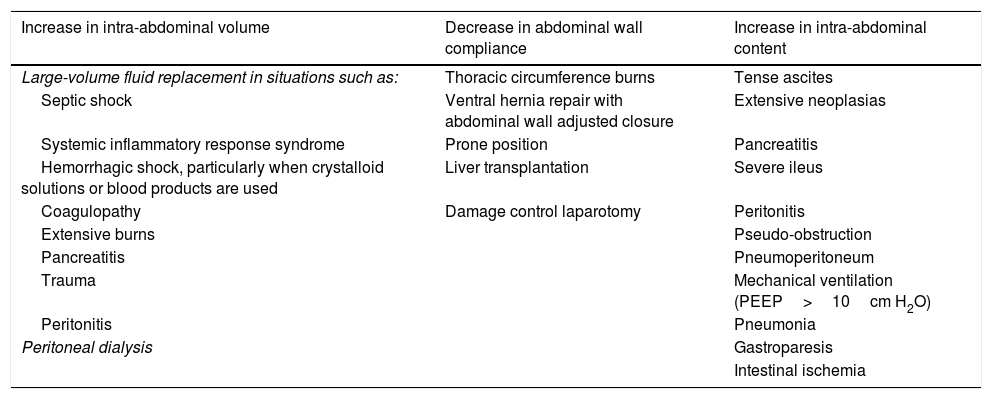
Etiology and risk factorsIAH has been reported to be an important mortality factor, with a relative risk (RR) of 1.85, even in the absence of ACS.11 Several risk factors are associated with the development of IAH, but it is difficult to predict which patients will present with it. In general, the increase in IAP can result in reduced abdominal wall compliance, an increase in abdominal content volume, or a combination of the two.1Table 1 presents a summary of the main risk factors for IAH and ACS.1,5,6,12–14
Risk factors for developing abdominal compartment syndrome.
| Increase in intra-abdominal volume | Decrease in abdominal wall compliance | Increase in intra-abdominal content |
|---|---|---|
| Large-volume fluid replacement in situations such as: | Thoracic circumference burns | Tense ascites |
| Septic shock | Ventral hernia repair with abdominal wall adjusted closure | Extensive neoplasias |
| Systemic inflammatory response syndrome | Prone position | Pancreatitis |
| Hemorrhagic shock, particularly when crystalloid solutions or blood products are used | Liver transplantation | Severe ileus |
| Coagulopathy | Damage control laparotomy | Peritonitis |
| Extensive burns | Pseudo-obstruction | |
| Pancreatitis | Pneumoperitoneum | |
| Trauma | Mechanical ventilation (PEEP>10cm H2O) | |
| Peritonitis | Pneumonia | |
| Peritoneal dialysis | Gastroparesis | |
| Intestinal ischemia |
The independent risk factors for ACS are massive transfusion (>10 red blood cell concentrates within 24h), a body mass index (BMI)>30kg/m2, hypothermia (<33°C), a pH<7.2, and resuscitation with >5000ml of crystalloids in 24h.2
Those independent risk factors are essential in many other conditions, such as burns, wounds, and pancreatitis, all of which require fluid resuscitation, and thus can also induce ACS.2
PathophysiologyIAP is determined by 2 elements: intra-abdominal volume and abdominal wall compliance.5 Both ACS and IAH are a result of altered tissue perfusion caused by increased pressure within a fixed volume of an anatomic compartment.1,2
ACS has a wide range of effects on different systems.
In the central nervous system, it leads to a decrease in cerebral blood perfusion, as well as to an increase in intracranial pressure when venous stasis is induced.2,14
At the cardiac level, the increase in IAP results in a decrease in venous return, caused by compression of the inferior vena cava, thus elevating intrathoracic pressure (ITP). Altered cardiac function is produced due to compression of the heart secondary to diaphragmatic elevation,14 for example, as a capillary leak that produces edema, leading to a hypovolemic state.14 A high IAP can also increase systemic vascular resistance by compressing the aorta and splanchnic circulation, resulting in an increased afterload and reduced stroke volume, producing a clear decrease in cardiac output, with the resulting hypotension.1
Increased permeability syndrome has been correlated in patients with persistent systemic inflammation that fail to curtail transcapillary albumin leakage. It can be a third hit (complication), after the initial injury and damage due to ischemia-reperfusion. Novel markers, such as the capillary leak index, extravascular pulmonary water, and pulmonary permeability index, can aid in the control of fluid administration directed toward each patient's individual needs, given that capillary leakage is an inflammatory condition resulting from a common pathway that includes ischemia-reperfusion, toxic oxygen metabolite production, cell wall injury, and enzymatic lesion that leads to a loss of capillary endothelial barrier function.14
At the pulmonary level, the increase in IAP leads to elevation of the diaphragm, thus reducing thoracic volume, residual functional capacity, and compliance, as it increases intrapleural pressure.1,5,14,15 Atelectasis due to the resulting compression, increases the physiologic dead space.2 The resulting imbalance of the ventilation-perfusion ratio often causes progressive worsening of hypoxemia, hypercapnia, and acidosis that can require invasive mechanical ventilation support.1,2,5,14 Increases in peak, plateau, and alveolar pressures elevate the risk for developing barotrauma and volutrauma. Ischemia associated with tissue hypoxia can cause the systemic release of proinflammatory cytokines, resulting in the accumulation of neutrophils and the production of oxygen free radicals at the pulmonary level. Those mechanisms are involved in the development of acute lung injury and acute respiratory distress syndrome.1,2,14,15
IAH gives way to pulmonary hypertension through an increase in ITP and a consequently direct compression of the pulmonary parenchyma and vessels, resulting in left and right ventricular dysfunction.2,16
Poor intestinal perfusion resulting from reduced splanchnic circulation, acute kidney injury secondary to decreased glomerular blood flow, and the decrease in venous return as a result of compression of the inferior vena cava can lead to systemic acidosis and cardiovascular collapse.1
Neither central venous pressure, nor the production of urine, reflects the potential volume depletion in patients with ACS.2,16 Thus, the end-diastolic volume index of the right ventricle is the diagnostic indicator of choice for gaining insight into the intravascular situation.2,16
An alteration of the micro-perfusion of the mucosa results in the elevation of serum lactate, which reduces the pH and causes mesenteric ischemia.2 Intestinal ischemia can manifest as interstitial edema and/or ileus, which increase intra-abdominal volume and worsen ACS. After 60min of sustained IAP above 25mmHg, mucosal blood flow has been reported to be altered, even in well-resuscitated patients, enabling the translocation of intraluminal bacteria through the damaged mucosal barrier. If ACS is not recognized and treated immediately, said bacterial translocation can cause sepsis or septic shock, with the subsequent rapid clinical deterioration or death of the patient.1
Kidney dysfunction is also common in ACS, manifesting as oliguria, when the IAP is >15mmHg, or as anuria, when the IAP is >30mmHg.1,5 IAH reduces the renal plasmatic flow and glomerular filtration rate through compression of the renal arteriole (vascular resistance of the renal vessels increases up to 500%, if the IAP is >20mmHg),2 venous circulation, and of the tubules themselves.1,14 In turn, the renin-angiotensin system (renal vessel vasoconstriction) is activated and serum levels of antidiuretic hormone (ADH) and aldosterone (antidiuretic effects) are elevated. That worsens ACS due to the additional increase in IAH and decrease in renal perfusion.1,2,14
Recognizing the essential role of fluid resuscitation in the pathogenesis of IAH and ACS, large-volume resuscitation with crystalloids should be avoided in patients that have ACS or are at risk of having it.5
Elevated IAP could cause hepatic hypoperfusion, given that it evokes a reduction in blood flow through the portal vein of up to 35%.1,2 Liver failure manifests through the altered elimination of lactate in plasma, resulting in metabolic acidosis. In addition, increased serum lactate levels depress serum pH, which can cause a decrease in myocardial contractility. Lactic acidosis can also cause the dilation of systemic arterioles, depressing systemic arterial pressure even more, affecting cell respiration, and further increasing lactic acid production, effectively creating a feedback cycle that accelerates physiologic decline.1
ClassificationAccording to its pathogenesis, ACS is classified as primary if IAH results from an intra-abdominal injury or disease (lesion in a parenchymal organ, such as the spleen or liver, rupture of an abdominal aortic aneurysm, pancreatitis, retroperitoneal bleeding).2,12,13,17
Secondary ACS is defined as IAH caused by a systemic or extraperitoneal pathology, such as sepsis or severe burns.2,12,13,17
Tertiary ACS is a recurrent disease after initially successful treatment of primary or secondary ACS.2
Clinical manifestationsClinical presentation generally includes a tense distended abdomen, dyspnea, orthopnea, abdominal pain, a sensation of fullness, hypotension or hypertension, hypercapnia, and oliguria.2,3
In contrast, mechanically ventilated patients have been described to present with edema and abdominal distension.2
DiagnosisThe most important requisite for the diagnosis and treatment of ACS is IAP measurement, which can be done directly or indirectly, as well as intermittently or continuously.16
Early recognition of IAH is the essential first step for preventing ACS. IAP should be measured in patients with 2 or more risk factors (Table 1).1 In such cases, IAP should be monitored at intervals of 4–6h, with hourly IAP monitoring restricted to patients with critical organ dysfunction.3
IAP measurement can be indirectly determined through intravesical or intragastric catheters, among others.18 Intravesical pressure measurement is the standard method for determining the presence of ACS or IAH, through the modified Kron method, given its simplicity, reliability, ease of use, and reproducibility, with the additional benefits of low cost, less invasion, and minimal side effects and complications.1–3,5,7,16,19
Intravesical pressure is measured through the instillation of 20ml of sterile water or saline solution in the bladder, through a urinary catheter connected to a manometer and zeroed at the midaxillary line. The procedure should be performed with the sterile technique to prevent iatrogenic urinary tract infections. Instillation of more than 50ml in the catheter artificially increases the IAP measurement. That technique is contraindicated in patients with a history of cystectomy, traumatic bladder injury, or pelvic packing.1
Variations of the original technique have been described and include utilizing a pressure transducer connected to a faucet, the U-tube technique, and the Y-set technique.3
According to the WSACS, IAP should be expressed in mmHg and measurements made at end-expiration, with the patient in the supine position, with no abdominal muscle contractions.1,15
Direct IAP measurement is theoretically the most accurate but requires access to the peritoneal compartment and includes the risks associated with invasive abdominal procedures. Therefore, those techniques are not widely used in IAH screening.1
Inferior vena cava pressure measurements have shown a good correlation with pressures obtained through other validated methods. Intragastric pressure is an alternative method for indirect IAP measurement and is achieved through nasogastric or orogastric tube manometry.1,5,16,19–21 However, intermittent measurements using that technique can be unreliable due to the confounding effects of the increase in pressure caused by gastric contractions. That particular limitation can be overcome through continuous pressure monitoring, using an intragastric balloon, but there are other possible confounding factors, such as the administration of enteral feeds, that have been insufficiently reported on in the literature. Other novel techniques have been described that utilize specialized catheters with embedded microchips for measuring intravesical, rectal, or intrauterine pressures, but they are less cost-effective than the simpler techniques described above.1
Sensitivity and positive predictive value of physical examination varies from 40% to 60%.1–3 The use of an abdominal perimeter is equally inaccurate. Imaging studies, such as plain abdominal x-ray, abdominal ultrasound, or abdominal computed tomography are also poorly specific for detecting an increase in IAP.13,15,16
In 2013, Kreiz et al. recommended the measurement of IAP every 6h in all patients in intensive care after acute abdominal surgery or fluid resuscitation.22
TreatmentMedical and surgical management have been described as treatment for ACS and need not be mutually exclusive.
The WSACS published a treatment algorithm based on 5 “columns”: (1) evacuate intraluminal contents, (2) evacuate intra-abdominal space-occupying lesions, (3) improve abdominal wall compliance, (4) optimize fluid administration, and (5) optimize systemic and regional perfusion.2,3,5,7
Evacuation of intraluminal contentsThe first step in intraluminal evacuation can be the insertion of a nasogastric or rectal drainage tube, which has been shown to be efficacious for reducing IAP.2
To prevent abdominal distension, prokinetics, such as metoclopramide, erythromycin, or neostigmine, are widely used. To treat opioid-induced constipation, methylnaltrexone can have prokinetic effects. If the patient does not respond, decompression of the colon through endoscopy should be considered.2
Space-occupying lesionsSpace-occupying lesions are commonly observed after major abdominal surgery, pancreatitis, or trauma. In addition, fluid overload during early resuscitation can result in fluid shift into the peritoneal cavity. The fact that those lesions lead to secondary infection can also cause or contribute to the increase in IAP in the ICU patient.2
For IAH grades I and II, fluid support helps maintain perfusion, which counterbalances the negative effects of IAH.23 Percutaneous catheter drainage or large-volume paracentesis are invasive techniques for achieving the reduction of intra-abdominal volume.2,5 Nevertheless, technical difficulties can arise with those methods, such as infection secondary to ascites and perforation of the intestine or other intra-abdominal structures, including vascular structures.24
Increased abdominal wall complianceNeuromuscular blockade is a rescue maneuver for reducing IAP by increasing abdominal wall compliance. However, the habitual use of sedatives should be avoided due to their side effects (atelectasis, pneumonia, etc.).1,2,25
Neuromuscular blockade has limited clinical usefulness in the definitive treatment of IAH and is ineffective in the treatment of true ACS. Notwithstanding, it can be useful as a temporary measure when definitive surgical management is not available or feasible.1
Optimized fluid administration and organ perfusionWhen early fluid resuscitation is employed in the septic patient, the intensivist has to balance the correction of hypovolemia to prevent iatrogenic secondary ACS. The WSACS recommends pharmaceutical diuresis in the initial phase, with the use of loop diuretics, such as furosemide, as well as renal replacement therapy.2,7,26 Furosemide not only reduces intravascular volume, but intestinal wall edema as well, leading to a decrease in IAP.27 The choice of technique depends on the luminal distension conditioner, whether through intestinal evacuation, hematoma evacuation, or nasogastric or rectal tube drainage, among other measures.28
According to the clinical situation, continuous hemofiltration may be necessary and should be installed early in the treatment of ACS. As stated above, central venous pressure is often artificially elevated. Therefore, the end-diastolic volume of the right ventricle could serve as a guide during volume therapy. In addition to depending on cardiac contractibility and the ejection fraction, a volume <90ml/m2 is associated with a high response rate to fluid administration. Once optimized, more fluid does not improve the end-diastolic volume of the right ventricle and could worsen intestinal edema and the IAH.2
Surgical decompression and modern concepts of open abdomen managementOpen abdomen poses a dilemma for the surgeon. On the one hand, the complication rate increases after 8 days of open abdomen therapy. On the other hand, abdominal cavity closure performed under excessive tension leads to the risk for tertiary ACS. Due to its risks, surgeons and intensivists should try to carry out rapid definitive fascial closure as soon as it is feasibly possible, because it significantly reduces the mortality rate and postoperative complications.2 Abdominal closure within the first 5 to 7 days after abdominal decompression has been suggested, if the patient underwent early decompression before developing organ injury.2,19
Definitive treatment of ACS due to the majority of causes, other than tense ascites, involves emergent surgical decompression of the abdomen through midline laparotomy, often performed at bedside in the ICU.2,7,25,28,29 However, that procedure can result in extreme morbidity (49.2%) and should only be used in the most severe forms of IAH or true ACS.1,2,7,19,21,22 Most authors agree that decompressive laparotomy should be performed on patients with new or progressive organ failure and IAP>20mmHg.7,19,21,22
The management of open abdomen involves several risks and complications: the surgical patient is exposed to permanent protein loss, fascial retraction, frozen abdomen, and enteroatmospheric fistulas.2,30
Abdominal decompression before developing ACS has been shown to improve survival.31
Importantly, there can be complications after a sudden decompression, such as ischemia-reperfusion syndrome, with the possible consequence of severe hypotension or even heart attack. To prevent that from occurring, the patient must receive sufficient parenteral fluid support before abdominal decompression.32
There are different temporary abdominal closure (TAC) techniques: skin only closure, Bogota bag, Opsite sandwich technique (polyethylene film, Opsite dressings, suction drains), absorbable mesh (Vicryl), nonabsorbable zipper/commercial zipper (Wittmann patch), and vacuum-assisted closure (VAC).2,33,34 According to Hecker et al. (2016), TAC techniques dramatically improved the clinical management of intensive care patients. The ideal TAC technique should prevent evisceration, enable intra-abdominal fluid extraction and the continuous washout of the peritoneal cavity, and prevent fascial retraction, loss of abdominal wall compliance, and enteroatmospheric fistulas.2 At present, there is no optimum TAC technique that meets all those requirements.2
Historically, the Bogota bag, so named by Mattox, who observed the procedure in Bogota, Colombia, has consisted of a bag with 3l of saline solution that covers the abdominal content and is sutured to the skin. The advantages of that technique are its low cost, the fact that it is not adherent, it prevents evisceration, is easy to apply, and is accessible. The disadvantages are skin loss, re-entry difficulty, the need to sterilize the materials, and poor control of fluid leaks into third spaces.35
Absorbable meshes are composed of polyglactin 910 and polyglycolic acid. Both can be secured to the skin or fascia and have great resistance strength. Absorbable meshes have the disadvantage of a greater incidence of enterocutaneous fistula.36 The most widely used nonabsorbable meshes are those made of polypropylene and polytetrafluoroethylene. Because they are nonabsorbable, they are prone to colonization and infections. They should be removed and cannot be used for definitive closure.37
The Wittmann patch is made of polyamide and polypropylene and has a Velcro zipper. The sheets are sutured to the edges of the fascia. When the patch is removed, definitive closure can be performed. Its advantages are easy re-entry and the prevention of abdominal domain loss. Disadvantages include its cost, manipulation of the fascia with the possibility of fascial necrosis, and reduced fluid control to third spaces.38
Assisted aspiration closure utilizes a non-adhesive drape over the abdominal content. The surgeon must first make fenestrations in the drape. Laparotomy pads are then placed over the drape, together with 2 drainage tubes packed between the laparotomy pads.39 A loban drape covers the entire open abdomen and suction is applied to the drains to create negative pressure to the wound. Dressings are generally changed every 48–72h, bringing the edges of the fascia together. The advantages of aspiration are that it reduces the probability of the development of ACS, there is less domain loss, it is removed and can quantify third space fluid, and it favors early closure of the fascia.40 Patients maintained with open abdomen are at risk for complications, such as systemic inflammatory response syndrome, multiorgan dysfunction syndrome, fistulas, and postoperative ileus.41
Laparotomy is generally followed by temporary abdominal wall closure, to create a functional peritoneal space to reduce the probability of ACS recurrence. Not even the VAC system prevents recurrence. Therefore, the physician must be attentive and carry out patient follow-up, to verify that there is no increase in IAP.42 Those patients require at least one re-exploration, and often several, before definitive abdominal closure, many of which can be performed in the ICU under deep sedation. For those in whom abdominal closure is not possible in the first re-examination, there are various management options that include fascial extension techniques, component separation techniques, with or without mesh reinforcement (biologic or synthetic), and temporary mesh followed by a split-thickness skin graft.43
The performance of decompressive laparotomy associated with temporary abdominal closure with expanded polytetrafluoroethylene (ePTFE) mesh, in patients with ACS that have severe acute pancreatitis, enables IAP reduction and is a therapeutic procedure to be considered. Likewise, that type of temporary abdominal closure allows early abdominal wall reconstruction.44 The main advantages of ePTFE mesh are: absence of adherents that impact fistula formation, the resistance of the material to high traction pressures, the possibility of re-examining the abdominal cavity through the mesh itself, cutting it and re-suturing it, and finally, progressive approximation of the mesh can be carried out to facilitate the later definitive abdominal wall closure.45
The time to perform abdominal wall reconstruction is generally 6–12 months after the last operation, to allow the inflammation to decrease. Reconstruction consists of skin graft extirpation, lysis of the adhesions, restoration of the gastrointestinal continuity if there is an ostomy, and frontal fascial closure.46
A recent literature review described the closure of abdominal fascia with TAC in 72% of the patients. Curiously, the closure rate was significantly lower in septic patients, compared with non-septic patients (e.g., trauma). When a dynamic TAC technique that combined negative pressure with fascial retraction (e.g., through mesh implantation) was chosen, the closure rate rose to 82%.2
For the VAC system, which consists of a spider-shaped sponge, a secondary fascial closure rate of 89% has been reported. Despite the success of vacuum-based TAC techniques, they present a risk for enterocutaneous fistula development.2
Late primary closure with no tension is the definitive closure of choice in open abdomen. Primary closure reduces the risk for infection, enterocutaneous fistula, and wound problems. Nevertheless, morbidity and mortality are associated with primary closure, in which there is tension on the fascia.46 If primary closure cannot be performed, definitive closure options include permanent prostheses, such as absorbable and nonabsorbable meshes, and closure with autologous tissue covered with a skin graft over a granulated tissue base.47
The patient should be kept in the ICU, in an effort to have better systemic tissue perfusion, including of the abdominal compartment, through rational fluid use, seeking the optimum dose according to the preferred method of each unit (invasive or noninvasive).48 Adequate vasopressor use, enabling appropriate perfusion pressure, and the management of mechanical ventilation support are essential key factors, along with adept monitoring, measuring intra-abdominal and esophageal pressures, to enable the maintenance of a satisfactory ratio of the pressures produced by the ventilator and consequently reduce the possibility of ventilator-induced damage. Therefore, based on lung protection with low volumes, positive end-expiratory pressure (PEEP), optimal for the situation of IAP, enables better pulmonary compliance, and if needed, the performance of alveolar recruitment maneuvers that correctly maintain gas exchange. Complementarily, the patient should receive comfort measures through sedation and analgesia, adjusted according to his/her situation (open abdomen vs. closed abdomen, initial medical management, etc.). Muscle relaxation is sometimes necessary for the strict control of IAP.49
ConclusionsThere is still no early diagnosis of ACS. Optimally, we must be able to diagnose ACS opportunely to carry out interventions, before the appearance of organ damage. Kidney injury frequently persists and can progress to acute kidney failure, which is one of the main parameters for contemplating abdominal decompression. As is true regarding other systems or organs, having an early and easily identifiable biomarker for damage would be ideal.
Surgical treatment of ACS is successful in the majority of cases, restoring adequate functional status to most patients. Numerous coadjuvant therapies are often necessary to optimize results in a given group of critically ill patients.
A multidisciplinary approach is generally needed to meet the patient's intensive care, convalescence, and reconstruction requirements. We highly recommend continuing the work directed at defining and implementing strategies to improve the quality of life of the patients affected. Clinical research studies and basic science studies are needed to enhance the comprehensive analysis of ACS.
The risk factors for developing abdominal compartment syndrome (ACS) are summarized. They are classified according to their impact on the abdominal region: increased intra-abdominal volume, reduced abdominal wall compliance, and/or increased intra-abdominal content. Different clinical situations are identified within those groups that can trigger said effects, for example: septic shock involves large-volume fluid replacement, which increases intra-abdominal volume, thus becoming a risk factor for developing ACS; damage control laparotomy conditions reduced abdominal wall compliance, equally becoming a risk factor for ACS; a case of pseudo-obstruction that leads to increased abdominal content becomes a risk factor for presenting with ACS; etc.
Ethical considerationsNo patients were recruited in relation to the present article, and so no statements of informed consent were requested. The patients mentioned in the manuscript are those involved in the studies cited.
Authorization by an ethics committee was not needed, given that no patients were recruited for or animal models utilized in the present literature review.
The authors declare that the present article contains no personal information enabling patient identification, given that no patients were recruited for the present review article.
Financial disclosureNo financial support was received in relation to the present article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the surgeons, Ericka Hazzel Contreras Flores and Alan I. Contreras Treviño, for their technical and editorial assistance.
Please cite this article as: Montalvo-Jave EE, Espejel-Deloiza M, Chernitzky-Camaño J, Peña-Pérez CA, Rivero-Sigarroa E, Ortega-León LH. Síndrome compartimental abdominal: conceptos actuales y manejo. Revista de Gastroenterología de México. 2020;85:443–451.