Treatment through endoscopic retrograde cholangiopancreatography (ERCP) in patients with modified anatomy is currently a challenge. There are 2 basic techniques: enteroscopy-assisted ERCP (EA-ERCP) and laparoscopy-assisted ERCP (LA-ERCP).1 The former has a low success rate (< 70%) and the latter has serious adverse events of up to 14.3%, limiting its use.2
A technique has recently been described that utilizes endoscopic ultrasound to anastomose the gastric pouch to the excluded stomach, placing a lumen-apposing metal stent between them, to then be able to perform conventional ERCP. The technique is known as endoscopic ultrasound-directed transgastric ERCP, or EDGE.3
A 63-year-old man had a diagnosis of choledocholithiasis and underwent Roux-en-Y gastric bypass surgery 5 years prior. Symptom onset began with fever, abdominal pain, jaundice, nausea, and vomiting. The laboratory work-up showed direct hyperbilirubinemia, cholestasis, and leukocytosis, for which magnetic resonance cholangiography was carried out that identified choledocholithiasis (Fig. 1A). The risks and benefits of the EDGE procedure were thoroughly explained, and after obtaining informed consent, its performance was proposed.
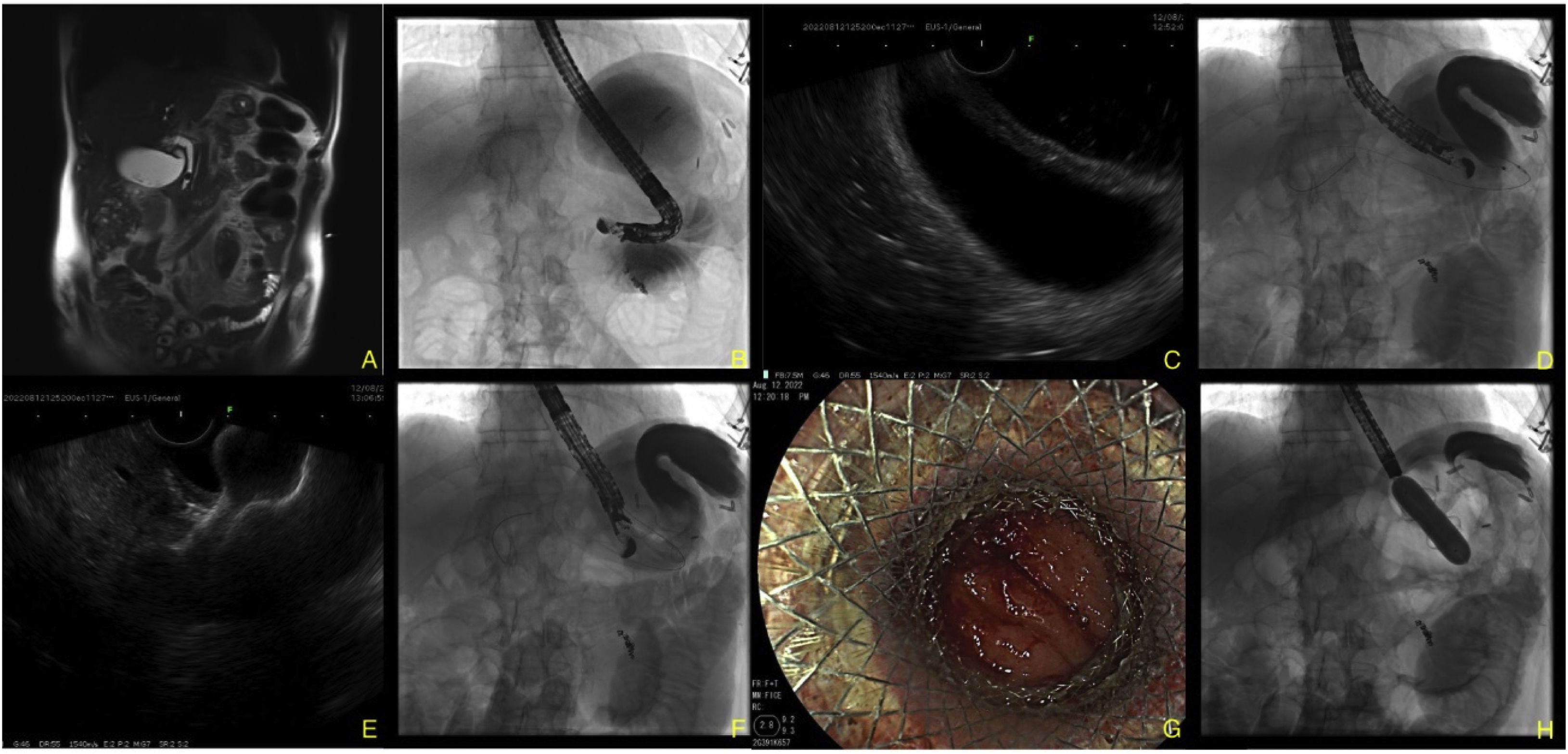
A) Magnetic resonance image showing a stone in the distal third. B) Distension of the excluded stomach seen through fluoroscopy. C) Endoscopic ultrasound (EUS) view of both gastric walls. D) 19G FNA puncture of the gastric remnant to the excluded stomach and advancement of the 0.035″ hydrophilic guidewire into the antrum of the excluded stomach. E) EUS image of the Hot AXIOS™ lumen-apposing stent placement. F) Fluoroscopic image of the placement. G) Endoscopic image of the gastrojejunal anastomosis. H) 18mm CRE™ balloon dilation of the anastomosis through the body of the stent.
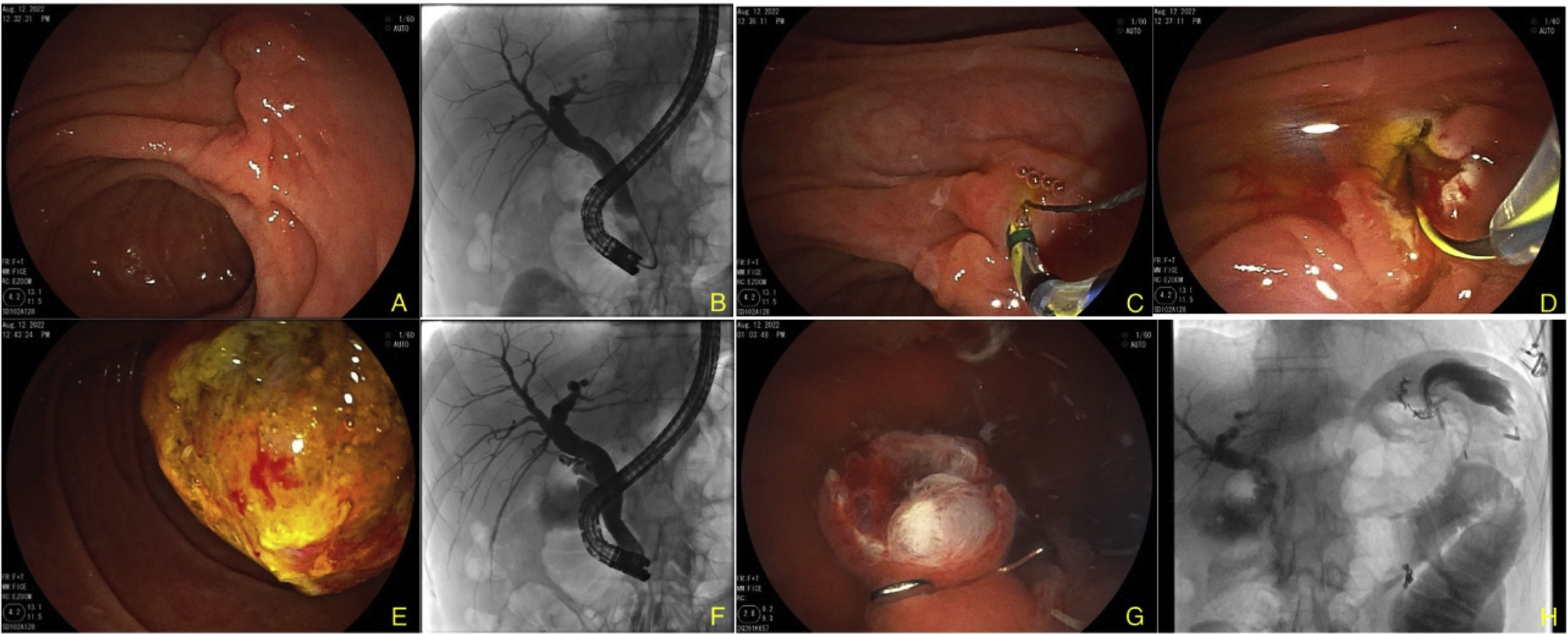
The gastric remnant and the gastrojejunal anastomosis were examined through conventional endoscopy, after which injectable water combined with 50% water-soluble contrast medium was instilled, in a retrograde fashion, filling the excluded stomach through the afferent jejunal segment. The results were corroborated by fluoroscopy and linear endosonography (EG-580UT, Fujinon, Tokyo, Japan), visualizing both gastric walls (Fig. 1B and C). The gastric remnant was punctured with a 19G fine needle (Expect™, Boston Scientific, Marlborough, MA, USA) and a 0.035″ guidewire (Jagwire™, Boston Scientific, Marlborough, MA, USA) was advanced, to corroborate adequate stent positioning into the gastric antrum (Fig. 1D). A 20mm×10mm HOT-AXIOS™ lumen-apposing stent (Boston Scientific, Marlborough, MA, EEUU) was then placed and pure cutting current (Autocut 120W, ERBE VIO® 3, Tübingen, Germany)3 was applied. A gastrogastric anastomosis was created (Fig. 1E–G). Hydrostatic balloon dilation to 18mm was carried out (CRE™, Boston Scientific, Marlborough, MA, USA) (Fig. 1H). Conventional ERCP was then performed with a duodenoscope (ED-580XT, Fujinon, Tokyo, Japan), which was introduced orally, after lubrication with mineral oil, reaching the second part of the duodenum by way of the lumen-apposing stent (Fig. 2A). The bile duct was selectively cannulated, confirming the presence of several filling defects in the distal third of the choledochus and the escape of purulent matter from the bile duct (Fig. 2B). Sphincterotomy was carried out (Fig. 2C and D) and the stones were extracted with no complications (Fig. 2E and F). The lumen-apposing stent was removed using a foreign body forceps and closure of the gastrogastric fistula site with a GC OVESCO clip (OTSC®, Tübingen, Germany) was satisfactorily performed (Fig. 2G and H). Patient progression was favorable, and he was released 48h after the procedure.
A) Image of the ampulla of Vater with the duodenoscope. B) Fluoroscopic image showing the duodenoscope through the lumen-apposing stent; upon contrast medium injection there is bile duct dilation and filling defects inside it. C) Biliary sphincterotomy. D) Passage of the guidewire. E) Stone extraction. F) Fluoroscopic view in which stones are no longer seen in the bile duct. G) Endoscopic image of closure with OTSC® clip. H) Fluoroscopic image corroborating adequate closure and no signs of leakage.
Roux-en-Y gastric bypass surgery is frequently performed due to the high prevalence of overweight and obesity worldwide. However, it is a complex procedure in cases of pancreaticobiliary disease. Described in 2014 by Kedia et al.,4 EDGE enables the creation of a temporary endoscopic ultrasound-guided gastrogastric anastomosis for accessing pancreaticobiliary disease. Current technical and clinical success is 100 and 91%, respectively (similar to LA-ERCP but superior to EA-ERCP).5,6 Shah-Khan et al.7 showed EDGE to be a more popular therapeutic option, compared with other techniques (86% EDGE, 56% LA-ERCP, 19% enteroscopy), with 67% considering it standard treatment. The technique carried out at our hospital center is similar to that described by other authors and can be performed in one or 2 sessions. Because of the cholangitis our patient presented with, he underwent a single session, and like other authors, we had no secondary events. Nevertheless, some authors favor 2 sessions (creation of the gastrojejunal anastomosis in the first session and ERCP in the second, so the fistula can mature), or even three (adding one for closure), to potentially provide a reduction in the risk for perforation.4,5,7 Prakash et al.8 retrospectively evaluated EDGE performed in a single session, reporting less time, lower cost, and no differences in adverse events, with respect to more sessions. The majority of adverse events are minor (18%), and some are potentially serious (migration 11%, perforation 5%). In a retrospective case series on 128 patients, Shinn et al.9 showed that a lumen-apposing stent diameter < 15 mm was associated with a higher risk for migration (OR=5.36, 95% CI 1.29-22.24; p<0.02), but dilation, double-pigtail stent placement, or anastomosis site were not (p=NS). Lastly, there is controversy about fistula closure, given that it could only potentially prevent weight regain in those patients, but with no significant differences in the prevention of adverse events.10 In our case, due to the technical difficulty of the procedure, bile duct manipulation with the lumen-apposing stent as a point of support, and dilation to 18 mm, we decided on primary closure with a GC clip.
In conclusion, EDGE is a safe and effective technique, with an acceptable adverse event rate (< 20%), for the approach in patients that have modified anatomy and that present with pancreaticobiliary disease. Thus, it is a viable alternative that should be a first consideration in that group of patients in our medical environment.
Ethical considerationsThe authors declare that a signed statement of informed consent was requested and that the images presented herein preserve patient anonymity.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Hernández-Mondragón OV, Bartnicky-Navarrete I. Coledocolitiasis resuelta en paciente con by-pass gástrico en Y de Roux por medio de ultrasonido endoscópico por técnica EDGE. Reporte de caso y revisión de la literatura. Rev Gastroenterol Méx. 2023;88:291–293.