To characterize a university hospital population of Chilean adult patients with celiac disease.
Patients and methodWe retrospectively reviewed the records of patients under control that were diagnosed with celiac disease through clinical characteristics, serology, and histology.
ResultsA total of 149 patients were included, 119 (79.9%) of whom were women. Mean patient age was 42 years at diagnosis and 13.4% of patients had a family history of celiac disease. Mean body mass index was 24.3kg/m2, 55.3% presented with normal weight, 37.9% with overweight and obesity, and 6.8% with underweight. The main reasons for consultation were diarrhea (47%), weight loss (31%), dyspepsia (43%), and fatigue (26.1%). Anemia (26.1%), elevated transaminases (17.4%), low ferritin (11.4%), and hypovitaminosis D (9.3%) stood out, among others, in the initial laboratory work-up. The more frequent associated diseases were hypothyroidism (15.4%) and depressive disorder (11.4%). Small intestinal bacterial overgrowth was found in 10.1% and lactose malabsorption in 15.4%. The primary histologic diagnosis was celiac disease, with Marsh stage 3a villous atrophy (34.9%).
ConclusionOur results were similar to those of other case series on adults, finding that celiac disease was more frequent in women, disease began in the fourth decade of life, extraintestinal symptoms predominated, and there was an association with other autoimmune diseases. An important percentage of patients were also overweight and obese.
Caracterizar una población chilena de enfermos celiacos adultos de un centro universitario.
Pacientes y métodoSe revisaron de manera retrospectiva los expedientes de pacientes en control con diagnóstico de EC por clínica, serología e histología.
ResultadosSe incluyeron 149 pacientes, 119 mujeres (79,9%), con edad promedio de 42 años al momento del diagnóstico. Los pacientes tenían antecedentes familiares de EC en 13,4%. Índice de masa corporal promedio de 24,3kg/m2, peso normal un 55,3%, sobrepeso y obesidad 37,9%, y bajo peso 6,8%. Los principales motivos de consulta fueron: diarrea (47%), baja de peso (31%), dispepsia (43%) y fatiga (26,1%). En el laboratorio inicial, destacaron anemia (26,1%), transaminasas elevadas (17,4%), ferritina baja (11,4%), hipovitaminosis D (9,3%), entre otros. Las patologías asociadas más frecuentes fueron hipotiroidismo (15,4%) y trastorno depresivo (11,4%). Se encontró sobrecrecimiento bacteriano en 10,1% y malabsorción de lactosa en 15,4%. El principal diagnóstico histológico fue EC con atrofia de vellosidades estadio 3a de Marsh (34,9%).
ConclusiónNuestros resultados son similares a otras series en adultos que reportan que la EC es más frecuente en mujeres, con inicio en la cuarta década de la vida, con predominio de síntomas extraintestinales y asociado con otras enfermedades autoinmunes. Existe un porcentaje importante en pacientes con sobrepeso y obesos.
Celiac disease (CD) is defined as an immune-mediated chronic enteropathy that occurs when there is exposure of the mucosa to gluten, in genetically susceptible patients1. Gluten, the Latin term from which the word “glue” is derived, is an ethanol-soluble prolamin that provides elasticity and enables wheat fermentation. It is resistant to the proteolytic activity of the gastric, pancreatic, and intestinal proteases due to its high levels of proline. Those polypeptides enter into the submucosa of the small bowel through transcellular and paracellular mechanisms that are not completely understood, starting an inflammatory cascade in susceptible patients. Even though the disease primarily affects the small bowel, its manifestations are systemic, with both intestinal and extraintestinal symptoms2. Unlike CD in children, in whom the classic symptoms are chronic diarrhea, malabsorption, and malnutrition, adults can present with varied symptoms, including extraintestinal ones, or even be asymptomatic. Quality of life is considerably affected in undiagnosed patients, compared with treated patients3.
At the beginning of the 1950s, an estimated one out of every 8,000 Europeans had CD, based on the classic clinical manifestations. However, with the appearance of serologic testing and histologic findings, actual prevalence is calculated at 1:70 to 1:300 in the majority of countries4. Given that its clinical presentation is sometimes mild and nonspecific, it is estimated that for every child diagnosed with CD, 7 are undiagnosed5. Studies show an increasing prevalence with age and up to 15% of cases are diagnosed in persons above 65 years of age6. Despite the fact that the estimated worldwide prevalence of CD is 1%, studies from different countries show a different prevalence.
Comparing the prevalence of CD in the different Latin American countries is currently a complicated endeavor because of the considerable variability in the diagnostic methods utilized. Some studies indicate the frequency of positive celiac serology, evaluating celiac immunity, but they do not constitute a confirmation of CD diagnosis. Therefore, when making comparisons, it is important to take into account the methods utilized to determine prevalence. In South America, prevalence fluctuates between 0.46 and 0.64%, and is reported as higher in Argentina, Brazil, Uruguay, and Chile, in studies based on autoantibody and HLA-DQ haplotype positivity7. Gandolfi et al.8 described a 0.54% prevalence of CD in blood donors in Brasilia. On the other hand, in blood donors in Mexico, a prevalence of 2.7%, based on seroprevalence of tissue transglutaminase-IgA antibodies was reported9. In Chile10, the 2009-2010 National Health Survey estimated a prevalence of 0.76%, based on levels of antibodies against tissue transglutaminase, and they were higher in women than in men (1.1 vs 0.4%, respectively)11.
In 2005, our group, together with a private medical center, reported the clinical characteristics of 37 adults diagnosed with CD12. The aim of the present study was to communicate the clinical characteristics of a group of adults with CD, treated at a university hospital.
Materials and methodsA retrospective, descriptive, cross-sectional study was conducted, based on the review of the medical records of patients treated at the Gastroenterology Service of the Hospital Clínico of the Universidad de Chile, within the time frame of March 2018 and March 2019, that were diagnosed with CD or suspected of presenting with it due to their clinical symptomatology (abdominal pain, abdominal distension, change in stool characteristics, anemia, elevated transaminases, weight loss, or dyspeptic symptoms), with serologic confirmation (tissue transglutaminase antibody determination through the ELISA, with a value above 30 IU, or positive endomysial antibodies through the IFI technique), in the presence of histology consistent with the Marsh type 2, 3a, 3b, and 3c classification of villous atrophy13. Patients whose information was incomplete, that did not have follow-up after diagnosis, and patients with a Marsh 1 histologic study were excluded.
The demographic variables (sex, age at diagnosis, current age, years of symptom progression at diagnosis) and clinical variables (body mass index [BMI], clinical presentation at diagnosis, the presence of anemia, hypertransaminasemia, and the levels of vitamin D, albumin, ferritin, cyanocobalamin, and folic acid) were collected. A history of infertility, the presence of the comorbidities of diabetes mellitus, hypothyroidism, spondyloarthritis affecting the pelvis (corroborated by magnetic resonance imaging), systemic lupus erythematosus, inflammatory bowel disease, autoimmune hepatitis (diagnosis demonstrated by autoimmune serology and liver biopsy), fatigue, depression, fatty liver (diagnosed through ultrasound or abdominal tomography and the ruling out of other causes), and the presence of mucocutaneous manifestations (oral ulcers and lesions consistent with dermatitis herpetiformis) were also registered.
The presence of osteopenia was evaluated through bone densitometry and the coexistence of small intestinal bacterial overgrowth (SIBO) and/or lactose malabsorption was assessed through the lactulose and/or lactose hydrogen breath test, analyzing the samples in gas chromatography (QuinTron MicroLyzer, USA) and expressing the results in parts per million (ppm). SIBO was considered when the hydrogen level was above 20ppm from the baseline, in 2 or more measures, in the first 60min, and lactase malabsorption was considered when there were figures above 20ppm from the baseline. The endoscopic characteristics of the duodenal mucosa were determined as normal; with a loss of or decrease in folds; as having a scalloped aspect; with a nodular aspect; having a mosaic pattern; or with the presence of submucosal vessels. Duodenal biopsies (2 samples taken from the bulb and at least 6 samples from the second part of the duodenum) were evaluated by an anatomopathologist/gastroenterology specialist, utilizing the Marsh classification.
Statistical analysisThe categorical variables were expressed as absolute value and/or percentage and the quantitative variables were expressed as mean±standard deviation. The analyses were carried out using the 2016 IBM program (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Ethical considerationsPatients above 18 years of age agreed to participate in the study and signed written statements of informed consent. The follow-up data were collected using the Excel program, without using the patients’ names, to maintain confidentiality. The authors declare that the present article contains no personal information that could identify the patients. This study was approved by the Scientific Ethics Committee of the Hospital Clínico of the Universidad de Chile.
ResultsOf the 165 patients treated for CD during the study period, 149 met the clinical picture suggestive of CD and had positive antibodies and biopsy consistent with CD with villous atrophy, equal to or greater than Marsh 2. Of the study patients, 119 were women (79.9%) and 30 were men (20.1%). Their mean age at diagnosis was 42 years (range: 16-86) and 10% of the patients were above 60 years of age at diagnosis. Twenty patients (13.4%) had a family history of CD, with a greater frequency in men. Mean BMI was 24.3 kg/m2 (range: 16-44.4 kg/m2, SD 5.04 kg/m2) and 55.3% of the patients presented with normal weight, as shown in Table 1. Upon correlating BMI with hemoglobin, 4 out of 8 patients with a BMI < 20 presented with anemia, whereas none of the 11 patients with a BMI < 30 were anemic. The most frequent comorbidities were hypothyroidism and depressive disorder, as shown in Table 2.
Characteristics of the study group
| Total | Men | Women | |
|---|---|---|---|
| Patients | 149 (100%) | 30 (20.1%) | 119 (79.9%) |
| Age at diagnosis | 42.4 (±13.8) years | 44 (±15.4) years | 42.4 (±13.4) years |
| Current age | 47.4 (±13.7) years | 47.1(±15.6) years | 47.3 (±13.3) years |
| Follow-up | 4.8 (±5.6) years | 3.9 (±5) years | 5 (±5.7) years |
| Family history of CD | 20 (13.4%) | 7 (23.3%) | 13 (10.9%) |
| Mean BMI | 24.3 (±5) kg/m2 | 25.1(±5.4) kg/m2 | 24.6 (±5.4) kg/m2 |
| Normal weight | 82 (55%) | 17 (58.8%) | 65 (54.7%) |
| Underweight | 18(12%) | 2 (6.6%) | 16 (13.4%) |
| Overweight | 41 (27.5%) | 11 (35.3%) | 30 (25.2%) |
| Obesity | 16 (10.7%) | 2 (6.6%) | 14 (11.7%) |
CD: celiac disease; BMI: body mass index.
Comorbidities found in the study patients
| Total n=149 | Men n=30 | Women n=119 | |
|---|---|---|---|
| Hypothyroidism | 23 (154%) | 1 (33%) | 22 (184%) |
| Depression | 17 (114%) | 3 (10%) | 14 (117%) |
| Hepatic steatosis | 7 (47%) | 3 (10%) | 4 (33%) |
| Autoimmune hepatitis | 4 (26%) | 0 | 4 (33%) |
| Systemic lupus erythematosus | 3 (2%) | 0 | 3 (25%) |
| Type 1 diabetes mellitus | 3 (2%) | 0 | 3 (25%) |
| Primary biliary cholangitis | 1 (06%) | 0 | 1 (08%) |
| Inflammatory bowel disease | 1 (06%) | 1 (33%) | 0 |
The following were the primary reasons for medical consultation: diarrhea (47%), dyspepsia (43%), weight loss (31%), and fatigue (26.1%) (Table 3). The laboratory work-up revealed anemia (26.1%), elevated transaminases (17.4%), and low ferritin (11.4%). A total of 8.7% of patients had osteopenia or osteoporosis, through bone densitometry, but no fractures were reported in any of our patients. Table 3 shows the differences by sex and the main reasons for consultation.
Reasons for consultation and initial laboratory work-up findings
| Total n=149 | Men n=30 | Women n=119 | |
|---|---|---|---|
| Diarrhea | 70 (47%) | 18 (60%) | 52 (44.1%) |
| Weight loss | 46 (31%) | 13 (43%) | 33 (27.7%) |
| Dyspepsia | 64 (43%) | 8 (26.6%) | 56 (47%) |
| Fatigue | 39 (26.1%) | 10 (33.3%) | 29 (24.3%) |
| Abdominal pain | 13 (8.7%) | 2 (6.6%) | 11 (9.2%) |
| Infertility | 6 (4%) | 2 (6.6%) | 4 (3.4%) |
| Mucocutaneous manifestations | 7(4.7%) | 2 (6.6%) | 5 (4.2%) |
| Constipation | 4 (2.6%) | 1 (3.3%) | 3 (2.5%) |
| Anemia | 39 (26.1%) | 5 (16.7%) | 34 (28.9%) |
| Elevated transaminases (as a reason for consultation) | 26 (17.4%) | 7 (23.3%) | 19 (15.9%) |
| Elevated transaminases (control finding) | 59 (39.5%) | 15 (50%) | 44 (36.67%) |
| Low ferritin | 17 (11.4%) | 2 (6.6%) | 15 (12.6%) |
| Hypovitaminosis D | 14 (9.3%) | 3 (10%) | 11 (9.2%) |
| Hypoalbuminemia | 8 (5.3%) | 3 (10%) | 5 (4.2%) |
| Low vitamin B12 | 8 (5.3%) | 4 (13.3%) | 4 (3.3%) |
| Low folic acid | 6 (4%) | 4 (13.3%) | 2 (1.7%) |
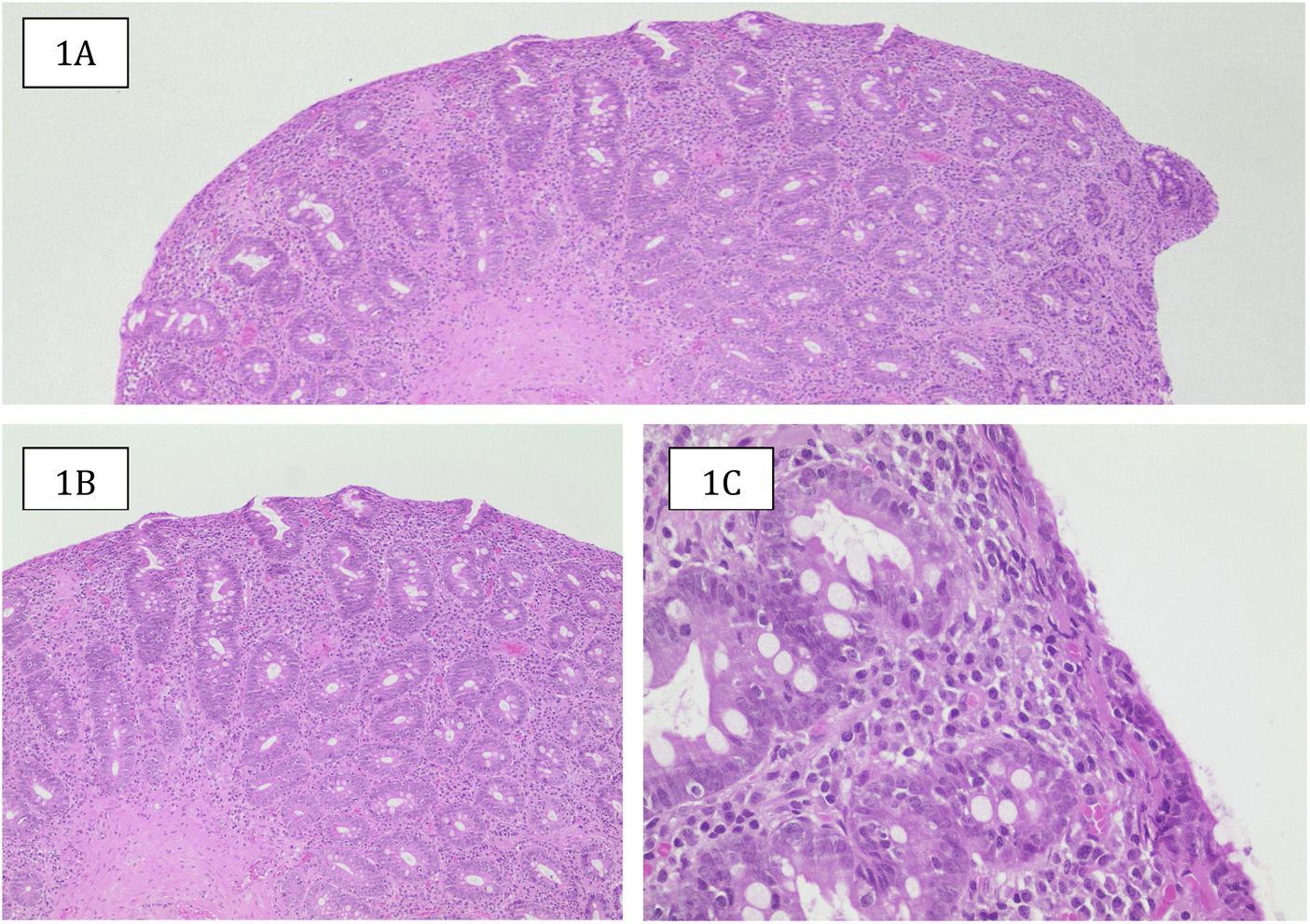
With respect to the lactulose hydrogen (H2) breath test, SIBO was found 10.1% of the patients and lactose malabsorption in 15.4%. Upper gastrointestinal endoscopy revealed a macroscopically normal duodenum in 40.3% of the patients (Table 4). In the cases with alterations, the most frequent were loss of or decrease in folds, scalloped aspect, nodular aspect, and mosaic pattern. In the histologic study, according to the Marsh classification, the most frequent pattern was 3a (34.9%), albeit a majority of patients were in the more advanced stages of 3b and 3c (Table 5) (Fig. 1).
Histologic sample from a female patient in the case series classified as Marsh 3b. A) Duodenal mucosa with a flat surface (absence of villi), with hyperplastic crypts (hematoxylin/eosin [H&E] ×4). B) Crypt hyperplasia and enlarged inflammatory infiltrate in the lamina propria (H&E ×10). C): Inflammatory infiltrate rich in plasma cells of the lamina propria, epithelial surface reduced in height, mucinous content, and numerous lymphocytes in its interior (H&E, ×40).
Source: Dr. Laura Carreño T.
An associated neoplasm was found in 10 patients: 3 presented with thyroid cancer, 2 with skin cancer, and 1 with jejunal lymphoma (it initially presented as mesenteric panniculitis, and in the study, ulcerative jejunitis associated with lymphoma was discovered), 1 with gastric cancer (diagnosed simultaneously with the endoscopic study of the patient’s CD), 1 with multiple intraductal papillary mucinous neoplasms (IPMNs), 1 with colon cancer (derived from post-colectomy persistent diarrhea and anemia), and 1 with a carcinoid cecal appendix. The diagnosis of the carcinoid case was made through tomography, 3 months after the diagnosis of CD, due to the persistence of abdominal pain and diarrhea, despite already having normal gliadin antibodies. The patient underwent surgery and is currently asymptomatic.
DiscussionIn our case series conducted at the Hospital Clínico of the Universidad de Chile, we reported an important number of adult patients with CD. It was more frequent in women (79.9%) and disease onset was around the fourth decade of life. Only 13.4% of the patients, more frequently men than women, stated they had a direct relative with the same diagnosis. In the study by Fasano et al.14 that included more than 13,000 persons, there was a prevalence of 1:22 in first-degree relatives, 1:39 in second-degree relatives, 1:56 in symptomatic patients, and 1:133 in low-risk patients.
An interesting finding in our study was that more than one-third of the patients were overweight or obese at diagnosis. Given the obesity pandemic, an increasing number of patients diagnosed with CD are overweight, and the initial description has been anecdotal. An Italian study on celiac children showed a 7.8% prevalence of obesity that was more frequent in older children, with lower tissue transglutaminase antibody levels15. Contrastingly, in adult patients, with positive histology and tissue transglutaminase antibodies, prevalence of overweight and obesity varied according to geographic zone, at 6.2% in a study from India16 and up to 38.3% in a study conducted in the United States17, with a mean BMI of 23.7 kg/m2, which was a bit lower than the mean BMI in our patients. A study carried out in Ireland described a BMI above 25kg/m2 in 39% of the patients and a BMI below 18.5kg/m2 in only 5%. Those authors reported that 81% of the patients had gained weight, at 2 years of control with a gluten-free diet18. They also found that diarrhea and low hemoglobin levels in women correlated with a lower BMI, as in our population.
To make the diagnosis of CD in adults requires a high level of suspicion, given the greater frequency of its atypical presentation. Some patients present with nonspecific symptoms, such as dyspepsia, nausea, meteorism, recurrent abdominal pain, and up to 20% of patients present with constipation19. Diarrhea and dyspepsia were predominant in our patients, but some had constipation and had previously been diagnosed with a functional disorder. Many adult patients with CD present with a clinical picture that includes abdominal pain, bloating, and mild constipation and diarrhea, resembling or simulating symptoms of irritable bowel syndrome (IBS). That complicates the differential diagnosis, especially in patients with latent CD, in whom the study of the mucosa can appear normal or minimally altered20. Nevertheless, having controlled CD does not invalidate presenting with IBS as a comorbidity.
In our patients, endoscopy revealed a normal macroscopic aspect in 40.3% of the patients, showing the need for high clinical suspicion before endoscopy. In their study on 70 Chilean patients with untreated CD, Weitz et al.21 reported that the histologic study showed a macroscopic lesion in 78.5% of the patients, compared with the suspicion of the endoscopists in 72%. Therefore, we believe that, even though the sensitivity of endoscopic screening for diagnosing CD is low, the procedure has a relevant role when duodenal biopsies are taken in the patients with clinical suspicion of the disease. In our case series, the patients described as having normal duodenal mucosa at endoscopy had the same histologic compromise as the patients with macroscopically altered duodenal mucosa.
With respect to the nutritional consequences of CD, anemia has been shown to be the most common extraintestinal manifestation in some case series, finding that 3 to 9% of patients present with iron deficiency and no gastrointestinal symptoms, with the figure increasing to 15% in symptomatic patients22. The figure was somewhat higher in our study, given that more than one-fourth of the patients presented with anemia. A study conducted in the Netherlands on 80 patients with untreated CD found deficiencies in vitamin B12, folic acid, iron, and vitamin D, among others, with only 10% of the patients presenting with underweight and 29% with overweight23. We found similar nutrient deficiencies in our population. Furthermore, chronic calcium and vitamin D malabsorption lead to osteoporosis, osteopenia, or osteomalacia. Metabolic bone disease is one of the primary causes of morbidity in CD, increasing the risk for fractures 2 to 3 times24, but no fractures were reported in our study population.
Other authors have described the coexistence of autoimmune diseases in 14% of the patients with CD, including type 1 diabetes mellitus25; autoimmune thyroid diseases, such as Hashimoto thyroiditis; and arthritis. The appearance of those diseases has been thought to depend on the period of exposure to gluten, concurring with what we observed in our population, in which there was an important number of cases of hypothyroidism, type 1 diabetes mellitus, spondyloarthritis affecting the pelvis, systemic lupus erythematosus, and psoriatic arthritis, among other autoimmune diseases. That is relevant because, for example, hypothyroidism has a prevalence of 0.1 to 7% in the general population11, compared with the 15.4% found in our case series.
Since the first reports on cases of CD, an association with hepatic alterations has been described, the most frequent being the increase in transaminases that presents in 20% of the patients. In addition to its association with autoimmune liver diseases, an association with nonalcoholic fatty liver disease (NAFLD), hepatitis C virus, hepatitis B virus, cirrhosis, and non-cirrhotic portal hypertension has also been described. Ludvigsson et al.26 showed an up to 6-times greater risk for presenting with a liver disease in patients with CD versus the control population. In our study, 17.4% of the patients had altered liver function tests and up to 39.5% presented with hypertransaminasemia during their disease progression. NAFLD is one of the main causes of liver disease worldwide and has greater prevalence in obese patients with CD27. A total of 4.7% of the patients in our analysis, and more frequently men, had a NAFLD diagnosis. Bardella et al.28 showed that, in a group of celiac patients, with NAFLD confirmed by liver biopsy, 3% of them improved at 6 months, with only a gluten-free diet. Our group did not study improvement through diet, but we believe it should be a future line of research. Although the relation between those two entities has not been established, it could be due to greater intestinal impermeability or an alteration of the microbiota. Given that CD is more frequent in patients with NAFLD than in the general population, we consider that antibody testing for ruling out CD should be performed on patients that present with liver disease of undetermined etiology and no metabolic risk factor.
In patients with autoimmune hepatitis (AIH), CD is 10-times more frequent than in the general population29. There were 4 patients with AIH in our case series. A genetic correlation between the two entities has been suggested because of class II HLA complex gene expression on chromosome 6. The mechanism is not fully known, but it is postulated that said increase could be due to the presence of CD autoantibodies that could trigger an autoimmune disease, as well as increased intestinal permeability. With respect to primary biliary cholangitis (PBC), the relation between the two entities is contradictory. Whereas some analyzes have found no mitochondrial antibodies in celiac patients, a population study in the United Kingdom showed a prevalence of 3% in 143 patients with CD and a 6% prevalence of CD in patients with PBC30, which was 10-times higher than the prevalence in the general population. One patient in our case series had PBC, a lower number than in the English study, and because of the small size of our sample, no conclusions could be drawn about that association. Many such patients have improved transaminase levels after 6 months of a gluten-free diet. Serial control transaminase quantification was carried out in our case series and there was improvement in the patients with good CD control. Unfortunately, not all patients had strict transaminase level follow-up, reflecting the lack of knowledge about the relation of the two entities.
Untreated CD has been related to T cell lymphoma that is associated with enteropathy and other non-Hodgkin lymphomas, with a 15 to 100-times higher risk than in the general population31. A population study showed a modest increase in gastrointestinal cancers at 5 years of follow-up, with a predominance of colorectal cancer32. Another clinical/pathologic study conducted in England showed that CD was a comorbidity in 39% of lymphomas and 13% of small bowel adenocarcinomas33. Such frequency of lymphoma in CD has not been reported in Chile, but there has been an association with other gastrointestinal and non-gastrointestinal malignant neoplasms, as seen in the present case series.
ConclusionOur results were similar to those found in other previously described international populations, showing a greater frequency in women, disease onset around the fourth decade of life, a predominance of extraintestinal symptoms, and an association with other autoimmune diseases. A high percentage of our patients with CD also presented with overweight and obesity and the histologic compromise was severe in the majority of the patients. A high level of suspicion of CD is required, regarding adult patients, because of the greater frequency of its atypical presentation and functional disorder overlap. Even though our study confirms some of the results observed in other parts of the world, it specifically provides relevant information on the behavior of CD in a Latin American country, where information on the disorder is scarcer. We believe larger population studies are needed that include all regions of Chile, given the imminent increase of CD diagnoses in our country.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Dr. Laura Carreño Toro, anatomopathologist at the Hospital Clínico of the Universidad de Chile for her collaboration and for providing the images of the histologic slides.
Please cite this article as: von Mühlenbrock-Pinto C, Madrid-Silva AM. Enfermedad celiaca en adultos chilenos. Rev Gastroenterol Méx. 2023;88:28–35.


![Histologic sample from a female patient in the case series classified as Marsh 3b. A) Duodenal mucosa with a flat surface (absence of villi), with hyperplastic crypts (hematoxylin/eosin [H&E] ×4). B) Crypt hyperplasia and enlarged inflammatory infiltrate in the lamina propria (H&E ×10). C): Inflammatory infiltrate rich in plasma cells of the lamina propria, epithelial surface reduced in height, mucinous content, and numerous lymphocytes in its interior (H&E, ×40). Source: Dr. Laura Carreño T. Histologic sample from a female patient in the case series classified as Marsh 3b. A) Duodenal mucosa with a flat surface (absence of villi), with hyperplastic crypts (hematoxylin/eosin [H&E] ×4). B) Crypt hyperplasia and enlarged inflammatory infiltrate in the lamina propria (H&E ×10). C): Inflammatory infiltrate rich in plasma cells of the lamina propria, epithelial surface reduced in height, mucinous content, and numerous lymphocytes in its interior (H&E, ×40). Source: Dr. Laura Carreño T.](https://static.elsevier.es/multimedia/2255534X/0000008800000001/v1_202302161607/S2255534X22000202/v1_202302161607/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w97o/wdEXW47bqlyT1CqG6R0=)

