Non-alcoholic fatty liver disease is the most prevalent hepatopathy, estimated at 30% in the general population. In the coming years, it will likely be the most common indication for liver transplantation and the most frequent cause of hepatocellular carcinoma. Current treatment for non-alcoholic fatty liver disease is based on dietary and exercise interventions that have been shown to be efficacious, even for reverting fibrosis. Unfortunately, compliance with general measures involving lifestyle modifications is very poor, making pharmacologic strategies a necessary option. At present, there are no treatments for non-alcoholic fatty liver disease approved by regulatory agencies, and the only ones with sufficient evidence and recommended by international societies are treatments with pioglitazone and vitamin E, which are not exempt from adverse effects. We review herein the current management of non-alcoholic fatty liver disease, including dietary and physical activity interventions, available treatments, equivocal therapies, emerging treatments, and treatments presently in clinical trials.
La enfermedad por hígado graso no alcohólico es la hepatopatía más prevalente, cercana al 30% de la población general, y se considera será en los siguientes años la indicación más común de trasplante hepático y la etiología más frecuente de carcinoma hepatocelular. El tratamiento actual de la enfermedad por hígado graso no alcohólico se debe basar en las medidas higiénico-dietéticas, que han demostrado ser eficaces incluso para revertir fibrosis. Desafortunadamente, el apego a las medidas generales es muy pobre, de ahí la necesidad de contar con estrategias farmacológicas. Hasta el momento no contamos con tratamientos aprobados por las agencias regulatorias para esta entidad, y los únicos tratamientos recomendados por las sociedades internacionales por tener suficiente evidencia son la pioglitazona y la vitamina E, que no están exentas de efectos adversos. En este artículo revisaremos el estado actual del tratamiento de la enfermedad por hígado graso no alcohólico, incluyendo las medidas higiénico-dietéticas, tratamientos disponibles, fármacos equívocos, tratamientos emergentes, y aquellos que actualmente se encuentran en ensayos clínicos.
Nonalcoholic fatty liver disease (NAFLD) is defined as a lipid deposit in more than 5% of hepatocytes. It is currently the most common hepatopathy, with an estimated prevalence of 30%.1 NAFLD is composed of 2 phenotypes: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), the latter having a worse prognosis because of its greater risk for progressing to cirrhosis of the liver and for having a closer association with unfavorable outcomes, such as cardiovascular mortality. It is estimated that 20-25% of the patients with NAFLD have NASH, and of those, 20% will progress to cirrhosis of the liver.2 NAFLD is a spectrum, and patients can pass from having NAFL to NASH, and vice versa, and it is one of the main factors involved in weight changes.3 On average, progression from one stage of fibrosis to another in patients with NAFL takes 14 years, whereas it takes only 7 years in patients with NASH.4
Given the natural history of NAFLD, specific pharmacologic treatments for that pathology should center around NASH, and not NAFL, because there is a low probability of morbidity and mortality due to hepatopathy with the latter.5 At present, there is no treatment for NASH that has been approved by the regulatory agencies, but hygienic-dietary measures should be at the center of all therapeutic regimens, given their efficacy, even for improving fibrosis.6 However, as occurs in patients with diabetes or high blood pressure, those measures are not efficacious in the long term in an important percentage of cases due to poor patient adherence. Surgical measures have also been shown to be highly efficacious, but they are not a viable alternative in a disease with such a high prevalence.7 Therefore, pharmacologic treatment is and will continue to be at the core of management of those patients. In the present review, we focus on current and future pharmacologic treatments, but we also provide a brief discussion of the hygienic-dietary measures, given their great relevance.
Hygienic-dietary measuresHygienic-dietary measures are very important because they modify disease progression and are usually the basis of treatment of the comorbidities that tend to accompany NAFLD, such as the different components of metabolic syndrome. A limitation to analyzing the effect of diet and exercise on NAFLD is that they are usually accompanied by changes in body weight, making it difficult to interpret results. In addition, most studies focus on the effect on steatosis, but do not have biopsies to determine the effect on the NASH components (inflammation, ballooning) and fibrosis.
Weight reductionThe majority of studies whose aim is to demonstrate the effect of weight loss on NAFLD have a before-and-after design, along with its inherent limitations. A study on 30 patients with paired biopsies showed that a body weight loss greater than or equal to 7% was required for significant improvement in the NAFLD Activity Score (NAS),8 a scoring system used in pathology that assigns points based on the grades of steatosis, inflammation, and ballooning found in liver biopsy. In a prospective study of 261 patients with paired biopsy, a relation between weight loss results and histopathologic improvement was observed after 52 weeks of lifestyle change: in particular, the necessary weight loss of at least 7% for significant improvement on the NAS (reduction of ≥ 2 points) was corroborated. In relation to fibrosis, in that same study, upon losing ≥ 7% of body weight, the stage of fibrosis was stabilized in 50% of the patients and there was improvement/resolution in the other 50%. Upon losing ≥ 10% of body weight, improvement/resolution of fibrosis was achieved in 80%.6 From the practical perspective, weight loss of only ≥ 3% is required to reduce steatosis, but to achieve resolution of NASH (absence of ballooning), weight loss must be ≥ 7%, and to improve fibrosis it must be ≥ 10%.
DietAny type of diet that results in reduced body weight will have potentially beneficial effects, such as those observed in the weight reduction section. However, an attempt has been made to determine whether the composition of the diet is important in NAFLD, regardless of changes in weight. In a study with a cross-over design that included 12 patients, greater reduction in steatosis, determined through magnetic resonance spectroscopy (MRS), was achieved through the Mediterranean diet than through an isocaloric low-fat, high-carbohydrate diet, regardless of body weight.9 It is also known that the Mediterranean diet reduces the risk for cardiovascular events, making it an attractive alternative in that group of patients.10
ExerciseIt is especially difficult to discern the effect of exercise from that of weight loss, but there are well-designed studies that show a beneficial effect of physical activity, regardless of changes in body weight. Regarding aerobic exercise, a before-and-after study should be mentioned, in which there was a significant reduction in hepatic steatosis, determined through MRS, in 48 subjects divided into 4 different exercise routines (control, low volume-high intensity, high volume-low intensity, low volume-low intensity). The effect was unrelated to changes in body weight.11 Similar results are described in studies that have evaluated resistance exercise.12 It was concluded in a systematic review, that both types of exercise are efficacious in reducing steatosis, with an average protocol consisting of 40-45min of exercise, 3 times a week, for 12 weeks. Nevertheless, with respect to resistance exercise, energy consumption and the intensity of exercise were lower (3.5 metabolic equivalents, compared with 4.8 metabolic equivalents in aerobic exercise), signifying that patients with poor cardiorespiratory condition may tolerate resistance exercise more easily.13
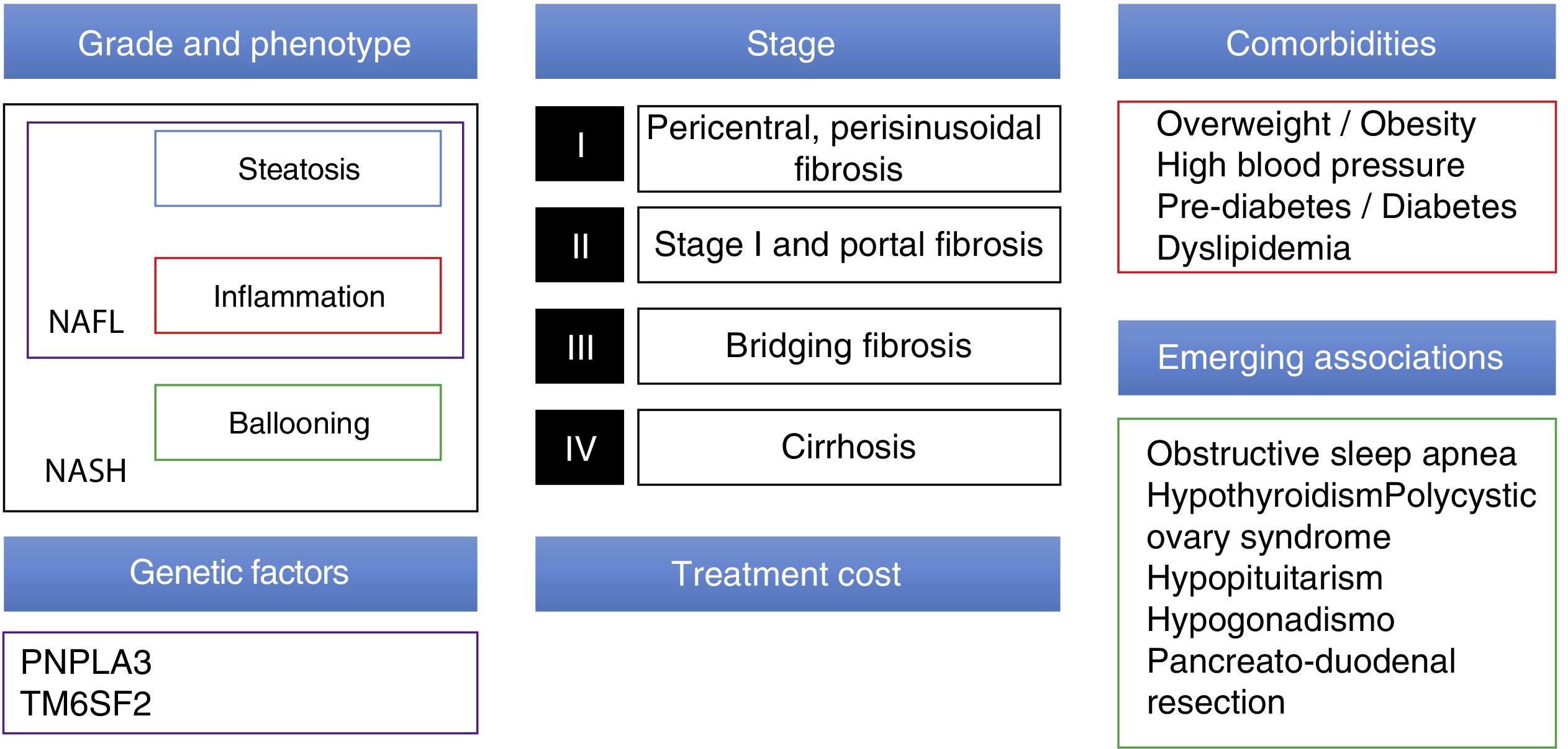
Pharmacologic treatmentCurrently, there are many drugs in development for NAFLD. Therapeutic strategy in the near future must take into account numerous factors, such as grade/stage of the disease (at present, liver biopsy continues to be the standard for determining those), comorbidities of each patient (mainly in relation to metabolic syndrome and emerging conditions associated with NAFLD), and genetic factors. In addition, it will be necessary to consider nonclinical factors, such as the price of the new medications and access to them (fig. 1).
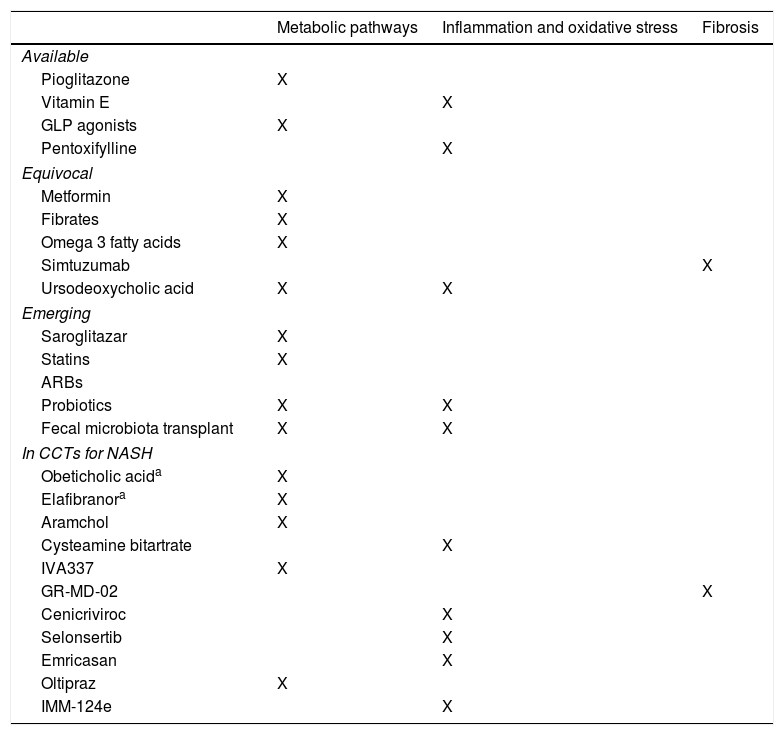
For their evaluation, we can divide treatment for NAFLD (Table 1):
Pharmacologic treatment classification.
| Metabolic pathways | Inflammation and oxidative stress | Fibrosis | |
|---|---|---|---|
| Available | |||
| Pioglitazone | X | ||
| Vitamin E | X | ||
| GLP agonists | X | ||
| Pentoxifylline | X | ||
| Equivocal | |||
| Metformin | X | ||
| Fibrates | X | ||
| Omega 3 fatty acids | X | ||
| Simtuzumab | X | ||
| Ursodeoxycholic acid | X | X | |
| Emerging | |||
| Saroglitazar | X | ||
| Statins | X | ||
| ARBs | |||
| Probiotics | X | X | |
| Fecal microbiota transplant | X | X | |
| In CCTs for NASH | |||
| Obeticholic acida | X | ||
| Elafibranora | X | ||
| Aramchol | X | ||
| Cysteamine bitartrate | X | ||
| IVA337 | X | ||
| GR-MD-02 | X | ||
| Cenicriviroc | X | ||
| Selonsertib | X | ||
| Emricasan | X | ||
| Oltipraz | X | ||
| IMM-124e | X | ||
ARBs: Angiotensin II receptor blockers; CCT: Controlled clinical trial; GLP: Glucagon-like peptide; NASH: Nonalcoholic steatohepatitis.
-Based on the pathophysiologic process in which they are involved
-Based on their availability and current evidence
The treatments described in the present review rely on that logic.
Available treatmentsAt present, the treatments recommended by the American Association for the Study of Liver Diseases (AASLD) are vitamin E and pioglitazone in patients with NASH,5,14 but they are not exempt from adverse effects. Pentoxifylline and glucagon-like peptide-1 (GLP-1) receptor agonists are also available, and although they are not recommended in the international guidelines, they can be used in individualized cases.
PioglitazoneA peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist, pioglitazone improves adipose tissue insulin sensitivity, promoting free fatty acid deposit in that tissue in a nonectopic manner (e.g., liver, pancreas). In addition, it increases adiponectin secretion by the adipose tissue, favoring hepatic free fatty acid beta-oxidation. It is also found in the Kupffer cells, where it exerts an anti-fibrotic and anti-inflammatory effect.15
In the PIVENS controlled clinical trial (CCT), 247 patients diagnosed with NASH, with no cirrhosis or diabetes, were randomized to pioglitazone (30mg qd), vitamin E (400U bid), or placebo, for 2 years. Pioglitazone improved insulin resistance, steatosis, inflammation, and resolution of NASH, but did not improve fibrosis, ballooning, or the primary outcome measure of the study (improvement in the NAS ≥ 2 points with no worsening of fibrosis).16 It was concluded in 3 meta-analyses that pioglitazone also improved fibrosis.17–19
Unfortunately, pioglitazone use is associated with an increase in body weight and therefore is not an attractive strategy in patients with metabolic syndrome. It has also been associated with reduced bone density,20 increased risk for hip fracture, and increased risk for bladder cancer, albeit the association with bladder cancer was not corroborated in a study on one million patients.15
Vitamin EVitamin E is an antioxidant, and the focus of its use is to improve the oxidative stress present in NASH. In the PIVENS trial, vitamin E had no effect on weight or insulin resistance and improved steatosis, inflammation, ballooning, and the main outcome measure. It did not improve fibrosis or resolve NASH.16 Given that the PIVENS study excluded patients with diabetes, the AASLD guidelines only recommend vitamin E in nondiabetic patients.5,14 However, vitamin E was reported to also be effective in patients with diabetes in a retrospective analysis that grouped together the results of the PIVENS study and the FLINT study,21 a CCT that compared obeticholic acid with placebo in patients with NASH, with or without diabetes.22 In an online meta-analysis that included obeticholic acid, pentoxifylline, vitamin E, and pioglitazone, the efficacy of vitamin E for improving steatosis and ballooning was corroborated, but no significant improvement in inflammation or fibrosis was found.19 The adverse effects that have been associated with vitamin E in observational studies are an increase in general mortality, prostate cancer, and hemorrhagic stroke.23,24 Vitamin E efficacy and safety in NASH after 96 weeks of treatment is currently being evaluated in a multicenter CCT (NCT02962297).
PentoxifyllinePentoxifylline is a phosphodiesterase inhibitor with hemorrheologic and antioxidant properties through reducing tumor necrosis factor alpha levels. Its evidence in NASH, albeit promising, is limited to studies with a small number of patients. Improvement in steatosis, inflammation, and fibrosis, but not in ballooning, was demonstrated in a meta-analysis,25 whereas a benefit in ballooning was described in an online meta-analysis.19 Pentoxifylline has a very acceptable safety profile and is economic. Unfortunately, there are no new CCTs registered, and the current evidence is too limited to be able to recommend its routine use.
Glucagon-like peptide 1 agonistsTreatment with GLP-1 agonists is approved for diabetes and obesity. They have been studied in NASH because, not only do they promote weight loss, they can also increase hepatic beta-oxidation, reduce appetite (the effect on leptin levels and gastric emptying delay), increase the secretion of insulin stimulated by glucose, and reduce glucagon secretion.26 A meta-analysis of 5 studies showed significant reduction in transaminase levels.27 A study with a before-and-after design on patients with MRS-determined steatosis that received liraglutide/exenatide showed improvement in steatosis as well as a gradient between the improvement obtained and baseline levels of steatosis.28 The more recently published LEAN study is a CCT that randomized 26 patients, the majority of whom were not diabetic, to placebo or liraglutide, and its primary outcome measure was histopathologic (resolution of NASH with no worsening of fibrosis). After one year, liraglutide was shown to be superior to placebo in the primary outcome measure (40 vs 10%). Unfortunately, the study did not have sufficient power to determine whether the effect was separate from the weight loss observed in the patients in the liraglutide arm of the study.29 In fact, the more recent Lira-NAFLD before-and-after study on 68 patients with poorly controlled diabetes, showed reduced hepatic steatosis, determined through MRS, only in the patients that had significant weight reduction. Those data question the hypothesis that GLP-1 antagonists have an additional effect other than the one derived from weight loss.30 A study on Asian patients that determined steatosis through magnetic resonance compared diet/exercise with liraglutide in 24 obese, nondiabetic patients. Both groups had significant and comparable weight reduction and reduced intrahepatic lipids, suggesting that the effect of liraglutide may be confounded by the weight loss it induces.31
Equivocal treatmentsWe reviewed treatments whose mechanisms of action would respond to the pathophysiology of NAFLD, but at present have not been shown to be efficacious. They include metformin, ursodeoxycholic acid, fibrates, omega 3 fatty acids, and the monoclonal antibody, simtuzumab.
MetforminThe expectations for metformin were big, given that it perfectly fits into the pathophysiology of NAFLD, by improving insulin resistance. However, there is no evidence to support its use in NASH. Although it reduces insulin resistance, it has not consistently been shown to be efficacious in improving liver function tests or the histopathologic components of NAFLD (steatosis, ballooning, inflammation, fibrosis).32
Of course, this does not mean that the patients with NAFLD in whom metformin is indicated (e.g., pre-diabetes or diabetes), and who are the majority, should not receive it. In fact, an association between metformin and reduced general mortality and the risk for hepatocellular carcinoma has been shown in observational studies on patients with cirrhosis of the liver, and thus it is important to consider metformin use in patients in whom it is clinically indicated.33
Ursodeoxycholic acidUrsodeoxycholic acid is a hydrophilic secondary bile acid utilized in the treatment of primary biliary cholangitis, cholelithiasis, and other forms of cholestasis. It has been studied in NAFLD because it has a potential anti-apoptotic and anti-oxidant effect, as well as having a weak interaction with the farnesoid X receptor (FXR). Nevertheless, there is good quality evidence in relation to NASH (a double-blind CCT with a satisfactory number of patients and histopathologic outcome), in which UDCA showed no benefit, and therefore it cannot be recommended.34,35
FibratesFibrates function as PPAR-alpha agonists, promoting hepatic and muscle beta-oxidation. Three non-controlled studies, 2 with histopathologic outcomes, had negative results for their use in NASH.36–38 A CCT that compared nicotinic acid, fenofibrate, and placebo in steatosis, determined through MRS, was also negative.39 Thus, there is no evidence supporting their use in NASH.
Polyunsaturated fatty acidsPolyunsaturated fatty acids are a plausible option that reduce triglyceride levels, increase adiponectin levels, improve endothelial dysfunction, and increase insulin sensitivity. The most widely studied are docosahexaenoic acid and eicosapentaenoic acid. There are at least 2 CCTs with histopathologic outcomes, one with 37 patients and the other with 41, in which there was no significant difference with respect to placebo.40,41 Their application as treatment of NASH cannot be sustained with the current evidence, but the AASLD guidelines emphasize considering them for the treatment of hypertriglyceridemia in patients with NAFLD.5,14
SimtuzumabSimtuzumab is a monoclonal antibody directed against lysyl oxidase-like 2 that participates in the interlacing of collagen, and its involvement in fibrosis progression has been observed. Studies on that molecule in patients with hepatitis C virus and with C virus and human immunodeficiency virus coinfection had negative results, and NAFLD was no exception. Two phase II studies of simtuzumab in patients with NASH and advanced fibrosis, and NASH and cirrhosis (NCT01672866, NCT01672879) were recently ended due to lack of efficacy.
Emerging treatmentsEmerging treatments are not indicated for NASH but could be beneficial for that pathology based on preliminary studies, albeit evidence is still insufficient.
Angiotensin II receptor blockersThe potential mechanisms through which angiotensin II receptor blockers could serve as treatment in NASH are: they increase adiponectin levels, they favor hepatic beta-oxidation, and they have an anti-inflammatory effect by reducing tumor necrosis factor-alpha. The results of a clinical trial with paired biopsies that randomized 54 patients with NASH to valsartan or telmisartan for 20 months showed significant improvement in fibrosis, the NAS, and transaminase levels in the telmisartan group. The lack of a control group limited the interpretation of those results.42 In another open clinical trial, 50 patients with NASH were randomized to telmisartan and hygienic-dietary measures or only to hygienic-dietary measures. The telmisartan group demonstrated histopathologic improvement in steatosis, inflammation, and ballooning, as well as in fibrosis.43 However, the study had no placebo group, the number of patients was small, and many patients were lost, limiting the conclusions.43
There is insufficient evidence for supporting telmisartan use as specific treatment of NASH. Nevertheless, if it is not contraindicated, it can be a first-line drug in patients with NASH that have high blood pressure.
StatinsApart from the fact that statins will be clinically indicated in many patients with NASH (e.g., dyslipidemia, cardiovascular risk), their pleiotropic properties suggest they would be a good treatment for that pathology. However, evidence is limited. A 2013 Cochrane collaboration study found only two eligible studies based on established inclusion criteria. Both analyses had a high risk for bias, and only one included paired biopsies for evaluating the histopathologic outcome. The conclusion reached was that further clinical trials were necessary.44 A before-and-after study with paired biopsies on 20 patients with NASH that received rosuvastatin for one year, reported resolution of NASH in 19 cases. The main limitation of that study was the lack of a control group.45 Statins are undoubtedly drugs that can have a place in NAFLD, but CCTs with adequate numbers of patients need to be conducted. It is important to point out that in the case of NAFLD, an elevated transaminase level, and even the presence of cirrhosis of the liver, are not contraindications for statin use. Therefore, they should be utilized in all patients in whom they are clinically indicated.
ProbioticsProbiotics are a feasible strategy, given the intimate relation between the gut microbiota, microbial products, and the liver through the portal system. In addition, they can regulate bacterial growth, and suppress bacterial pathogens. They also have immunomodulating properties and are capable of reinforcing the mucosal barrier. Patients with NAFLD have dysbiosis, mainly characterized by a decrease in diversity and a reduced Bacteroides to Prevotella ratio. In a CCT with paired biopsies in which 66 patients were randomized to placebo or Bifidobacterium longum, the results showed a significant reduction in steatosis and in the NAS. At present, the greatest limitations of studies on probiotics is the small number of patients, lack of histopathologic outcome evaluation, and the use of different strains and doses.46 More robust evidence is required to corroborate the beneficial effect of probiotics and to identify the ideal strain for patients with NAFLD.
Fecal microbiota transplantUnder the same principle that probiotics can be useful in NAFLD, fecal microbiota transplant is a plausible option. There are at least 4 clinical trials on its use in NASH (NCT02469272, NCT02868164, NCT02721264, NCT02496390).
Sodium glucose co-transporter type 2 inhibitorsSodium glucose co-transporter type 2 inhibitors are drugs that were recently approved for the treatment of diabetes mellitus type 2. Their mechanism of action consists of inhibiting glucose resorption at the level of the kidneys, favoring glycemic control. Preclinical studies showed that dapagliflozin, a medication with that mechanism of action, reduced the intrahepatic triglyceride content. A study is currently being conducted in which the effect of dapagliflozin on hepatic steatosis determined through MRS in diabetic patients is being evaluated (NCT02696941).
Drugs for non-alcoholic fatty liver disease found in controlled clinical trialsAt present there are several CCTs evaluating new drugs in NASH. We will review some that are phase III studies, and then others that are phase II.
Obeticholic acidObeticholic acid is a semi-synthetic bile acid that is a chenodeoxycholic analog and an FXR agonist. It is currently approved in patients with primary biliary cholangitis that are non-responders to or do not tolerate ursodeoxycholic acid. It stimulates FXR in the distal ileum, with the consequent secretion of fibroblast growth factor 19 (FGF-19) into the portal system, which takes it to the liver, where it reduces bile acid production, stimulates beta-oxidation, and decreases lipogenesis and gluconeogenesis. In the FLINT study, 282 patients with NASH were randomized to obeticholic acid or placebo for 72 weeks. The main outcome measure was improvement in the NAS of at least 2 points, with no worsening of fibrosis. Obeticholic acid was significantly superior to placebo regarding the primary outcome measure and it also improved steatosis, inflammation, ballooning, and even fibrosis. However, one of the most common adverse effects was pruritus, and an increase in low density cholesterol and insulin resistance were observed, which are worrisome aspects in patients with metabolic syndrome.21 Obeticholic acid is presently in a phase III study (REGENERATE, NCT02548351) that will study patients with NASH and significant fibrosis. A phase II study (CONTROL, NCT02633956) is also open, whose aim is to evaluate the impact of the joint administration of different doses of obeticholic acid and atorvastatin on patient lipid profiles.
ElafibranorElafibranor is a dual PPAR agonist (delta and alpha) that stimulates hepatic and muscle beta-oxidation and at the hepatic level it reduces gluconeogenesis and triglyceride production and has an anti-inflammatory effect, promoting a favorable metabolic profile, unlike the abovementioned effects observed in the FLINT study. In a phase IIa CCT on patients with NASH (GOLDEN 505), the primary aim (resolution of some of the NASH components, with no worsening of fibrosis) was not met, but histopathologic inflammation was improved, along with liver function tests, insulin resistance, and the lipid profile. However, it was associated with a transitory increase in creatinine.47 Elafibranor is currently being evaluated in a phase III study (RESOLVE-IT, NCT02704403) on patients with NASH and fibrosis.
Other peroxisome proliferator-activated receptor gamma agonistsIVA337IVA337 is a pan-PPAR agonist. Patients are presently being recruited for the NATIVE study, a phase IIb CCT that will evaluate two different doses of that compound, compared against placebo, with a histopathologic outcome (NCT03008070).
SaroglitazarSaroglitazar is a dual PPAR agonist (gamma and alpha) that was originally approved for the treatment of dyslipidemia. At present, patients with NASH are being recruited for the EVIDENCES II study, in which 3 different doses of the compound will been evaluated. Outcome will be histopathologic (NCT03061721).
AramcholAramchol is a conjugate of arachidonic acid and cholic acid that inhibits the enzyme, stearoyl coenzyme A desaturase 1 (SCD1), causing reduced fatty acid synthesis, reduced triglyceride reserve, and increased intracellular elimination of cholesterol into ApoA1 particles. It reduced the grade of steatosis determined through MRS in a phase II study.48 It is currently in a phase IIb study (ARREST, NCT02279524) that will compare doses (400mg and 600mg). The primary outcome measure will be evaluated through nuclear magnetic resonance, as well as through paired biopsies.
Others-Cysteamine bitartrate is a glutathione precursor with antioxidant capacity that has primarily been studied in children with NAFLD, but no histologic improvement was shown in a CCT.49
-GR-MD-02 is a galectin-3 inhibitor that modulates the binding of macrophages to residual galactose. It improved serum markers of fibrosis in a phase I study and presently is in a phase IIa study on patients with cirrhosis of the liver due to NASH, but patient recruitment has not yet begun (NCT02462967).
-Cenicriviroc is an antiretroviral agent that inhibits the CCR2 and CCR5 chemokine receptors. A beneficial effect of the compound was observed in the preliminary results of a phase IIb study (CENTAUR, NCT02217475). A total of 289 patients with NASH were randomized to cenicriviroc or placebo. At one year of follow-up there was no significant improvement in the primary outcome measure (improvement of at least 2 points on the NAS, with no worsening of fibrosis), but there was improvement in the secondary outcome measure of improvement in fibrosis, with no worsening of NASH. The results of the follow-up at 2 years are expected.
-Selonsertib is an apoptosis signal-regulating kinase 1 (ASK-1) inhibitor that is involved in the liver fibrosis pathways. In a phase IIa study on patients with NASH and significant fibrosis, 72 patients were randomized to two different doses of selonsertib (18mg and 6mg) or placebo, with or without simtuzumab. The preliminary results showed significant improvement in fibrosis with a dose-response gradient. Publication of the definitive results of that study is pending (NCT02466516).
-Emricasan is a caspase inhibitor that is in a phase IIb study (ENCORE-NF, NCT02686762) that will include paired biopsies. It previously produced improvement in transaminases in a phase IIa study.
-Oltipraz is a liver X receptor-alpha (LXR-alpha) inhibitor that is capable of inhibiting the synthesis of intrahepatic lipids. In a controlled CCT with placebo that evaluated 2 different doses (60mg and 120mg) and included 64 patients, oltipraz was associated with reduced steatosis determined through MRS.50
-IMM-124e is a compound of anti-lipopolysaccharide antibodies and adjuvants, mainly glycosphingolipids, whose theoretic basis is to modify the microbiota, as well as the innate immune response at the intestinal level. In an open phase I/II study on 10 patients with NASH and prediabetes/diabetes, the oral administration of that compound for 30 days improved insulin resistance, liver function tests, and adiponectin levels. It is currently in phase a phase II study that will compare the effect of 2 different doses of IMM-124e with placebo in hepatic steatosis detected through magnetic resonance (NCT02316717).
ConclusionsHygienic-dietary measures have been shown to be efficacious for treating NAFLD and should be at the core of any therapeutic strategy. Current pharmacologic treatments are limited, and they are only recommended in patients with NASH. In addition, they are not free from adverse effects. Therefore, each case must be individualized, and the risks/benefits must be explained to the patients before they are prescribed. It is important to always keep in mind that the main cause of mortality in those patients is cardiovascular, and so management must be integrated, treating each of the components of metabolic syndrome. There are numerous CCTs on new drugs for the treatment of NASH that will result in novel alternatives in the near future. It is possible that more than one drug will be needed for the satisfactory treatment of NASH, and for each therapeutic regimen, the phenotype of the disease and its stage, the comorbidities of each patient, the presence of emerging conditions, and genotype, as well as the important aspect of the economy of every patient, must be contemplated.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe author received a grant from Gilead Canada for completing his hepatology training.
Please cite this article as: Moctezuma-Velázquez C. Tratamiento actual de la enfermedad por hígado graso no alcohólico. Revista de Gastroenterología de México. 2018;83:125–133.