The prevalence of cow’s milk protein allergy in the first year of life varies from 1.8 to 7.5%. The Cow’s Milk-related Symptom Score (CoMiSS) was published in 2014 and facilitates the diagnosis of cow’s milk protein allergy. It is not meant to replace the clinical diagnosis, but rather to guide the treating team in the diagnostic process and reduce unnecessary diets. The aim was to translate the CoMiSS from English to Spanish and culturally adapt and validate the resulting Spanish version.
Materials and methodsAn adaptation and validation study on the CoMiSS questionnaire was carried out in two phases: First, the CoMiSS was translated from English to Spanish, after which interrater reliability of the translated score was assessed. Second, interrater reliability tests were carried out on 32 pediatric patients under 7 years of age that were treated for the first time at the Food Allergy Clinic of the Hospital Italiano de Buenos Aires, were suspected of having cow’s milk protein allergy, and had not received any treatment, within the time frame of May 2018 and May 2019.
ResultsThirty-two patients were evaluated, 14 of whom were females (45%), and the median patient age was 3 months (IQR 2-4). The median result of the first measurement of the scale was 7.0 (IQR 4.5-9.0) and the median of the second measurement was 5.0 (IQR 4.0-8.0). The final intraclass correlation coefficient was 0.80 (95% CI 0.63-0.9).
ConclusionThe Spanish translation of the CoMiSS was comparable to the original English version, with excellent interrater reliability. This simple and little-known tool has the benefit of being a noninvasive, rapid, reliable, and easy-to-use strategy.
La prevalencia de alergia a la proteína de leche de vaca en el primer año de vida varía entre el 1.8% y el 7.5%. En el a˜no 2014 se publicó The Cow’s Milk-related Symptom Score (CoMiSS, Score de síntomas de Alergia a la Proteína de Leche de Vaca) para facilitar su diagnóstico. Con ello no se pretende reemplazar el diagnóstico clínico sino orientar al equipo tratante en el proceso diagnóstico y disminuir dietas innecesarias. El objetivo de este estudio fue traducir, adaptar y validar de forma transcultural la versión traducida del inglés al español del CoMiSS.
Materiales y métodosSe realizó un estudio de adaptación y validación del cuestionario CoMiSS en dos fases: primero, la traducción de la escala CoMiSS del inglés al español y luego la evaluación de la confiabilidad interevaluador de la escala traducida. Secundariamente, se realizaron las pruebas de confiabilidad entre evaluadores en 32 pacientes pediátricos menores de 7 años que fueron atendidos por primera vez en la Clínica de Alergia Alimentaria del Hospital Italiano de Buenos Aires con sospecha de alergia a la proteína de leche de vaca y que no habían recibido ningún tratamiento entre mayo de 2018 y mayo de 2019.
ResultadosSe evaluaron 32 pacientes, de los cuales 14 fueron mujeres (45%) y la mediana de edad presentada fue de 3 meses (IQR 2-4). La mediana del resultado de la escala en la primera medición fue 7.0 (IQR 4.5-9.0) y en la segunda medición fue 5.0 (IQR 4.0-8.0). El coeficiente de correlación intraclase final de la escala fue 0.80 (IC 95% 0.63-0.9).
ConclusiónLa traducción al espa˜nol del CoMiSS mostró resultados homologables a su version original y una excelente fiabilidad entre los evaluadores. El beneficio de utilizar esta sencilla y poco conocida herramienta radica en ser una estrategia no invasiva, rápida, confiable y de fácil implementación.
Cow’s milk proteins are among the first antigens the infant comes into contact with; they commonly are the source of the first non-homologous antigen the child receives in significant quantities.
Cow’s milk allergy can be defined as a reproducible adverse reaction of an immunologic nature, induced by cow’s milk proteins. It can be classified as IgE-mediated, with immediate appearance after ingestion; non-IgE-mediated, with symptoms of delayed onset; or as mixed, when both types are present.1–3
The prevalence of cow’s milk protein allergy (CMPA) during the first year of life varies from 1.8 to 7.5% in different case series.4 That wide range of prevalence is due to differences in diagnostic criteria, as well as to genetic and environmental factors.1 However, overdiagnosis is also a factor, given that food allergy perceived and reported by the patient could be up to 10-times higher than its confirmation through the pertinent tests.2
A thorough clinical history that includes a family history of atopy and a detailed physical examination is essential for making the correct diagnosis. In addition, the majority of affected children have one or more symptoms that involve one or more organs or systems, primarily the gastrointestinal tract or the skin.5,6 Approximately 50-70% of patients present with cutaneous symptoms, 50-60% with gastrointestinal symptoms, and close to 20-30% with respiratory symptoms.7 Regarding gastrointestinal symptoms, blood-streaked stools are alarming for the parents, but recent evidence suggests that it is a benign entity and a self-limited phenomenon, mostly occurring in exclusively breastfed infants.
A study was conducted in 2014, in which clinicians with experience in the management of children with gastrointestinal problems and/or atopic diseases attended a workshop to review the literature and determine the usefulness of a clinical scoring system derived from symptoms associated with cow’s milk protein ingestion. The scoring of general symptoms, dermatologic symptoms, gastrointestinal symptoms, and respiratory symptoms was developed as a tool to be used when CMPA is suspected, called the Cow’s Milk-related Symptom Score (CoMiSS). It can also be used to evaluate and quantify symptom progression during therapeutic interventions, but it does not diagnose CMPA or replace the customary diagnostic methods.8,9 The CoMiSS provides the primary care physician with a simple, rapid, and easy-to-use instrument that is an awareness tool for symptoms related to CMPA.8
Elimination diets and food challenges are the gold standard for diagnosing CMPA in breast-fed or formula-fed infants. The CoMiSS questionnaire does not make the diagnosis, but rather guides the physician toward making it.
The aim of the present work was to translate the CoMiSS from English to Spanish and culturally adapt and validate the resulting Spanish version, to provide a tool for Spanish-speaking physicians.
MethodologyAn adaptation and validation study on the CoMiSS questionnaire was carried out. The protocol was approved (No. 3836) by the Research Protocol Ethics Committee (CEPI, the Spanish acronym) of the Hospital Italiano de Buenos Aires. It was also registered at the Centralized Register of Research Projects of the General Administration of Teaching and Research of the City Government of Buenos Aires, as stipulated by provision 139/DGDOIN/14 Annex 1. Authorization by the father, mother, or guardians of the patient was requested through verbal informed consent to conduct the study and was registered accordingly in the clinical history.
The study was carried out in two phases: First, the CoMiSS was translated from English to Spanish, after which interrater reliability of the translated score was assessed. The study was conducted at the outpatient consultation offices of the Food Allergy Clinic of the Pediatric Gastroenterology Service of the Hospital Italiano de Buenos Aires, which provides interdisciplinary care in gastroenterology, allergy and immunology, and nutrition.
Phase 1: Translation and linguistic equivalence of the scoreThe CoMiSS was translated from the original English into Spanish and culturally adapted, based on the ISPOR18 norms. Linguistic equivalence was achieved through a series of stages.
Before the present study was begun, Dr. Yvan Vandenplas, the author of the original version of the CoMiSS, was contacted and he authorized the use of the score for this study.
For the initial translation, two authors (one from Argentina and the other from the United States) fluent in the two languages, translated the score from English to Spanish, and compared their versions. For the final agreement, a third translator (from Argentina) resolved the differences between the original and the two Spanish translations. The resulting Spanish version was then retro-translated into English by a bilingual physician (Table 1).
The Cow’s Milk-related Symptom Score for cow’s milk protein allergy.
| Symptom | Score | |||
|---|---|---|---|---|
| Crying | 0 | < 1 hour/day | ||
| 1 | 1 to 1.5 hours/day | |||
| 2 | 1.5 to 2 hours/day | |||
| 3 | 2 to 3 hours/day | |||
| 4 | 3 to 4 hours/day | |||
| 5 | 4 to 5 hours/day | |||
| 6 | > 5 hours/day | |||
| Regurgitation | 0 | 0 to 2 episodes/day | ||
| 1 | > 3 < 5 episodes of small volume | |||
| 2 | > 5 episodes with a volume greater than 1 coffee spoon | |||
| 3 | > 5 episodes with a volume ± half of the feeds, in fewer than half of the daily feeds | |||
| 4 | Continuous regurgitation of small volumes > 30 minutes after feeding | |||
| 5 | Regurgitation of half of the feed, in at least half of the feeds | |||
| 6 | Regurgitation of the complete feed after each feeding | |||
| Stools (Bristol stool scale) | 4 | Type 1 and 2 (hard stools) | ||
| 0 | Type 3 and 4 (normal stools) | |||
| 2 | Type 5 (soft stools) | |||
| 4 | Type 6 (liquid stools, not related to infection) | |||
| 6 | Type 7 (watery stools) | |||
| Cutaneous symptoms | 0 to 6 | Atopic eczema | Head-neck-trunk | Arms-hands-legs-feet |
| Absent | 0 | 0 | ||
| mild | 1 | 1 | ||
| Moderate | 2 | 2 | ||
| Severe | 3 | 3 | ||
| 0 to 6 | Urticaria | No | Yes | |
| 0 | 1 | |||
| Respiratory symptoms | 0 | No symptoms | ||
| 1 | Slight symptoms | |||
| 2 | Mild symptoms | |||
| 3 | Severe symptoms | |||
The research group reviewed the quality of the retro-translation, as well as the harmonization of the conceptual discrepancies of the items. A cognitive revision was carried out that evaluated comprehension through the application of questionnaires to 6 members of the Food Allergy Clinic’s team that included physicians and nutritionists. This was done to determine the correct understanding of the score, review its drafting, grammar and spelling, and to determine any difficulties arising upon the application of the translated score. Lastly, the grammatical and typographic errors were corrected.
Phase 2: Interrater reliability of the Spanish version of the Cow’s Milk-related Symptom ScoreThe interrater reliability tests were performed on 32 pediatric patients under 7 years of age that were treated for the first time as pediatric gastroenterology outpatients at the Food Allergy Clinic of the Hospital Italiano de Buenos Aires, within the time frame of May 2018 and May 2019. The patients were suspected of presenting with CMPA and had not received any kind of treatment prior to answering the questionnaire.
The Spanish version of the CoMiSS was administered by two different physicians to the same patient in two periods separated by an interval of 2 to 7 days. We felt that no substantial symptom changes, as a consequence of the indication to exclude cow’s milk proteins after the first questionnaire was applied, would occur during said interval. The evaluations were carried out consecutively and independently to prevent bias. The healthcare professionals involved in administering the scores in the second period were blinded to the results of the scores in the first period.
Statistical analysisThe numerical data were summarized as mean and standard deviation (SD) or median and interquartile range (IQR), according to the Kolmogorov-Smirnov normality tests and their graphs. The categorical data were expressed as relative frequencies and percentages. The CoMiSS results range from 1 to 33 points, according to the symptoms. That numerical value was analyzed quantitatively. A correlation analysis was performed between the final result of the score in the two periods evaluated through the Spearman’s test, and the intraclass correlation coefficient was utilized to measure the interobserver reliability. An intraclass correlation below 0.4 was considered poor, between 0.41-0.60 moderate, between 0.61-0.80 substantial, and above 0.81 almost perfect. The information was analyzed with the R version 3.5.1 statistics program.
Ethical considerationsParticipation in the present study was voluntary and certified by the process of verbal informed consent. The study was conducted according to current national and international regulations: The Declaration of Helsinki of the World Medical Association and the ICH E6 Good Clinical Practice Guidelines.
ResultsThirty-two pediatric patients under 7 years of age were evaluated. Fourteen of the patients were females (45%) and the median patient age was 3 months (IQR 2-4).
Table 2 shows the distribution of the items of the CoMiSS.
Distribution of each item of the scale.
| Item | First period score | Second period score |
|---|---|---|
| Crying | 0.0 (0.0-2.5) | 1.0 (0.0- 2.0) |
| Regurgitation | 1.0 (0.0-3.0) | 0.0 (0.0-2.0) |
| Stools (Bristol stool scale) | 4.0 (2.0- 4.0) | 4.0 (2.0- 4.0) |
| Cutaneous symptoms- Atopic eczema | 0.0 (0.0-0.5) | 0.0 (0.0-0.0) |
| Cutaneous symptoms - Urticaria | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
| Respiratory symptoms | 0.0 (0.0- 0.0) | 0.0 (0.0-0.0) |
Values are expressed as median and interquartile range (IQR).
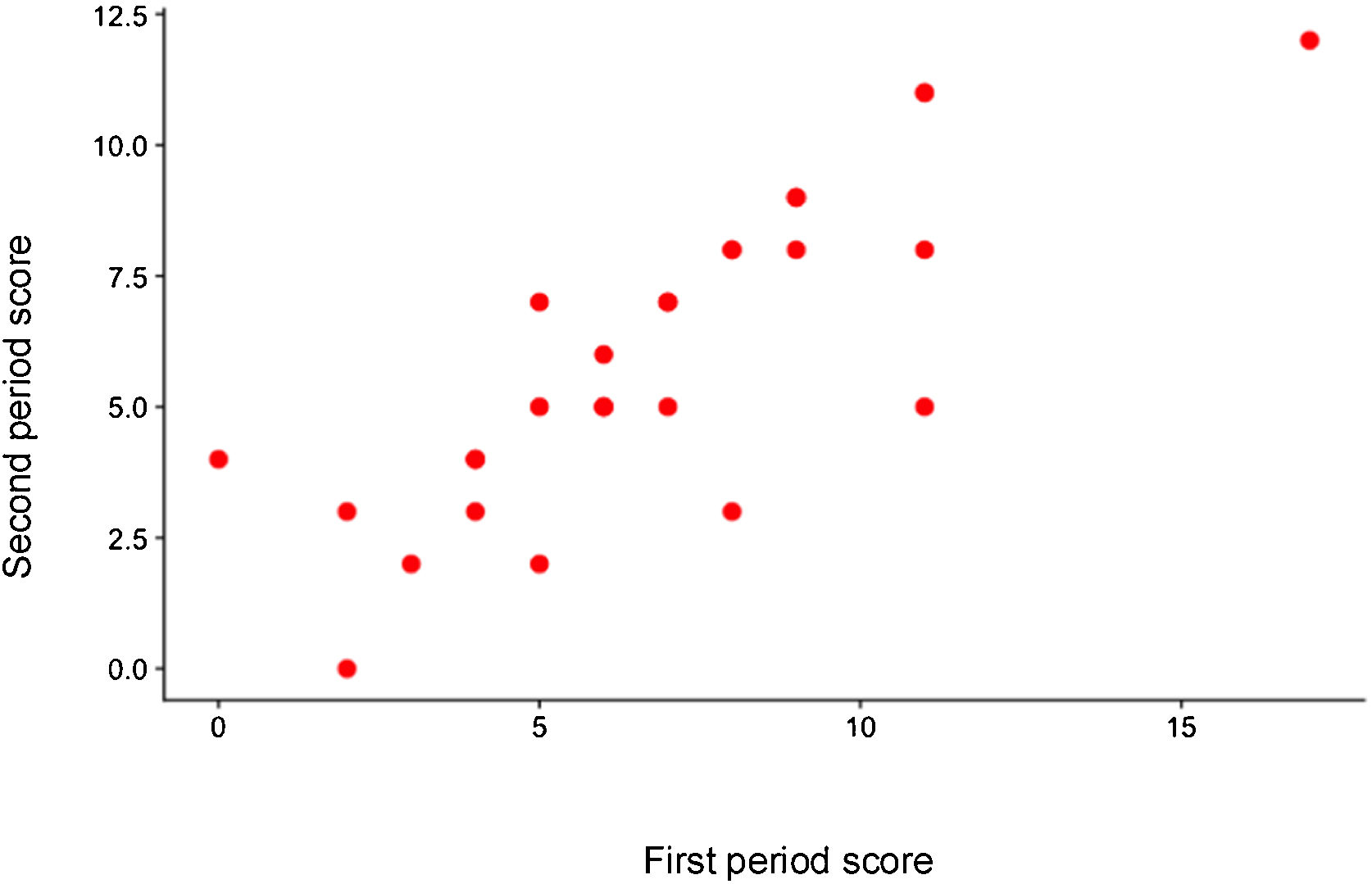
The median of the result of the first measurement of the score was 7.0 (IQR 4.5-9.0) and the median of the second measurement was 5.0 (IQR 4.0-8.0).
Fig. 1 shows the correlation between the final score result of the two measurements.
The intraclass correlation of the final score result was 0.80 (95% CI 0.63-0.9).
DiscussionThe present work describes the process of cultural adaptation of an instrument as an aid in the diagnosis of CMPA. The use of the Spanish version of the CoMiSS in our study demonstrated excellent interrater reliability. Studies have been conducted with the CoMiSS, but only in the original English version. No studies have been conducted with the Spanish version nor have any other authors translated it into Spanish.10,11
In that context, the importance of the CoMiSS lies in its usefulness as a guide for general pediatricians and primary care physicians that aids in the diagnosis and treatment monitoring of CMPA. Elimination diets and food challenges are the gold standard for diagnosing CMPA in breastfed or bottle-fed infants. The CoMiSS is not meant to replace the diagnostic methods but rather to guide the clinician toward the diagnosis.
A prospective study is needed to assess the cutoff value of the score in the Mexican environment. Said evaluation was not carried out in the present work because it would have required a larger sample size, to have statistically significant data.
ConclusionHaving a useful and reliable instrument to evaluate CMPA is important in our environment due to the impact of exclusion diets, that are often unnecessary, on growth and development. Given that there is no diagnostic test for CMPA, management and follow-up can be difficult for the clinician, especially with respect to symptom recognition and overestimation. The present study demonstrated the reliability of the CoMiSS, which is a simple and rapid tool that is easy to apply. Because it is a relatively new instrument, it is not widely known.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Ursino FG, Orsi M, Mehaudy R, Micheletti ME, Parisi C, Petriz N, et al. Adaptación transcultural y validación de la versión en español del Cow’s Milk-related Symptom Score (CoMiSS) para alergia a la proteína de leche de vaca. Rev Gastroenterol Méx. 2023;88:44–49.