The first clinical guidelines on hepatic encephalopathy were published in 2009. Almost 14 years since that first publication, numerous advances in the field of diagnosis, treatment, and special condition care have been made. Therefore, as an initiative of the Asociación Mexicana de Gastroenterología A.C., we present a current view of those aspects. The manuscript described herein was formulated by 24 experts that participated in six working groups, analyzing, discussing, and summarizing the following topics: Definition of hepatic encephalopathy; recommended classifications; epidemiologic panorama, worldwide and in Mexico; diagnostic tools; conditions that merit a differential diagnosis; treatment; and primary and secondary prophylaxis. Likewise, these guidelines emphasize the management of certain special conditions, such as hepatic encephalopathy in acute liver failure and acute-on-chronic liver failure, as well as specific care in patients with hepatic encephalopathy, such as the use of medications and types of sedation, describing those that are permitted or recommended, and those that are not.
La primera guía clínica sobre encefalopatía hepática se publicó en el año 2009, a casi catorce años de esta primera publicación, numerosos avances en el campo del diagnóstico, tratamiento y cuidados en condiciones especiales, han ocurrido; por ello, a iniciativa de la Asociación Mexicana de Gastroenterología A.C presentamos este manuscrito elaborado por 24 expertos que han trabajado en seis mesas de trabajo analizando, discutiendo y resumiendo los siguientes tópicos: Definición de encefalopatía hepática, clasificaciones que se recomiendan utilizar, panorama epidemiológico mundial y en México, herramientas para el diagnóstico, condiciones que ameritan hacer un diagnóstico diferencial, tratamiento y profilaxis primaria y secundaria. Así mismo, esta guía hace énfasis en el manejo de ciertas condiciones especiales como encefalopatía hepática en insuficiencia hepática aguda y en falla hepática aguda sobre crónica y cuidados específicos en pacientes con encefalopatía hepática como medicamentos que se deben evitar, por ejemplo, uso de analgésicos permitidos y no permitidos, y en caso de sedación para procedimientos cuales se permiten o sugieren y cuáles no.
Hepatic encephalopathy (HE) is a complex disorder with different degrees of severity that negatively impacts patient quality of life and is associated with a significant healthcare burden, not only for patients, but also their primary caregivers. In addition, the prevalence of cirrhosis, the most common risk factor for having HE, has been on a constant and gradual rise in recent years. As a result, an uptick in the clinical and healthcare burdens related to this complication is expected in the coming years that will greatly affect the healthcare systems.1
In Mexico in 2009, the “Clinical guidelines on HE diagnosis and treatment” were published in 3 parts, divided into (1) generalities,2 (2) pathophysiology and diagnosis,3 and (3) treatment and future perspectives.4 After nearly 14 years, an update is now indispensable, given the scientific advances that have come about over that period of time, resulting in numerous new developments in diagnostic and therapeutic tools that are now essential for daily clinical practice. Thus, the Asociación Mexicana de Gastroenterología A.C. (AMG) has taken the initiative of formulating updated clinical guidelines on HE that integrate the new concepts on epidemiology, diagnosis, treatment, and follow-up of patients presenting with the disease. Table 1 summarizes the recommendations made in these clinical guidelines.
| Recommendation | Quality of evidence | Strength of recommendation |
|---|---|---|
| Definitions and classifications | ||
| 1. HE is defined as brain dysfunction caused by liver failure and/or portosystemic shunting; it is manifested by a wide spectrum of neurologic or psychiatric abnormalities, ranging from subclinical alterations to coma. | NA | NA |
| 2. MHE is neurocognitive and motor dysfunction that cannot be diagnosed in a standard physical examination and can only be detected through psychometric and/or neurophysiologic tests. | NA | NA |
| 3. HE can be classified based on different criteria: 1) etiology, 2) manifestation severity, 3) presentation over time, 4) precipitating factors. | NA | NA |
| 4. We suggest classifying HE according to severity into covert HE (MHE or grade I HE [according to the WHC]) and overt HE (starting from WHC grade II). | NA | NA |
| Pathophysiologic mechanisms, clinical manifestations, and precipitating factors | ||
| 5. The pathophysiology of HE is multifactorial. Hyperammonemia (a determining factor in its pathogenesis) leads to brain dysfunction associated with neuropsychiatric and neurologic complications. | NA | NA |
| 6. Systemic inflammation, oxidative stress, alterations in intestinal permeability and gut microbiota composition, sarcopenia, zinc deficiency, and neurogliovascular unit dysfunction are other factors that contribute to the presence and severity of HE. | NA | NA |
| 7. HE is characterized by neurologic, neuropsychiatric, and musculoskeletal alterations, whose degree of clinical presentation is variable and heterogeneous. | NA | NA |
| Tools for the diagnosis of HE and the differential diagnosis | ||
| 8. The WHC grade the severity of clinically overt HE and guide therapeutic decision-making. | II-B | 1 |
| 9. Routine determination of ammonia in the diagnosis and follow-up of HE is not indicated. | II-C | 2 |
| 10. The PHES is considered accessible, sensitive, and cost-effective for diagnosing MHE, and is also standardized. | II-B | 1 |
| 11. CFF and the Stroop test are objective tests that can be useful for diagnosing MHE. | II-B | 1 |
| 12. In the differential diagnosis of the patient with cirrhosis and HE, laboratory (serum electrolytes, infection markers, kidney function, liver function, acid-base balance, and peritoneal fluid and cerebrospinal fluid analyses), imaging (CT, head MRI), and microbiologic (cultures) studies are recommended. | II-B | 1 |
| Treatment and prophylaxis | ||
| 13. Sufficient caloric and protein intake must be ensured in patients with HE to prevent malnutrition and sarcopenia. | II-B | 1 |
| 14. Lactulose is the therapeutic strategy of choice for overt HE. | I-A | 1 |
| 15. Lactulose is useful as a secondary prophylaxis strategy, after a first bout of overt HE. | I-A | 1 |
| 16. Lactulose can be recommended as primary prophylaxis in patients with cirrhosis that present with high-risk factors for developing episodic overt HE. | I-A | 1 |
| 17. Lactulose can be recommended as treatment in patients with MHE. | I-A | 1 |
| 18. Rifaximin is useful as an adjuvant to lactulose in patients with overt HE and suboptimal clinical response and as an adjuvant in the context of secondary prophylaxis, particularly after a second bout of overt HE. | I-A | 1 |
| 19. L-ornithine L-aspartate is an effective therapeutic strategy in patients with overt HE, both as monotherapy and as a coadjuvant to lactulose and rifaximin. | I-A | 1 |
| 20. L-ornithine L-aspartate can be used as primary and secondary prophylaxis to prevent the development of overt HE in high-risk conditions. | I-A | 1 |
| 21. L-ornithine L-aspartate is a superior strategy to placebo for treating MHE and can be prescribed as an alternative to lactulose or rifaximin. | I-A | 1 |
| 22. Supplementation with branched-chain amino acids can improve the clinical manifestations of HE. | II-A | 2 |
| 23. Currently, there is insufficient evidence for recommending the use of zinc, L-carnitine, probiotics, or fecal microbiota transplantation. | II-A | 2 |
| 24. Polyethylene glycol has shown efficacy in the treatment for HE, but more evidence is needed for its recommendation. | I-A | 2 |
| Special conditions: HE in acute liver failure and acute-on-chronic liver failure | ||
| 25. The clinical appearance of HE is a criterion for the diagnosis of ALF. | II-B | 1 |
| 26. In the context of ALF, the exclusion of other causes of neurologic alteration is the recommended diagnostic approach. | II-B | 1 |
| 27. Patients with ALF that have serum ammonia levels > 200 µmol/l have a higher risk for developing cerebral edema and intracranial hypertension. | II-B | 1 |
| 28. Anti-cerebral edema measures should be contemplated in the management of HE in patients with severe ALF. | II-B | 1 |
| 29. The presence of HE in a patient with ACLF confers poor prognosis, increasing the risk for mortality in the short term and long term. | II-B | 1 |
| Additional specific care in patients with cirrhosis and hepatic encephalopathy | ||
| 30. The use of certain drugs in patients with cirrhosis can precipitate HE. | II-B | 1 |
ACLF: acute-on-chronic liver failure; ALF: acute liver failure; CFF: critical flicker frequency; CT: computed tomography; HE: hepatic encephalopathy; MHE: minimal hepatic encephalopathy; NA: not applicable; PHES: psychometric hepatic encephalopathy score; MRI: magnetic resonance imaging; Stroop test: semantic interference test; WHC: West Haven Criteria.
In March 2022, the executive board of the AMG, in coordination with the association’s scientific committee, presented the initiative of updating the Clinical Guidelines on HE, and it was approved. Two expert physicians were designated to act as the guideline coordinators and their functions were:
- 1
To carry out a thorough search of the literature on the Pubmed, Embase, Medline, Trip Database, Clinical Evidence, and Cochrane Library databases, to compile all current and relevant information. The search criteria included the terms: HE, minimal HE (MHE), definition, classifications, epidemiologic panorama in the world and in Mexico, pathophysiology, risk factors, clinical manifestations, diagnosis, differential diagnosis, treatment, primary and secondary prophylaxis. The special conditions of HE in acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) were included, as well as the specific care in patients with HE: medications to avoid, analgesics that can and cannot be used, and types of sedation that can be used in procedures and those that cannot.
- 2
To divide the panel of 24 experts, according to the participants’ areas of greater expertise, into one of the 6 distinct working groups that were responsible for formulating the different statements and recommendations, as well as for grading the evidence through the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Table 2).5
Table 2.Strength of recommendation and quality of evidence (modified GRADE).
Strength of recommendationStrong, in favor of the intervention: the quality of evidence influences the strength of recommendation, from which important positive results are derived, with respect to the patient or to costs.Weak, in favor of the intervention: variability in preferences and values or uncertainty. Little or low-quality evidence that shows benefit to the patient or requires high costs or resource use. Quality of evidenceI-A. Randomized, controlled, clinical trials II-A. Nonrandomized clinical trials II-B. Observational studies: cohort or case-control II-C. Observational studies: case series. Noncontrolled experimentsIII. Expert opinion Source: Manterola et al.5
Worldwide, the accumulated incidence of HE in cirrhotic patients at 1, 5, and 10 years varies from 0 to 21%, 5 to 25%, and 7 to 42%, respectively. Within 2 years after transjugular intrahepatic portosystemic shunt (TIPS) placement, the incidence of HE ranges from 20 to 55%. The prevalence of MHE varies from 20 to 80%, with respect to the grade of decompensation of the cirrhosis. In patients with cirrhosis, the main risk factors for developing overt HE include MHE, sarcopenia, hyponatremia, epilepsy, type 2 diabetes, elevated creatinine, elevated bilirubin, and hypoalbuminemia.1
Because overt HE is a manifestation of decompensated liver failure, it has a negative impact on survival. Cohort studies show that median survival in cirrhotic patients that present with overt HE is only a few months and they have a 2-times higher risk for death at one year of follow-up, compared with cirrhotic patients without HE.6,7
In Mexican patients with cirrhosis, the prevalence of MHE has been reported at 11.5%.8 According to an October 2018 press release, HE accounts for 10% of all hospital admissions at a tertiary care referral center in Mexico City.9
Definitions and classification- 1
HE is defined as a brain dysfunction caused by liver failure and/or portosystemic blood shunting; it is manifested by a wide spectrum of neurologic or psychiatric abnormalities that range from subclinical alterations to coma.
Panel level of agreement: In total agreement 100%
Brain dysfunction can occur under multiple conditions, with or without liver disease. Therefore, HE should not be defined by specific symptoms, but rather by the presence of cirrhosis or ALF and the presence of hyperammonemia, the causal agent. Many other cofactors can act synergically with hyperammonemia, to produce or aggravate HE, but experts do not consider them to be exclusive causes of HE, on their own or in isolation, i.e., in the absence of hyperammonemia.10 Patients with portosystemic shunts (PSSs) and no liver disease can also present with hyperammonemia-associated brain dysfunction despite having a functional or normal liver11; those patients can also benefit from specific therapies that detoxify ammonia/ammonium.
- 2
MHE is a neurocognitive and motor dysfunction that is impossible to diagnose through a standard physical examination; it can only be detected through psychometric and/or neurophysiologic tests.
Panel level of agreement: In total agreement 100%
MHE is the mildest form of HE. It is relevant because it significantly influences quality of life, prognosis, and incidence of complications, in a negative manner. Its diagnosis requires a wide spectrum of psychometric and neurophysiologic tests discussed in these guidelines, in detail, further ahead. Treatment is based on the same principles as overt or clinical HE.12
- 3
HE can be classified based on different criteria: (1) etiology, (2) severity of manifestations, (3) presentation over time, and (4) precipitating factors.
Panel level of agreement: In total agreement 100%
According to the underlying liver disease, HE can be classified as11:
- •
Type A, resulting from ALF.
- •
Type B, resulting from a PSS.
- •
Type C, caused by the presence of cirrhosis.
With respect to its presentation over time, HE can be11:
- •
Episodic, which presents as a short event, generally triggered by infection, dehydration, or dietary transgression, at intervals of more than 6 months.
- •
Recurrent, which presents with episodes that occur more frequently, at intervals under 6 months.
- •
Persistent, which presents with altered behavioral patterns all the time, interspersed with relapses of overt HE that occur within days or weeks.
With respect to its precipitating factor, it is classified as11:
- •
Nonprecipitated, in which the triggering factor cannot be identified through an adequate clinical history or pertinent laboratory tests.
- •
Precipitated, in which the etiology is identified and must be treated adequately to improve the HE episode. The main precipitating factors are infections, gastrointestinal bleeding, diuretic overdose, insufficient fluid intake, altered electrolytes, constipation, and dietary transgression.
- 4
We suggest classifying HE according to its severity, as covert (MHE and grade I of the West Haven Criteria [WHC]) and overt (starting at grade II of the WHC).
Panel level of agreement: In total agreement 91%, in partial agreement 9%
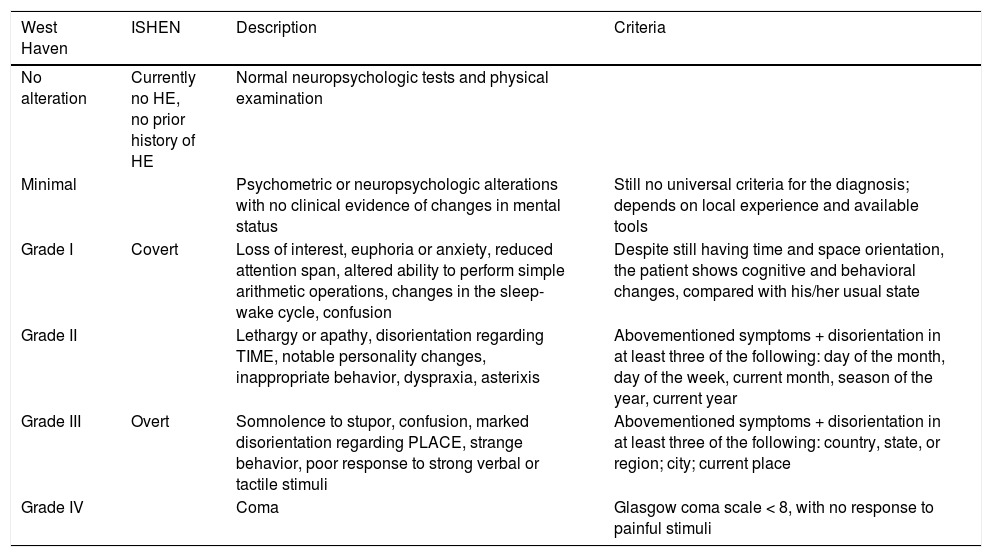
In general, the initial classification we suggest applying distinguishes between patients with MHE and those with marked signs or symptoms, called “overt” HE. Overt HE is graded from II to IV, based on the WHC13 (Table 3).
Covert and overt HE and its integration into the West Haven Criteria.
| West Haven | ISHEN | Description | Criteria |
|---|---|---|---|
| No alteration | Currently no HE, no prior history of HE | Normal neuropsychologic tests and physical examination | |
| Minimal | Psychometric or neuropsychologic alterations with no clinical evidence of changes in mental status | Still no universal criteria for the diagnosis; depends on local experience and available tools | |
| Grade I | Covert | Loss of interest, euphoria or anxiety, reduced attention span, altered ability to perform simple arithmetic operations, changes in the sleep-wake cycle, confusion | Despite still having time and space orientation, the patient shows cognitive and behavioral changes, compared with his/her usual state |
| Grade II | Lethargy or apathy, disorientation regarding TIME, notable personality changes, inappropriate behavior, dyspraxia, asterixis | Abovementioned symptoms + disorientation in at least three of the following: day of the month, day of the week, current month, season of the year, current year | |
| Grade III | Overt | Somnolence to stupor, confusion, marked disorientation regarding PLACE, strange behavior, poor response to strong verbal or tactile stimuli | Abovementioned symptoms + disorientation in at least three of the following: country, state, or region; city; current place |
| Grade IV | Coma | Glasgow coma scale < 8, with no response to painful stimuli |
HE: hepatic encephalopathy.
- 5
The pathophysiology of HE is multifactorial. Hyperammonemia (a determining factor in its pathogenesis) leads to brain dysfunction associated with neuropsychiatric and neurologic complications.
Panel level of agreement: In total agreement 91%, in partial agreement 9%
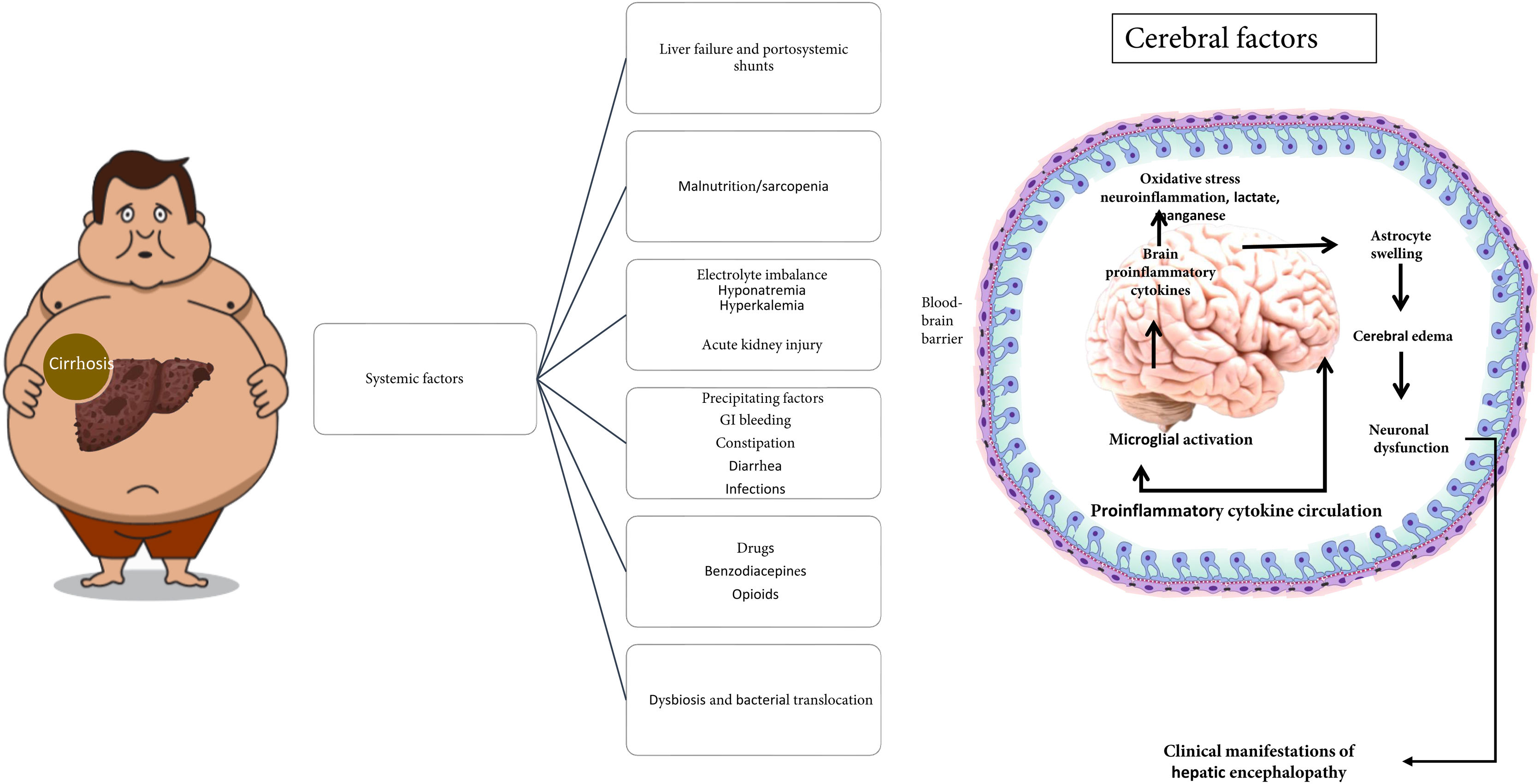
The main precipitating factors that commonly contribute to the development of HE in patients with cirrhosis are shown in Fig. 1.
Elevated levels of ammonia, proinflammatory cytokines, and the accumulation of false neurotransmitters, among others, are factors involved in the development of HE. Hyperammonemia is considered the main etiologic factor. Ammonia is the product of the metabolism of nitrogenated substances, derived mostly from proteins, and its primary elimination pathway is via the urea cycle in hepatocytes. Another metabolic pathway involves the enzyme, glutamine synthetase, present in several tissues, which converts ammonia with glutamate into glutamine, achieving its renal secretion through the synthesis of urea from glutamine. On the other hand, at the level of the brain, neurons metabolize the glutamine that comes from astrocytes into glutamate (a neurotransmitter), which is then converted back into glutamine by astrocytes, to be reused.14
In chronic liver disease, urea and glutamine metabolism are altered. Hepatocytes have a reduced capacity to eliminate ammonia through the urea cycle, leading to hyperammonemia that stimulates glutamine synthesis in extrahepatic tissues (muscle, brain, heart, and lungs). Glutamine is degraded into ammonia in the intestine and kidneys, achieving its partial excretion.15 At the level of the brain, hyperammonemia conditions an increase in glutamine synthetase activity, and by exceeding the metabolic capacity of the astrocyte, conditions intracellular osmolarity alterations with later edema, cytolysis, and proinflammatory cytokine release. Those cell changes inhibit glutamine synthesis and glutamate receptor expression, reducing its neuronal uptake and triggering an imbalance in the glutamate-glutamine cycle with the consequent increase in brain blood flow, edema, and intracranial pressure, leading to the neuropsychiatric and neurologic clinical manifestations seen in HE.16
- 6
Systemic inflammation, oxidative stress, alterations in intestinal permeability and the gut microbiota, sarcopenia, zinc deficiency, and neurogliovascular unit dysfunction are other factors that contribute to the presence and severity of HE.
Panel level of agreement: In total agreement 91%, in partial agreement 9%
The pathophysiology of HE is complex. Hyperammonemia is the cardinal detonator of an additional series of alterations that perpetuate and aggravate HE, such as oxidative stress, systemic inflammation, neurogliovascular unit dysfunction and microglia, increase in the permeability of the blood-brain barrier, astrocytic edema, and hyponatremia.17,18
Dysbiosis also plays a role in HE. Compared with healthy subjects, changes in the gut microbiota in patients with cirrhosis and HE have been seen; for example, the translocation of Stenotrophomonas pavanii and Methylobacterium extorquens into peripheral blood increases the risk for HE.17
Sarcopenia is an important complication in cirrhosis and develops in nearly 50–70% of cirrhotics;19 it also increases the risk for HE in those patients.20 Dasarathy et al. have shown that elevated levels of serum ammonia and myostatin contribute to sarcopenia in cirrhosis.21 And so a vicious circle is formed when a cirrhotic patient develops sarcopenia, given that there is a decrease in the elimination of ammonia in sarcopenic patients, which in turn, leads to a higher risk for developing hyperammonemia and HE.22 The detoxification of ammonia through the administration of L-ornithine L-aspartate (LOLA) in a murine model of sarcopenia and steatohepatitis significantly improved mean body mass, hand grip strength, and mean diameter of muscle fiber, in the group supplemented with LOLA vs. the placebo group, suggesting that LOLA may be efficacious in improving sarcopenia.23
Zinc, an active coenzyme center for more than 300 types of enzymes that mediate cell functions, is an essential trace element. The prevalence of zinc deficiency in patients with advanced liver disease due to increased excretion in urine and reduced absorption can reach 80%. Zinc plays an important role in ammonia metabolism and its detoxification in the liver.24,25 Other factors that contribute to the development of HE are lactate and excess manganese.26
- 7
HE is characterized by neurologic, neuropsychiatric, and musculoskeletal alterations, whose clinical presentation is variable and heterogeneous.
Panel level of agreement: In total agreement 83%, in partial agreement 5%, in partial disagreement 12%
The diagnosis of HE is principally clinical, and for its staging, the clinical characteristics are grouped, based on the WHC25 (Table 3).
Covert HE, which encompasses MHE (detectable only through altered neuropsychometric tests that are described in these guidelines further ahead) plus grade I HE, is typically associated with frequent falls, altered motor skills (e.g., in driving), the presence of fatigue, disinterest, distraction with fluctuating attention span and response inhibition. The cognitive capacities of memory, fine motor skills, attention, and calculation ability are affected in patients.25,27
The most characteristic sign of the neuromuscular manifestations of HE of unknown pathogeny is asterixis or flapping tremor. It progresses to stupor that can require a very painful stimulus to get a sustained response. In that stage, patients have tachypnea, with loss of habitual respiratory control, often leading to respiratory problems of alkalosis. In noncomatose patients with HE, motor system anomalies can be seen, such as hypertonia, hyperreflexia, and positive Babinski sign. Contrastingly, in coma, deep tendon reflexes can decrease, and even disappear.28
In patients with ALF, the development of HE is a rapidly progressing process of cognitive decline and abrupt confusion, with limb rigidity and resistance to passive movements. Extensor posture suggests structural brain damage, characteristically occurring in WHC grade IV HE and can be completely reversible after hyperammonemia correction. The pupils of the patients with early HE are normal, with preserved responses, but in grade III or IV HE, the pupillary reaction becomes sluggish and is accompanied by a periodic lateral or dysconjugate gaze, or fixed gaze. Involuntary muscle movements or spasms in the limbs can also occur and may merge with myoclonus.11
Tools for the diagnosis of hepatic encephalopathy and the differential diagnosis- 8
The WHC are used for grading the severity of clinically overt HE and guide therapeutic decision-making.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 96%, in partial agreement 4%
The WHC have traditionally been used for diagnosing and grading the severity of clinically overt HE, but they are a subjective tool, with limited interobserver reliability, especially for a grade I classification; they are more useful in grade II HE, and higher. The WHC should be complemented with the Glasgow coma scale, but nevertheless they continue to be useful and are valuable in clinical practice due to their simplicity and utility in guiding therapeutic decision-making. Ideally, WHC grades III and IV should be treated in the intensive care unit, particularly due to the potential need for orotracheal intubation to protect the airway during neurocognitive decline.29,30
- 9
Routine ammonia determination is not indicated for diagnosing HE or at HE follow-up.
Quality of evidence: II-C, Strength of recommendation: 2
Panel level of agreement: In total agreement 96%, in total disagreement 4%
Ammonia is central in the pathophysiology of HE, but its role in the diagnosis and follow-up of those patients in clinical practice is relatively uncertain. Classically, it should not be used as a screening method for diagnosing asymptomatic patients and its use in patients with signs or symptoms is a subject of debate, given that ammonia is not specific for diagnosing HE. Thus, the clinical evaluation is the most important aspect. Ammonia values do not correlate with severity and are easily influenced by the measuring method utilized. High serum ammonia levels add no diagnostic, staging, or outcome value in patients with HE and a normal value requires diagnostic re-evaluation. When there are no signs or symptoms of HE, high ammonia levels, alone, should not indicate treatment for HE.30,31
Despite the above, much more recent evidence reveals that, in addition to aiding in making the differential diagnosis of HE, normal ammonia values, even though possible in HE, can justify reconsidering the diagnosis.32,33 Serum ammonia testing offers little additional benefit in clinical settings with a high or low pre-test probability for HE. However, if the pre-test probability for HE is uncertain, a low ammonia level might reduce the post-test probability of HE. In such a setting, other causes of mental status alterations should be explored.33
Ammonemia could also be a prognostic marker, not only in patients with HE, but also in patients with no neurologic symptoms, suggesting a possible toxic role of ammonia beyond the brain.32 Tranah et al. conducted a prospective cohort study that evaluated the association of plasma ammonia levels in 754 patients with compensated cirrhosis at 3 independent liver units. They found that hyperammonemia was an independent predictor of hospitalization with liver-related complications and mortality, superior even to the Child-Pugh classification and the Model for End-stage Liver Disease (MELD) score. Those results were confirmed in an independent patient cohort.34
Lastly, focusing on ammonemia, while monitoring the therapeutic response, could be a way to improve results in patients with HE.32 And so, even though for the time being routine ammonia determination cannot be recommended, we agree with other expert groups that future research should concentrate on developing a standardized focus for the collection and processing of serum ammonia and the interpretation of its results.33
- 10
The psychometric hepatic encephalopathy score (PHES) is considered accessible, sensitive, and cost-effective for the diagnosis of MHE, in addition to being standardized.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 96%, in partial agreement 4%
PHES has standardized values and is adjusted for age and educational level. It requires that the patient know how to read and write, examines attention and fine motor skills, and is used for MHE screening. PHES has been validated in healthy controls vs. cirrhotics, with and without HE. Each patient can obtain a score between −15 and +3 points. The limit defining the existence of MHE is −4 points. Patient age and educational level should be taken into consideration in the result. The score is obtained after taking 5 paper-and-pencil tests, and thus, is accessible and cost-effective.35
- 11
Critical flicker frequency (CFF) and the semantic interference effect (Stroop test) are objective tests that can be useful for diagnosing MHE.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 87%, in partial agreement 13%
CFF measurement has been shown to be useful in diagnosing MHE. The patient must press a button when he/she notices the flicker of a luminous point inside a visor. If the frequency detected is above 39 Hz (in a mean of 10 measurements), the test is considered normal. Cirrhotics with MHE register a mean below 39.36 CFF, as a diagnostic criterion, has a sensitivity of 83.3%, compared with other psychometric tests. Agreement between the CFF and PHES is not absolute and one-third of patients present alterations in only one of the tests. The result of the CFF has the advantage of not being influenced by age or educational level and presents no learning phenomenon; its disadvantage is that there are very few devices in Mexico.37
Another recently validated cognitive test for MHE is the semantic interference effect, or Stroop test. It is taken by downloading the application (EnchephalApp strooptest) onto a smartphone or electronic tablet. It consists of Ontime and Offtime states, for practice and correct execution, combining changing signs, words, and colors, and the patient must touch the corresponding button. The resulting score is entered on the webpage https://www.encephalapp.com/test1.html, along with the patient’s age, educational level, and sex, to get a final score and a diagnosis or not of MHE. It has a sensitivity of 85% and is an available, objective test.38
Electroencephalograms, as well as auditory evoked potentials and visual evoked potentials, are not routinely used in the diagnosis of MHE. They are more useful in research protocols.39
- 12
In the differential diagnosis of the patient with cirrhosis and HE, laboratory (serum electrolytes, infection markers, kidney function, liver function, acid-base balance, peritoneal fluid, and cerebrospinal fluid), imaging (brain tomography or magnetic resonance imaging), and microbiologic (cultures) studies are recommended.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 100%
The precipitating factors of HE should be identified and corrected whenever possible (Fig. 1). Infections have been identified as the most common precipitating factor for developing HE, and the most relevant are spontaneous bacterial peritonitis, respiratory tract infections, and urinary tract infections. Performing urinalysis, cytologic analysis, ascites cytochemistry, chest x-ray, and cultures are recommended for the timely identification of infectious processes. Indirect markers, such as serum leukocytes, procalcitonin, C-reactive protein, globular sedimentation rate, etc., can also be useful. We must not forget that hyponatremia and hypokalemia are the fluid and electrolyte imbalances most commonly associated with the development of episodic HE, thus serum electrolyte determination is useful.40
Other studies, such as arterial blood gas (acid-base balance, amounts of oxygen and carbon dioxide in blood), blood glucose, serum creatinine, serum urea, and blood urea nitrogen determination, are relevant for making the differential diagnosis.40,41
A differential diagnosis should be carried out in patients with atypical data suggesting focalization (facial hemiparesis or involvement of a cranial nerve, hemiparesis or hemiplegia, no response to sudden stimuli, convulsive crises, aphasia, decorticate posture, Babinski sign) and a history of trauma, or in patients that a priori have neurologic examination data suggestive of other structural alterations of the central nervous system, as well as in patients that show no improvement after anti-ammonia therapy. A cerebrovascular event, intraparenchymal bleeding, and tumor are among the most frequent imaging study findings included in the differential diagnosis.40,41
Regarding infectious encephalopathy markers, cerebrospinal fluid analysis to determine leukocytes, proteins, glucose, and microbiology should be considered.40,41
A small percentage of neurologic alterations can be explained by the ingestion of toxic agents. Whenever possible, a differential-directed clinical history should be carried out. Lastly, psychiatric disorders, such as hypoactive or hyperactive delirium, particularly in older adults, should be considered in the differential diagnosis.40
Treatment and prophylaxis- 13
Sufficient caloric and protein intake should be ensured in patients with HE, to prevent malnutrition and sarcopenia.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 100%
Sarcopenia frequently presents in patients with cirrhosis, with a prevalence as high as 65–90%.42 Inadequate nutrient intake, compromised nutrient absorption, and poor nutrient metabolism and utilization secondary to liver failure are factors that have been identified in the pathogenesis of sarcopenia.43 Added to those are liver necrosis with cytokine release, biomolecules of the host that include damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), and PSSs. All contribute to hyperammonemia and endotoxemia, and together with the cause of the liver disease itself, promote anabolic resistance, in which the nutrients and physical activity are incapable of increasing protein synthesis and decreasing proteolysis.44 Once the liver is unable to metabolize ammonia, it can only be done by astrocytes and muscle cells, converting ammonia into glutamine through the glutamine synthetase enzyme. However, with sarcopenia, the compensating mechanism of ammonia metabolism in the muscle is reduced, playing a relevant role in favor of HE. In the study by Nardelli et al.,45 58% of the patients presented with sarcopenia. A history of HE was more frequent in those patients, compared with the patients with no sarcopenia (43 vs. 15%, respectively, p = 0.02), and the same was true with respect to serum ammonia levels (62.6 ± 17.7 vs. 41.4 ± 16.1 μg/dl, p < 0.001), as well as to MHE (73 vs. 18.5%, p < 0.001). Treatment should be guided and monitored, preferably by a service specializing in nutrition. Caloric intake should be at least 35 kcal/kg for the nonobese patient and 500–800 kcal/day for the obese patient (intake can also be stratified according to body mass index [BMI]: 25−35 kcal/kg for patients with a BMI of 30−40 kg/m2 and 20−25 kcal/kg for patients with a BMI > 40 kg/m2. With respect to protein intake in the patient with cirrhosis, the quantity needed to prevent muscle mass loss, or reverse the existing loss, is 1.2–1.5 g/kg, and whenever possible, the oral or enteral route should be preferred over the parenteral route.46
- 14
Lactulose is the therapeutic strategy of choice for overt HE.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 96%, in partial agreement 4%
Lactulose is the most widely used nonabsorbable disaccharide for the treatment of HE. Its mechanism of action has been attributed to the suppression of proteolytic bacteria, by favoring an increase in acidophilic bacteria (e.g., Lactobacillus) and ammonia ion uptake, thanks to the acidification of the content of the colon (the increase in bowel movement rhythm and osmotic effect due to reduced pH) and bacterial nitrogen metabolism alteration, stimulating the use of ammonia by those bacteria for protein synthesis.47 All those mechanisms explain its efficacy.48,49
A series of randomized clinical trials (RCTs),50–53 as well as observational studies, have shown the benefit of lactulose compared with the absence of therapy, albeit there are no truly double-blind studies, given that it is extremely difficult to blind the laxative effect and the typical sweet taste of lactulose. Among those studies, an open RCT showed that patients that had recovered from an episode of overt HE, and were receiving lactulose, had a recurrence risk at 14 months of 20 vs. 47% in the patients that did not receive lactulose.50
In general, lactulose is administered orally as a syrup (15−30 ml), with dose titration, for a goal of 2–4 soft bowel movements daily. Lactulose can also be administered rectally (300 ml in 700 ml of saline solution) and is the preferred route for patients for whom oral administration is difficult (grade III or IV HE).54 Common secondary effects of lactulose include flatulence, abdominal discomfort, and diarrhea, resulting in a lack of treatment adherence in some patients.
- 15
Lactulose is useful as a strategy in secondary prophylaxis after a first episode of overt HE.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 96%, in partial agreement 4%
Recurrent HE occurs in 47–57% patients per year and is related to poor prognosis in patients with cirrhosis, making its control an important goal. An online systematic review and meta-analysis that included a total of 1,828 participants showed that lactulose was efficacious for preventing bouts of overt HE, with mild gastrointestinal adverse effects.55
A systematic review and meta-analysis52 on eight RCTs (705 participants) with a low risk for bias showed a beneficial effect of the nonabsorbable disaccharides vs. placebo/no intervention, regarding mortality (relative risk [RR] 0.63; 95% confidence interval [CI]: 0.41 to 0.97). Compared with placebo/no intervention, the nonabsorbable disaccharides were associated with beneficial effects on HE (RR 0.58; 95% CI: 0.50 to 0.69; 1,415 participants; 22 RCTs; I2 = 32%). The additional analyses showed that the nonabsorbable disaccharides helped reduce the severe adverse events associated with the underlying liver disease, including liver failure, hepatorenal syndrome, and variceal bleeding (RR 0.47; 95% CI: 0.36 to 0.60; 1,487 participants; 24 RCTs; I2 = 0%). The nonabsorbable disaccharides were mainly associated with nonsevere gastrointestinal adverse events. With respect to recurrent hospital admissions for HE, the importance of individualized lactulose dose titration stands out, given that 22% of readmissions at 30 days can be prevented.50
- 16
Lactulose can be recommended as primary prophylaxis in patients with cirrhosis that present with high risk factors for developing episodic overt HE.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 87%, in partial agreement 13%
Lactulose has been shown to be efficacious in preventing HE in patients with cirrhosis and variceal bleeding (VB). The relation between gastrointestinal bleeding and the increase in serum ammonia is well established and considered multifactorial. An open randomized study from a single center showed that treatment with lactulose significantly reduced the incidence of HE in patients with gastrointestinal bleeding (14 vs. 40%, p < 0.03), with no effect on survival (8.5 vs. 14%, p = NS).56 Another open randomized study also showed that lactulose significantly reduced the incidence of HE (3.2 vs. 16.9%, p < 0.02); Child-Pugh grade was one of the most relevant factors independently associated with the appearance of HE.57 In a Mexican double-blind, placebo-controlled clinical trial that included cirrhotic patients with VB, with no overt HE or MHE at admission, lactulose and other anti-ammonia treatments (rifaximin, LOLA) were compared with placebo. The development of HE was less frequent in the lactulose group vs. placebo (27.3 vs. 54.5%, respectively); the difference had a trend towards clinical significance (odds ratio [OR] = 0.3; 95% CI: 0.09–1.0; p = 0.06) and there was no significant difference between the three groups that received any anti-ammonia treatment, but there were greater gastrointestinal adverse effects in the group treated with lactulose.58 There is insufficient quality evidence for recommending prophylaxis with lactulose in the context of active infectious processes, as well as in a post-TIPS scenario. Therefore, we believe it is prudent to evaluate each case individually, assessing the risk-benefit of lactulose administration in those clinical contexts.
- 17
Lactulose can be recommended as treatment in patients with MHE.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 91%, in partial agreement 9%
According to different clinical trials, patients with cirrhosis that develop MHE have improved health-related quality of life, neurophysiologic variables, and psychometric test performance after therapy with lactulose, albeit no decrease in mortality was reported.59–61 Both lactulose and lactitol have effects on the gut microbiota and are considered intestinal prebiotics. Lactulose can produce a bifidogenic effect related to a favorable effect on the metabolism of ammonia in the colon.62
In a network analysis of 25 trials and 1,563 participants, lactulose was an effective treatment for reversing MHE, decreasing ammonia levels, and improving quality of life, with tolerable adverse effects.55
- 18
Rifaximin is useful as an adjuvant to lactulose in patients with overt HE and a suboptimal clinical response, as well as in the context of secondary prophylaxis, particularly after a second episode of overt HE.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 91%, in partial agreement 9%
The prevalence of overt HE is 10–14% at diagnosis of cirrhosis, in general, 16–21% in cases of decompensated cirrhosis, and 10–50 % in patients with TIPS. The accumulated prevalence indicates that 30−40% of the patients with cirrhosis can present with it throughout their clinical progression.63,64 Rifaximin-α is a poorly absorbed antibiotic that reduces ammonia-producing bacteria in the gut, in patients with HE.65 Recently, an RCT showed the efficacy of the combination of rifaximin plus lactulose vs. lactulose alone in patients with grades II to IV overt HE. There was a high probability for HE resolution, shortened hospital stay, and improved survival.66 In a study utilizing two US databases that included 11,205 patients with HE treated with rifaximin plus lactulose, hospital admissions related to HE were reduced by 33–34%, compared with lactulose.67 A randomized study showed reduced mortality (p < 0.05) with rifaximin plus lactulose vs. lactulose alone. The majority of patients had a MELD score of 24.6 and 81.7% presented with grade III or IV HE according to the WHC.68
- 19
L-ornithine L-aspartate is an effective therapeutic strategy in patients with overt HE, both as monotherapy and as a coadjuvant to lactulose and rifaximin.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 91%, uncertain 4.5%, in partial disagreement 4.5%
A clinical trial conducted by Ahmad et al. showed that the administration of LOLA in monotherapy at a dose of 20 g per day/for 5 days was superior to placebo for improving mental status in cirrhotic patients with hyperammonemia and overt HE.69 Two more recent double-blind, randomized, placebo-controlled clinical trials evaluated the efficacy of intravenous LOLA at 30 g per day/for 5 days. In the first study, LOLA was added to lactulose and ceftriaxone (standard care) vs. placebo plus standard care, to reverse bouts of overt HE in patients with cirrhosis. Overt HE grade was significantly lower in the LOLA group, compared with placebo, from the first 24–48 h and sustained up to day 4. Mean recovery time was lower in the LOLA group, compared with the placebo group (1.92 ± 0.93 vs. 2.50 ± 1.03 days, p = 0.002; 95% CI: –0.852 to –0.202). Venous ammonia on day 5 and hospital stay were significantly lower in the LOLA group.70 The second study showed that the combination of LOLA with lactulose and rifaximin was superior to exclusive standard care with lactulose plus rifaximin, achieving greater reduction in serum levels of ammonia, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). There were higher improvement rates in HE grade (92.5 vs. 66%, p < 0.001), shorter recovery time (2.7 ± 0.46 vs. 3.0 ± 0.87 days, p = 0.03), and a lower 28-day mortality rate (16.4 vs. 41.8%, p = 0.001), in the LOLA group compared with placebo.71
- 20
L-ornithine L-aspartate can be used as primary and secondary prophylaxis to prevent the development of overt HE in high-risk conditions.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 91%, uncertain 4.5%, in partial disagreement 4.5%
In an open RCT, Bai et al. found that intravenous LOLA administered at a dose of 30 g/day for 7 days after TIPS placement significantly reduced ammonia levels on days 4 and 7, in both the fasting and postprandial periods, compared with placebo. During the study period, the patients in the LOLA group had better performance on psychometric tests, compared with the control group. Even though no difference was observed regarding the frank development of overt HE during treatment, there was a trend towards lower frequency of overt HE in the LOLA group (one patient in the LOLA group and 3 patients in the placebo group; p = 0.331). LOLA administration was safe, given that there were no differences in complications, adverse events, or death, between the 2 groups.72 Another risk condition is VB, in which up to 40% of patients with said complication also develop HE. Higuera-de la Tijera et al. found that the intravenous administration of LOLA at a dose of 10 g/day/for 7 days, in that clinical context, prevented the development of HE more efficiently than placebo (22.7 vs. 54.5%; OR 0.2, 95% CI: 0.06−0.88; p = 0.03), and was not inferior to other anti-ammonia treatments (lactulose or rifaximin; p = 0.94) when administered as monotherapy.58
With respect to secondary prophylaxis, Varakanahalli et al. conducted a double-blind, placebo controlled RCT that consisted of administering 6 g of LOLA orally 3 times a day/for 6 months to patients that had recently recovered from a bout of overt HE. Recurrence of overt HE was significantly lower in the group that received LOLA vs. placebo (12.3 vs. 27.7%, p = 0.02). That same study also showed objective improvement in different MHE parameters and in serum ammonia levels in the group that received LOLA vs. placebo: PHES (2.53 ± 2.18 vs. –0.01 ± 1.92; p < 0.001), ammonia (–23.58 ± 14.8 vs. 1.41 ± 13.34 μmol/l; p < 0.001), CFF (5.85 ± 4.82 vs. 0.58 ± 4.53; p < 0.001), and score on the disease impact profile questionnaire (–7.89 ± 5.52 vs. –0.95 ± 4.25; p < 0.001).73
- 21
L-ornithine L-aspartate is a superior strategy to placebo for treating MHE and could be prescribed as an alternative to lactulose or rifaximin.
Quality of evidence: I-A, Strength of recommendation: 1
Panel level of agreement: In total agreement 87%, uncertain 13%
In the study by Mittal et al., the administration of oral LOLA 6 g/3 times a day/for 3 months was superior to placebo for improving neuropsychiatric alterations characteristic of MHE, reducing serum ammonia levels, and improving quality of life in patients with cirrhosis. There was also no difference between LOLA and the standard treatment with lactulose.74 In their placebo-controlled RCT, Sharma et al. reported improved neuropsychometric parameters evaluated through the Number Connection Test-A (NCT-A), Figure Connection Test-A (FCT-A), the Digit Symbol Test (DST), and the CFF, compared with placebo, and noninferiority to rifaximin (in that study there was no comparison with lactulose).68 Alvares-da-Silva reported that the number of patients that progressed from MHE to overt HE was significantly lower in the group receiving oral LOLA at a dose of 6 g/3 times a day/for 6 months vs. placebo (5 vs. 37.9%; p = 0.016).75
- 22
Supplementation with branched-chain amino acids can improve the clinical manifestations of HE.
Quality of evidence: II-A, Strength of recommendation: 2
Panel level of agreement: In total agreement 82.6%, in partial agreement 13%, uncertain 4.3%
Branched-chain amino acids (BCAAs) serve as a substrate for the synthesis of proteins in skeletal muscle and the 3 main ones are: leucine, isoleucine, and valine. BCAA metabolism is associated with an increase in the activity of glutamine synthetase, favoring the formation of glutamine and the subsequent detoxification of ammonia.76–78
There is evidence related to the usefulness of BCAAs in preventing HE recurrence.76–80 In a 2015 Cochrane review that included 16 studies with a total of 827 patients, the usefulness of BCAAs with lactulose, neomycin, diet, placebo, or no intervention was compared. Gluud et al. reported a beneficial effect in favor of BCAAs on HE, but those benefits were not observed in the quality of life, mortality, or nutritional outcome parameters.80 Therefore, their use has been proposed only as an alternative or additional agent, in patients with refractory HE.11
- 23
Currently there is insufficient evidence for recommending the use of zinc, L-carnitine, probiotics, or fecal microbiota transplantation.
Quality of evidence: II-A, Strength of recommendation: 2
Panel level of agreement: In total agreement 87%, in partial agreement 8.7%, uncertain 4.3%
Zinc is a cofactor in the enzymatic reactions associated with the conversion of ammonia into urea via ornithine transcarbamylase in the liver and via glutamine synthetase in the skeletal muscle. Taking two systematic reviews with meta-analyses as a reference, Chávez-Tapia et al. evaluated the studies published on the use of zinc, compared with no treatment or placebo. Its use was associated with improvement on the number connection test in patients with HE but no improvement in other clinical or biochemical outcomes.81
Shen et al. evaluated studies on zinc supplementation compared with placebo or other treatments and concluded that the performance on the number connection test also improved with the combination of zinc plus lactulose for 3–6 months, compared with the use of lactulose alone, in patients with MHE.82
Acetyl-L-carnitine (ALC) is an endogenous ester of L-carnitine that has been associated with a reduction in concentrations of ammonia in the blood and brain. The applicability of the oral or intravenous formulations of L-carnitine was evaluated in a 2019 Cochrane review that included 5 studies that compared the use of L-carnitine with standard therapy in patients with HE. Despite the fact that its use appeared to be associated with a decrease in serum ammonia levels, there was no benefit over placebo in the reduction of fatigue or quality of life. There are no data evaluating adverse effects, hospital readmissions, or mortality.83
The potential benefit in the use of probiotics for HE management is based on the effect they exert through the decrease in the pathogenic intestinal bacterial load, promoting a healthy intestinal barrier and attenuating the toxic effect of bacterial translocation. The majority of studies have evaluated the applicability of VSL3 (Visibiome®).84 Despite the fact that probiotics have shown promising results in early phases, there is insufficient evidence of cognitive improvement in patients with HE. A 2017 Cochrane review that evaluated 21 studies (more than 1,400 patients) concluded that probiotics appear to have no beneficial effect on HE, when compared with placebo. Importantly, most of the evidence was considered low-quality.85 In addition, there is still concern as to whether the use of probiotics is associated with alterations in the composition of the microbiota that could favor resistances.
Fecal microbiota transplantation (FMT) was first proposed in 2016 as a case report, but the beneficial effect described therein appears to have been transitory in a patient with grade I to II HE.86 One year later, a clinical trial was conducted that evaluated the therapeutic effect of FMT combined with lactulose and rifaximin in 10 patients, compared with 10 patients that received only rifaximin and lactulose. There was a significant difference in the number of hospitalizations associated with recurrent HE and overall improvement in cognition.87 That study paved the way for a similar trial with no pre-treatment antibiotics that utilized a FMT capsule instead of a FMT enema. The intervention was shown to be safe and well tolerated but there was insufficient statistical power for evaluating its efficacy with respect to improvement in HE.88
- 24
Polyethylene glycol has shown efficacy in treatment for episodic HE, but more evidence is needed for its recommendation.
Quality of evidence: I-A, Strength of recommendation: 2
Panel level of agreement: In total agreement 82.6%, in partial agreement 13%, uncertain 4.3%
Polyethylene glycol (PEG) is a safe osmotic laxative. Several RCTs evaluated the effectiveness of PEG vs. lactulose in the treatment of episodic HE. However, the results have been inconsistent.89–92 A first systematic review with a meta-analysis included four trials with 229 patients. The Hepatic Encephalopathy Scoring Algorithm (HESA) combines clinical indicators with those derived from simple neuropsychologic tests and attempts to standardize and objectify the classification of the different grades of HE, but it lacks greater validation. Compared with lactulose, according to the HESA, the size of the pooled effect showed a significantly lower mean score at 24 h (mean difference [MD] = −0.68; 95% CI: −1.05 to −0.31), p < 0.001), a higher number of patients with a decrease in the HESA score by ≥ 1 grade at 24 h (RR = 1.40; 95% CI: 1.17–1.67; p < 0.001), a higher number of patients with a HESA score of grade 0 at 24 h (RR = 4.33; 95% CI: 2.27–8.28; p < 0.001), and a shorter time to resolution in the HE group (MD = −1.45; 95% CI: −1.72 to −1.18; p < 0.001), in favor of the patients treated with PEG.93
In a more recent meta-analysis that included a total of 434 patients that participated in 7 randomized studies, a significant advantage was found in therapy with PEG vs. lactulose, with respect to increased clinical efficacy (RR = 1.46; 95% CI: 1.26–1.68; p = 0.001; I2 = 0%), and shorter hospital stay (DM = −1.78; 95% CI: −2.72 to 0.85; p = 0.001; I2 = 90.1%). There were no significant differences in the incidence of adverse events or in the serum ammonia level after 24 h, between the 2 groups. Those authors concluded that PEG can lead to faster HE resolution in the first 24 h and shorten hospital stay, without increasing the adverse effect rate, but they also indicated there are several limitations to keep in mind. First, the meta-analysis is limited by the small number and deficient quality of the studies included. Second, there is still significant heterogeneity in the study results, the source of which includes age, sex, culture, and many other corresponding factors. In addition, the follow-up of the studies evaluated were only short-term. In the future, more prospective studies with long-term follow-up should be conducted.94
Special conditions: hepatic encephalopathy in acute liver failure and acute-on-chronic liver failure- 25
The clinical appearance of HE is a criterion for the diagnosis of ALF.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 95.7%, in partial agreement 4.3%
ALF is a syndrome characterized by the rapid decline of normal liver function after an acute insult, in a patient with no previously known liver disease.95 It is typified by deterioration on liver function tests, with the potential involvement and dysfunction of other organs. Jaundice, coagulopathy, and HE are present in the clinical development of liver failure.
The most widely accepted definition of ALF is that established by O’Grady, which divides it into 3 types, according to the onset of HE. The definition is important, given that, by virtue of the diagnosis, the possible etiology and prognosis are established:95
- a
Hyperacute: HE in the 7 days after the onset of jaundice, from causes such as acetaminophen, hepatitis A, hepatitis E, and hepatic hypoperfusion. It usually presents with an important increase in transaminases, severe coagulopathy, an important risk for cerebral edema, and high mortality without transplant.
- b
Acute: HE that appears between 7 and 28 days after the onset of jaundice and is mainly attributed to hepatitis B. It presents with moderate elevation of transaminases, bilirubin, and coagulopathy, with an intermediate risk for cerebral edema. Its survival rate is better than that of the hyperacute type.
- c
Subacute: HE that presents between 28 days and 26 weeks after the onset of jaundice and is attributed to idiosyncratic liver damage due to drugs. Its manifestation is a slight elevation of transaminases, important elevation of bilirubin, low risk for cerebral edema, and low mortality.96
- 26
In the context of ALF, the exclusion of other causes of neurologic alteration is the recommended diagnostic approach.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 95.7%, in partial agreement 4.3%
The differential diagnosis should mainly include infectious etiologies, such as malaria, leptospirosis, dengue, rickettsia, typhoid fever, and hemophagocytic syndrome, as well as the neurologic diseases that present with altered mental status, coagulopathy, and jaundice.97
The differential diagnosis should mainly include infectious etiologies, such as malaria, leptospirosis, dengue, rickettsia, typhoid fever, and hemophagocytic syndrome, as well as the neurologic diseases that present with altered mental status, coagulopathy, and jaundice.97
- 27
Patients with ALF that present with serum ammonia levels >200 µmol/l have a higher risk for developing cerebral edema and intracranial hypertension.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 87%, in partial agreement 8.7%, in partial disagreement 4.3 %
Cerebral edema in the patients with ALF causes an increase in intracranial pressure (ICP). Patients at high risk for increased ICP are those with the acute or hyperacute phenotypes characterized by a shorter period of HE after the onset of jaundice, as well as young patients, and those with renal involvement and increased ammonia levels. Having levels persistently >200 µmol/l, even after the start of treatment and anti-edema measures, is highly suggestive of an increase in ICP.98
- 28
The use of anti-cerebral edema measures should be considered in the management of HE in patients with severe ALF.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 87%, in partial agreement 13%
Patients with ALF, HE, and at risk for an increase in ICP should be managed in a calm environment, with serum sodium monitoring (maintaining levels between 140 and 145 mmol/l) and HE treatment with lactulose and rifaximin. General recommended measures are raising the head of the bed to 30 degrees and preventing fever, hypoglycemia, or hyperglycemia. ICP should be monitored, maintaining it between 20 and 25 mmHg, with a brain perfusion pressure above 50 mmHg.99 When ICP is above 25 mmHg, boluses of hypertonic saline solution or intravenous mannitol at 20% should be added to the treatment.100
- 29
The presence of HE in a patient with ACLF confers a poor prognosis, increasing the risk for mortality in both the short and long terms.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 100%
ACLF is a dynamic syndrome that involves prognostic scores for making the diagnosis. The tracts, systems, and organs involved in the disease are the liver, kidney, brain, coagulation, circulation, and respiratory tract.101 The Chronic Liver Failure – Consortium – Acute-on-Chronic Liver Failure (CLIF-C-ACLF), available at: www-clifconsortium.com, is a prognostic model that has been developed to stratify patients with ACLF, and the Chronic Liver Failure – Consortium – Acute Decompensation (CLIF-C-AD), for patients that do not meet the ACLF criteria.102 At the brain level, 1 point is established in patients with WHC grade 0 HE, 2 points for grades I to II, and 3 points for grades III to IV. Two factors in particular, the presence of acute kidney injury and brain failure, have been established as independent predictors for mortality at 28 days and at 90 days, in patients with ACLF.103 Thus, mortality in patients with grade 1 ACLF varies from 23 to 41%, from 31 to 55% in patients with grade 2, and from 74 to 78% in patients with grade 3, at 28 days and 90 days, respectively.104
Additional specific care in patients with cirrhosis and hepatic encephalopathy- 30
The use of certain drugs can precipitate HE in patients with cirrhosis.
Quality of evidence: II-B, Strength of recommendation: 1
Panel level of agreement: In total agreement 100%
Patients with cirrhosis habitually undergo endoscopic examinations. Esophagogastroduodenoscopy (EGD) is crucial in patients with cirrhosis for screening and follow-up of esophageal or gastric varices, which are manifestations of portal hypertension. Midazolam is the drug usually utilized to sedate patients undergoing EGD, and its adequate application produces anterograde amnesia, causing the patient to forget the discomfort during the procedure. Nevertheless, because midazolam and the majority of benzodiazepines are mainly metabolized in the liver, its metabolic rate can decrease in patients with cirrhosis. Midazolam has also been described as a risk factor for developing overt and covert HE.105 On the other hand, propofol is a drug that can be used as a sedative in endoscopy and is not known to have pharmacokinetic differences in patients with kidney failure or liver failure. Propofol has been shown to not produce significant cognitive changes, to have a shorter recovery period compared with midazolam, and to have a better safety and efficacy profile.106–108
Importantly, in endovenous sedation, lorazepam and oxazepam can be used with caution, if a benzodiazepine is needed to manage generalized anxiety disorder or sleep disorders, given that they are metabolized in the liver, mainly in biotransformation phase II. That phase includes the conjugation with endogenous compounds, a mechanism that tends to be preserved even in cirrhotic patients, unlike the rest of the benzodiazepines that are transformed in phase I (which includes hydrolysis, reduction, or oxidation) and are not recommendable.109
All patients with WHC grade III or grade IV HE and a score <8 on the Glasgow coma scale should be considered for intubation as an airway protection measure. Short duration drugs, such as propofol or dexmedetomidine should be utilized to sedate intensive care patients. The latter drug is associated with preserved cognitive function and reduced mechanical ventilation duration in patients in the intensive care unit. It can also be used to treat abstinence from alcohol, enabling a decrease in benzodiazepine administration. Both dexmedetomidine and propofol are associated with similar secondary hemodynamic effects.110,111 Dexmedetomidine has been well tolerated in patients with chronic liver disease, without increasing side effects, albeit patients require more time before extubation, once the medication is suspended.112
Opioids are frequently employed in combination with a benzodiazepine for moderate sedation. Those drugs bind to central nervous system receptors and increase the pain threshold, altering its perception. The combination of those agents increases the probability of adverse effects in patients with cirrhosis.113
In general, opioids should be avoided in cirrhotic patients. They have well established adverse effects, such as respiratory depression, sedation, and constipation, which can generate or exacerbate HE, and so should be used with caution. When an opioid agent is required as part of palliative care in patients with liver diseases, prophylactic lactulose use, to prevent constipation, is recommended, along with close patient and adverse effect follow-up. The preferred first-line opioids are: hydromorphone at low doses (1 mg oral or 0.4 mg intravenous) or oxycodone (2.5 mg oral) with more extended dose intervals, as well as intravenous fentanyl in hospitalized patients or patches in outpatients. Morphine and codeine should be avoided because they have been associated with numerous adverse effects, including HE and hypoglycemia.114 Despite recent reports against the use of tramadol and buprenorphine, more research is needed on patients with cirrhosis. In pain management, tramadol at low doses of 25–50 mg every 12 h is an adequate option in Mexico, as long as adverse effects are closely monitored.
Meperidine elimination is prolonged in the liver in patients with advanced liver disease and so should not be used, but fentanyl elimination is not appreciatively altered in patients with cirrhosis, and thus is a good option, as a sedative or in pain management, as long as it is used with caution.113
In the past, methadone was an attractive analgesic for patients with liver disease, but today it has been shown to have a long half-life and to accumulate in cirrhotic patients, and so its use is not recommended.115
The use of proton pump inhibitors (PPIs) in patients with cirrhosis has been associated with dysbiosis, in small intestinal bacterial overgrowth, C. difficile infection, and the development of spontaneous bacterial overgrowth and therefore is a potential risk for HE. PPI use in those patients should have a clear indication and be used at the lowest dose for the least amount of time necessary.116,117
Furosemide and spironolactone are the most widely used diuretics in patients with chronic liver disease and water retention. All patients that start on diuretics should be monitored to detect adverse events, whose prevalence varies between 19 and 33%. Almost half of adverse events require the suspension of the diuretic or a reduced dose. HE has been observed in 25% of hospitalized patients treated with diuretics117,118 (Table 4).
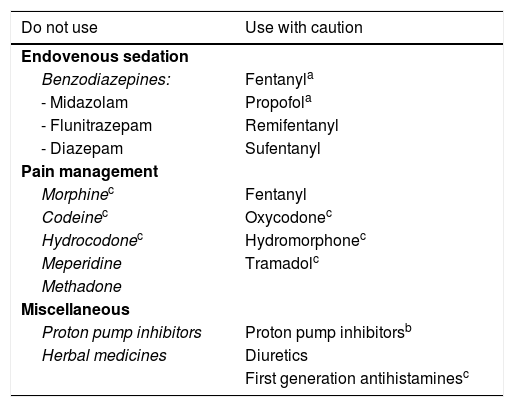
Drugs that can precipitate hepatic encephalopathy.
| Do not use | Use with caution |
|---|---|
| Endovenous sedation | |
| Benzodiazepines: | Fentanyla |
| - Midazolam | Propofola |
| - Flunitrazepam | Remifentanyl |
| - Diazepam | Sufentanyl |
| Pain management | |
| Morphinec | Fentanyl |
| Codeinec | Oxycodonec |
| Hydrocodonec | Hydromorphonec |
| Meperidine | Tramadolc |
| Methadone | |
| Miscellaneous | |
| Proton pump inhibitors | Proton pump inhibitorsb |
| Herbal medicines | Diuretics |
| First generation antihistaminesc | |
HE: hepatic encephalopathy; PPIs: proton pump inhibitors.
These clinical guidelines were carried out with funds of the Asociación Mexicana de Gastroenterología A.C. and with the logistical support of Grünenthal de México.
Conflict of interestFátima Higuera de la Tijera is a speaker for Gilead, Abbott, Grünenthal, and Merz Pharma.
José A. Velarde-Ruiz Velasco is a speaker for Megalabs.
Ricardo H. Raña Garibay has no conflict of interest.
Graciela E. Castro Narro has no conflict of interest.
Juan Miguel Abdo Francis is a speaker for Medix, Abbott, and Grünenthal.
Rosalba Moreno Alcántar is a speaker for Grünenthal, Gilead, and Abbvie.
José L. Pérez Hernández is a speaker for Gilead and Grünenthal.
Aldo Torre is a speaker for Grünenthal, Alfa Wassermann, Viatrix, Grifols, Medix, and Astra.
Raúl Contreras Omaña is a speaker for Megalabs.
Ana Cano Contreras has no conflict of interest.
Mauricio Castillo Barradas has no conflict of interest.
Juanita Pérez Escobar has no conflict of interest.
Juan Manuel Aldana Ledesma has no conflict of interest.
Eira Cerda Reyes is a speaker for Gilead, Abbvie, Medix, Bayer, MSD, and Sanfer.
Nicolás Fernández Pérez is a speaker for Gilead and Megalabs.
Javier Meza Cardona has no conflict of interest.
Nayelli Cointa Flores García is a speaker for Gilead, Abbott, Grünenthal, and Merz Pharma.
Mónica Reyes Bastidas has no conflict of interest.
Jorge Emilio Lira Vera has no conflict of interest.
Edgar Santino García Jiménez has no conflict of interest.
Daniel Santana Vargas is a speaker for Grünenthal.
Victor Manuel Páez Zayas is a speaker for Alfa Wassermann.
Norberto Chávez Tapia has no conflict of interest.
Ernesto Márquez Guillén has no conflict of interest.
Please cite this article as: Higuera-de-la-Tijera F, Velarde-Ruiz Velasco JA, Raña-Garibay RH, Castro-Narro GE, Abdo-Francis JM, Moreno-Alcántar R, et al. Visión actual sobre el diagnóstico y cuidados integrales en la encefalopatía hepática. Rev Gastroenterol Méx. 2023;88:155–174.