Inflammatory bowel disease is considered an immune-mediated entity that particularly involves the digestive tract, but extradigestive manifestations frequently occur. Crohn’s disease usually presents with deep transmural involvement and can affect any part of the gastrointestinal tract, from the oral cavity to the anus. At diagnosis, the majority of patients with Crohn’s disease present with a predominantly inflammatory pathology. Esophagogastroduodenal involvement is rare, with a low incidence estimated from 0.5 to 4% of the cases of Crohn’s disease,1 and so is considered a diagnostic challenge. Its diagnosis is based on the summation of clinical, endoscopic, histologic, and radiologic findings. A long list of differential diagnoses must first be ruled out, including lymphoma, adenocarcinoma, and tuberculosis.2
We present herein a previously healthy 24-year-old woman, coming from a rural area. She sought primary care attention due to 6 months of daily, colicky abdominal pain. It increased after meals, with moderate intensity, and was associated with frequent postprandial nausea and vomiting. Three months prior to her medical consultation, she experienced watery diarrhea, on average 4-6 times daily, with a consistency between 6 and 7 on the Bristol stool scale. The patient stated having had recurrent episodes of melena and a 17 kg weight loss. Upon physical examination, her health status was regular, and she was cachexic. She measured 154 cm in height, weighed 32 kg (her normal weight was 49 kg), and her BMI was 13.5 kg/m2. She had moderate, colicky abdominal pain, with no peritoneal irritation.
The initial endoscopic studies described poor distension of the gastric walls, abundant fluids, and the retention of food remnants, as well as an infiltrating ulcerated lesion affecting almost the entire gastric body and antrum, with pyloric stricture. Probable gastric cancer was diagnosed. The endoscopic biopsies showed severe inflammatory changes due to the lymphoplasmacytic infiltrate and the presence of ulcerated material. Given the initial endoscopic suspicion of gastric cancer, staging was undertaken with abdominal computed axial tomography, which identified severe parietal thickening encompassing the entire gastric wall, predominantly affecting the corpus and antrum, and multiple adenopathies that were interpreted as neoplastic perigastric adenopathies. Because the first biopsies showed no malignancy, a new endoscopy was ordered, and it revealed the same endoscopic pattern. Due to the high suspicion of neoplasia, multiple biopsy samples were again taken, even utilizing the same-site biopsy technique. The results were similar to those of the first biopsies, and so a gastric endo-ultrasound study was ordered to evaluate the gastric walls, and a later transmural fine-needle aspiration of the gastric wall and the perigastric adenopathies was carried out. The lymph node and gastric FNA samples were insufficient and the pathology report was negative for malignancy.
The patient was evaluated by surgeons from the general surgery service, who recommended the diagnosis and staging of the supposed gastric cancer, with laparoscopic biopsies taken from the peritoneum and the perigastric lymph nodes. Those results were also negative. With that scenario, the patient was referred to our hospital to complete her diagnosis and provide palliative management.
Upon admission, the patient was identified in poor general condition, with marked cutaneous/mucosal pallor, and the sensation of a mass in the epigastrium. The admission laboratory test results showing altered acute phase reactants were striking: CRP 18.51 mg/dl (RV up to 1 mg/dl), erythrocyte sedimentation rate of 83 mm/h; hemogram with leukocytosis of 12,700 mm3, neutrophilia of 81.6% (absolute neutrophil count 10,400 mm3), hemoglobin of 7.8 g/dl, hematocrit of 23.7%, thrombocytosis of 747,000mm3, severe hypoalbuminemia at 2.3 mg/dl, and nonreactive ELISA for HIV.
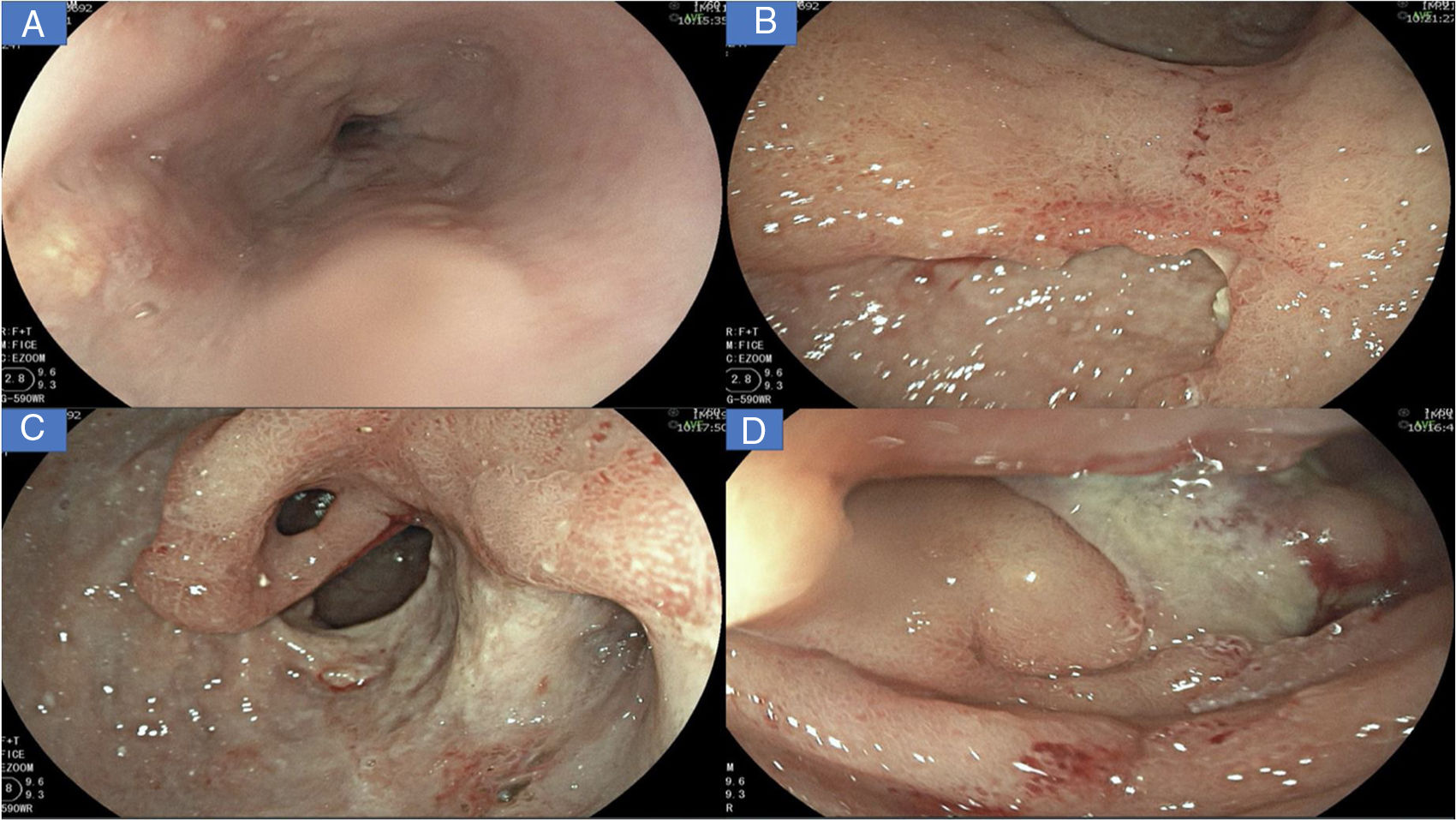
A new gastrointestinal endoscopy was performed that showed notching of Kerckring’s folds in the esophagus (Fig. 1A); multiple deep segmental ulcers with irregular edges in the stomach, with extensive involvement of the antrum and corpus (Fig. 1B-C); and severe segmental inflammatory changes in the duodenum, with greater compromise at the second part (Fig. 1D). Inflammatory disease was immediately suspected.
A) Linear ulcers, aphthous erosions, and notching of Kerckring’s folds in the esophagus. B) Severe inflammatory changes that affect the mucosa of the corpus, with signs of erythema, edema, nodularity, and deep, segmental ulcers. C) View of the gastric antrum, showing a deep ulcer affecting more than 70% of the circumference, with punched-out areas, pyloric deformity, and mucosal bridging (black arrow). D) Severe inflammatory changes in the duodenum, with serpiginous ulcers and a fibrin-covered dominant ulcer in DII.
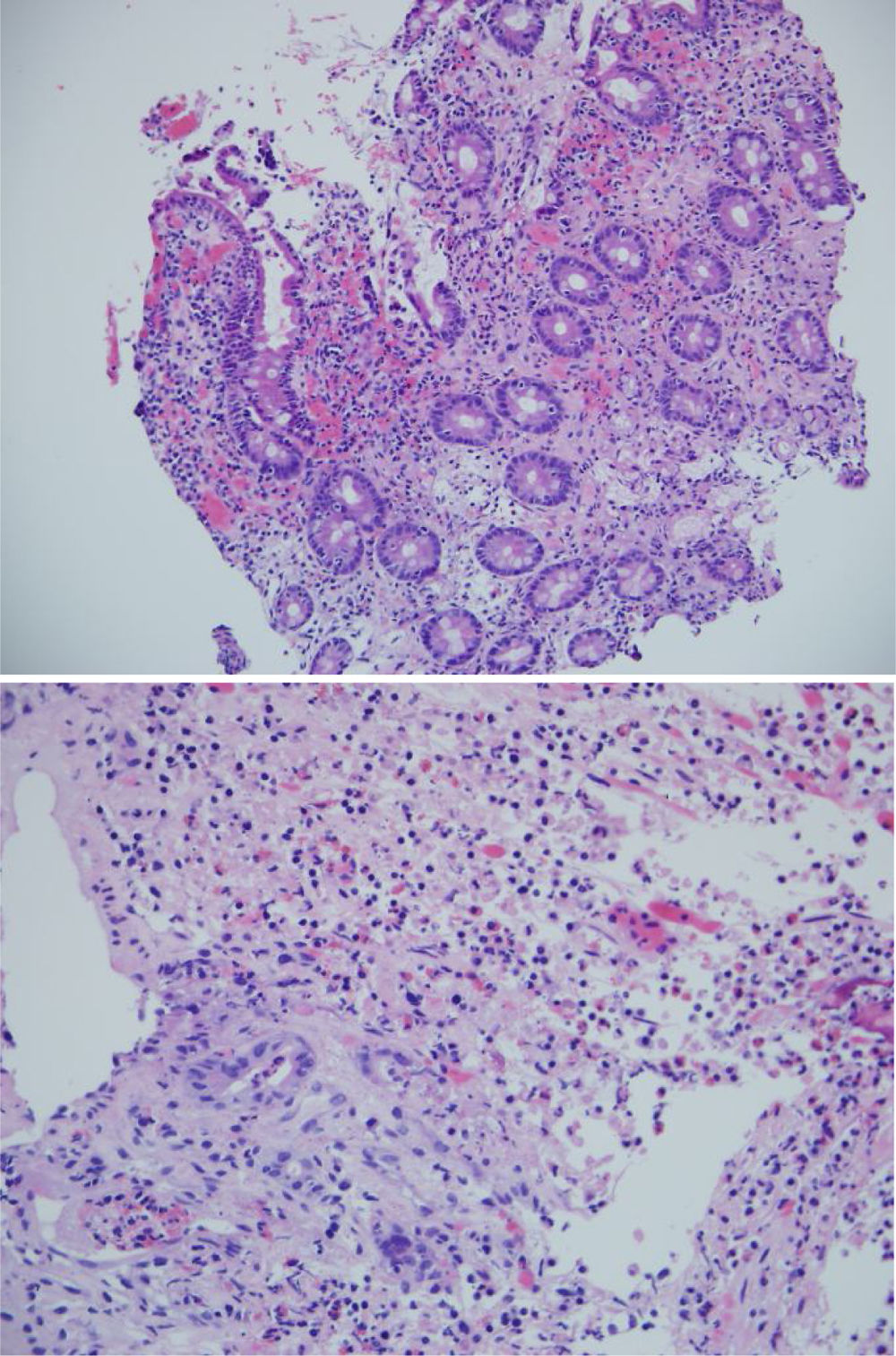
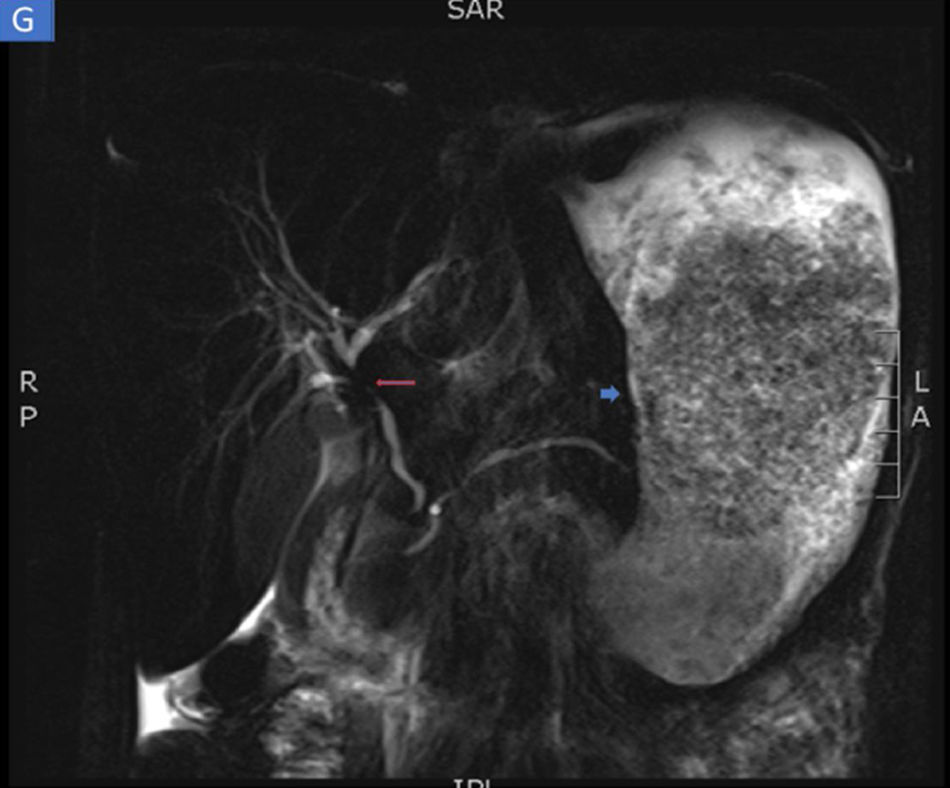
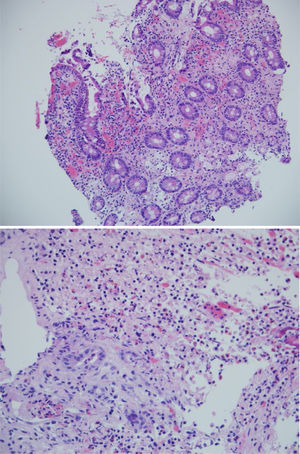
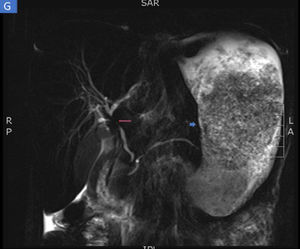
The biopsies taken at upper endoscopy showed severe chronic inflammatory changes, lymphoplasmacytic infiltrate, with deep, extensive ulcerated areas in the esophagus, stomach, and duodenum, and no evidence of dysplasia or malignancy. The immunohistochemical study ruled out a neoplastic process of adenocarcinoma or lymphoma and the Ziehl-Neelsen and methenamine silver stains were negative for tuberculosis and a fungal infection, respectively (Fig. 2). Due to the high suspicion of Crohn’s disease, we ordered a magnetic resonance enterography (MRE) study to evaluate disease extension, ruling out involvement of the rest of the small bowel and lower digestive tract. The extent and depth of the gastric lesions were striking in the MRE study, as was the presence of an inflammatory stricture at the confluence of the hepatic ducts and the proximal common bile duct (Fig. 3).
Magnetic resonance enterography identifying severe inflammatory changes and thickening of the stomach (short arrow), as well as the incidental identification of a severe stricture, with an inflammatory aspect, at the confluence of the hepatic ducts and the proximal common bile duct, consistent with primary sclerosing cholangitis (long arrow).
With the clinical, endoscopic, and radiologic findings, the negativity of the biopsies for malignancy, and the lack of deep infections, the diagnostic conclusion was Crohn’s disease with esophagogastroduodenal involvement. With a CDAI score of 449 upon admission, added to the presence of a dominant biliary stricture, we considered the high probability of primary sclerosing cholangitis, as an extradigestive manifestation. In the complementary immunologic studies, negative antinuclear antibodies, negative anti-smooth muscle antibodies, negative P ANCA, and positive anti-Saccharomyces IgA and IgG antibodies stood out.
Given that the pathology study ruled out malignancy and deep infections, treatment with systemic steroids (intravenous hydrocortisone 100 mg every 8 h) and anti-tumor necrosis factor-alpha therapy (infliximab at 5 mg/kg at induction doses at weeks 0-2-6) were begun. The initial response was satisfactory, with early improvement of abdominal pain and reduced nausea, and after one week of treatment, the patient had no more episodes of vomiting or diarrhea. Afterwards, the patient was given prednisolone at 40 mg daily for 2 weeks, with a gradual reduction, until its suspension after 2 months. She also took esomeprazole at 40 mg every 12 h for 2 months, which was then reduced to 40 mg daily. The patient received 2 vials of parenteral iron (ferric carboxymaltose) for severe anemia.
Because of the biliary findings in the MRE study, liver tests were carried out. They showed elevated total bilirubin levels of 2.45 mg/dl, with a predominance of direct bilirubin (1.9 mg/dl), alkaline phosphatase of 802 mg/dl, GPT of 37 U/l, and GOT of 50 U/I (RV up to 34). Endoscopic retrograde cholangiopancreatography was carried out as a complementary study and for bile duct diversion. The presence of an inflammatory stricture in the proximal common bile duct was confirmed by the endoscopic retrograde cholangiopancreatography. No other strictured areas or beading of the intrahepatic bile ducts were found. The common bile duct was diverted through the placement of a 10 cm x 10 Fr plastic stent. Ursodeoxycholic acid was used at 10 mg/kg/day due to the high suspicion of primary sclerosing cholangitis.
The patient was hospitalized for 58 days in general wards. Given her very poor nutritional status, the nutritional support group ordered treatment with a stringent hypercaloric diet and additional total parenteral in-hospital nutrition, to supply 1,737 kcal/day and 1.44 g/kg of protein. The patient’s nutritional recovery was acceptable, and at the time of her release, she weighed 43 kg and had improved nutritional parameters, with hemoglobin of 10.1 g/dl, hematocrit of 31%, and albumin of 3.4 mg/dl.
In the outpatient follow-up, 2 months after her release from the hospital, the patient felt better, tolerated an oral diet well, and had no more episodes of vomiting or new episodes of diarrhea. She had also gained 2 more kg of weight and her CDAI score was 135. Maintenance treatment was infliximab at 5 mg/kg every 2 months and ursodeoxycholic acid. Removal of the plastic biliary stent was ordered.
CommentaryEsophagogastroduodenal location of Crohn’s disease is rare. Some authors have stated that outcome could be worse in those patients.3,4 Endoscopic studies have shown that the antrum and the duodenum are the most commonly affected areas in proximal Crohn’s disease.5 Endoscopic findings are varied, from patchy erythema, nodular thickening, and focal erythematous spots, to varying degrees of superficial or deep ulcers. In the duodenum, irregular thickening, a cobblestone appearance, polypoid lesions, and focal ulcerations are common findings.6 Notching of Kerckring’s folds may be the only pathognomonic sign of upper gastrointestinal involvement in Crohn’s disease.7
When digestive tract involvement is proximal, the differential diagnosis is broad. If the lesions are in the esophagus, they must be differentiated from strictures secondary to gastroesophageal reflux, tumor, fistula, mediastinal abscess, or tuberculosis. If they are in the stomach, they must be differentiated from gastritis due to Helicobacter pylori (H. pylori), granulomatous gastritis, granulomatosis with polyangiitis, and other types of vasculitis. Adenocarcinoma, linitis plastica, lymphoma, and other malignancies that compromise the upper digestive tract must always be ruled out.7 With respect to histology, focal or diffuse lymphocytic gastritis, with no H. pylori, is the most frequent. The presence of granulomas, the most specific anatomopathologic datum, is detected in only 30% of cases. At the level of the duodenum, the presence of infections, such as tuberculosis, syphilis, and Whipple’s disease, and neoplasias, such as adenocarcinoma and MALT lymphoma, are the main differential diagnoses to be ruled out.8
In the present case, the initial suspicion from the endoscopic findings was locally advanced gastric cancer versus lymphoma. However, due to clinical progression, as well as to detailed endoscopic evaluation, the radiologic findings, and the ruling out of the differential diagnoses, the final diagnosis of Crohn’s disease with esophagogastroduodenal involvement could be made.
There was also involvement of the proximal extrahepatic bile ducts in our patient, resulting in the suspicion of primary sclerosing cholangitis (given the clinical context of Crohn’s disease, the presence of cholestasis, and an inflammatory stricture in the imaging studies). Strict future follow-up should be carried out, given that primary sclerosing cholangitis is considered a rare extradigestive manifestation in Crohn’s disease.
Ethical disclosuresThe authors declare that no experiments were performed on humans or animals for this study.
The authors declare that they have treated all patient data with confidentiality and anonymity, following the protocols of their work center.
Informed consent was not requested for the publication of this case because the article contains no personal data that could identify the patient.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that they have no conflict of interests.
Please cite this article as: Betancur Salazar K, Mosquera-Klinger G. Enfermedad de Crohn con compromiso esofagogastroduodenal. Revista de Gastroenterología de México. 2020;85:481–484.