Emerging concepts in the pathophysiology of gastroesophageal reflux disease (GERD) and the constant technologic advances in the diagnosis and treatment of this clinical condition make it necessary to frequently review and update the clinical guidelines, recommendations, and official statements from the leading academic groups worldwide. The Asociación Mexicana de Gastroenterología (AMG), aware of this responsibility, brought together national experts in this field to analyze the most recent scientific evidence and formulate a series of practical recommendations to guide and facilitate the diagnostic process and efficacious treatment of these patients. The document includes algorithms, figures, and tables for convenient consultation, along with opinions on GERD management in sensitive populations, such as pregnant women and older adults.
Los nuevos conceptos en la fisiopatología de la enfermedad por reflujo gastroesofágico (ERGE) y los constantes avances tecnológicos aplicados al diagnóstico y el tratamiento de esta condición clínica hacen necesarias la revisión frecuente y la actualización de guías clínicas, recomendaciones y posturas oficiales de los principales organismos académicos a nivel mundial. La Asociación Mexicana de Gastroenterología, consciente de esta responsabilidad, reúne a los expertos nacionales de este tema para analizar la evidencia científica más reciente y construir una serie de recomendaciones prácticas para orientar y facilitar el proceso diagnóstico y el tratamiento eficaz de los pacientes afectados por esta enfermedad. Se incluyen algoritmos, diagramas de flujo, cuadros y tablas que concentran estas recomendaciones y se agregan opiniones sobre el manejo de la ERGE en poblaciones sensibles como las mujeres embarazadas y las personas de la tercera edad.
GERD is a result of the ascent of the gastric or gastroduodenal content above the gastroesophageal junction, which causes symptoms and/or esophageal lesions that affect the health and quality of life of the individuals that suffer from it.1,2
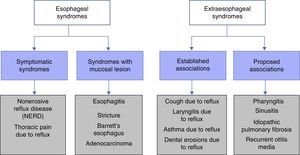
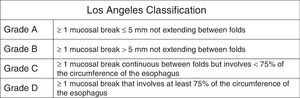
Classification and definition of the main gastroesophageal reflux disease phenotypesThe most widely used classification of GERD, worldwide, is the Montreal Classification3 (Fig. 1). It divides GERD into 2 large groups: esophageal syndromes and extra-esophageal syndromes. Esophageal syndromes are, in turn, subdivided into those characterized by being purely symptomatic, such as nonerosive reflux disease (NERD) and noncardiac chest pain, and those that in addition to symptoms, present with lesions that are macroscopically visible at conventional endoscopy. Traditionally, and from a practical perspective, 3 phenotypic varieties of GERD are recognized that can be diagnosed through endoscopy alone: NERD, erosive reflux disease (ERD), and Barrett's esophagus (BE). However, care must be taken to rule out functional heartburn in NERD patients. The symptoms are the common denominator in all these variants, and each one has a different clinical behavior.
Montreal Classification for GERD.
Taken from Vakil et al.3.
The extra-esophageal syndromes are subdivided into those with sufficient clinical evidence to relate the symptom to GERD, such as chronic cough, posterior laryngitis, difficult-to-control asthma, and dental erosions, and in those in which it has not been possible to relate the symptom to GERD, such as pharyngitis, sinusitis, recurrent otitis media, and pulmonary fibrosis.
Prevalence of gastroesophageal reflux diseaseGERD is a frequent disease, but its prevalence is difficult to estimate, especially when taking symptom frequency into account. Different studies on the topic generally speak of the prevalence of symptoms suggestive of the disease, but very few clearly demonstrate GERD.
Analyzing only the studies that consider the presence of heartburn or regurgitation once a week, the reported prevalence varies from 18.1 to 27.8% in the United States, from 8.8 to 25.9% in Europe, from 2.2 to 7.8% in the Far East, 11.6% in Australia, 23% in Argentina, and from 19.6 to 40% in Mexico (Table 1). Based on the results from a recent national survey (SIGAME),4 it was found that in an open Mexican population, according to the Rome III criteria, the frequency of heartburn or regurgitation at least once a week is 12.1% (95% CI, 11.09 to 13.1), it is 1.2% (95% CI, .09 to 1.3) in those that present with symptoms daily, and it is 49.1% (95% CI, 47.5 to 50.6) in those that present with symptoms at least once a month (Table 2). That study concludes that GERD is a disease with a high prevalence in Mexico, with regurgitation as the main symptom, followed by heartburn and a bitter taste in the mouth. The subjects with symptoms of GERD were significantly older. A multivariate analysis showed that the greatest prevalence was found in the male sex, with studies on university students or professionals, and that they belonged to medium-high and high economic strata.
The international DIGEST study5 estimated that the prevalence of GERD symptoms is 7.7%, with heartburn as the most frequent (13.5%) of the digestive symptom group. It also showed that regurgitation represents an important percentage (10.2%).
In Latin America, there are very few reports in the literature on the prevalence of GERD. A systematic review evaluated prevalence studies in the region (a total of 8 studies, one in Argentina, 5 in Brazil, and 2 in Mexico), concluding that there was a 3 to 11.9% prevalence.6
National and worldwide incidenceThere is very little information on GERD incidence, with no national reports and very few foreign ones. A 5% incidence of reflux esophagitis in endoscopies carried out on an open Chinese population was reported,7 along with a 22.5% incidence in patients with heartburn, whereas a 1.77 to 2.80% incidence of reflux was reported in Iran.8
Risk factors and susceptible populationSome of the most important risk factors for developing GERD are: heredity, overweight, central obesity, smoking, alcohol, and pregnancy.
The above should not be confused with the factors that favor or exacerbate gastroesophageal reflux (GER), such as fats, chocolate, coffee, alcohol, and gastric banding. However, evidence is poor and controversial and so must be individualized for each patient.9
Helicobacter pylori does not directly participate in the pathophysiology of GERD and thus its eradication should not be considered part of GERD treatment.
Pathophysiology of gastroesophageal reflux diseaseGERD pathophysiology is multifactorial. Its main pathophysiologic mechanism is transient lower esophageal sphincter relaxation (TLESR), defined as a LES relaxation of>1mmHg/s lasting at least 10 s and a pressure nadir of<2mmHg in the absence of a swallow 4 s before and 2 s after the beginning of the LES relaxation.
Other mechanisms that participate in GERD are the esophageal clearance disorders, whether mechanic (peristalsis or Earth's gravity) or chemical (saliva), antireflux barrier alterations (hiatal hernia, reduced LES pressure), delayed gastric emptying, or duodenal-gastric reflux.10
The extra-esophageal pathophysiologic manifestations are based on the direct acid damage to the pharyngeal mucosa and possible bronchial microaspiration episodes, as well as esophageal distension with a vagovagal reflex that produces bronchial spasm and associated symptomatology.
DiagnosisSymptomsThe typical GERD symptoms are heartburn and regurgitation. The presence of typical symptoms 2 or more times a week in a young patient (under 50 years of age) with no alarm symptoms establishes the presumptive diagnosis of GERD. A therapeutic trial with a proton pump inhibitor (PPI) is recommended for these patients.2,11,12
Therapeutic trialA PPI trial can be utilized to diagnose GERD in patients that have typical symptoms with no red flags. There is no consensus as to the type of PPI, dose, duration, or result evaluation. In general, a double PPI dose is recommended for a minimum of 2 weeks and is considered positive when symptom improvement is above 50%. Even though this test is easy to perform and widely available, its sensitivity and specificity are low.12–14
Symptom questionnairesSymptom questionnaires are instruments that identify GERD patients. The ReQuest, Carlsson-Dent, and RDQ have been validated in Spanish. They are frequently used in research studies, but their usefulness in daily practice is limited because of sub-optimal sensitivity and specificity.15–18
Conventional endoscopy and biopsiesEndoscopy should not be routinely used as a screening study for GERD due to its poor diagnostic sensitivity.
Endoscopy is useful for detecting GERD complications, such as esophagitis (Fig. 2), stricture, BE, and adenocarcinoma. Therefore, it is indicated in cases of GERD with more than 5 years of progression or refractory GERD, in patients with alarm symptoms such as dysphagia, gastrointestinal bleeding, chest pain, and unintentional weight loss, and in patients with risk factors for BE and in cases of suspected eosinophilic esophagitis.2,12,19
The taking of biopsies during endoscopy is indicated in cases with lesions suggestive of BE and suspicion of eosinophilic esophagitis. They should not be taken to confirm GERD diagnosis.
Magnifying endoscopy and the use of optical filtersMagnifying endoscopy with conventional or electronic chromoendoscopy (NBI, FICE, i-Scan) enables the identification of microerosions and alterations in the vascular pattern of the esophageal mucosa in patients with NERD. Confocal laser endomicroscopy evaluates esophageal mucosal histology at the cellular level in real time and enables directed biopsies to be performed to detect dysplasia in BE. These techniques are not recommended in the routine evaluation of GERD patients.20–23
EsophagogramEsophagogram is not useful for diagnosing GERD. It is indicated in the evaluation of esophageal strictures, large hiatal hernias, and suspicion of short esophagus in patients that are candidates for anti-reflux surgery.24
Esophageal pH monitoring (pH study)The ambulatory 24 to 48h measuring of esophageal pH (pH study) is indicated in patients with typical or extra-esophageal symptoms of GERD and negative endoscopy that do not respond to PPI therapy, and to confirm the presence of pathologic reflux in patients that are candidates for anti-reflux surgery with no evidence of esophageal mucosal lesions at endoscopy. Care should be taken to suspend acid-blocking medications at least 7 days before the study. The routine performance of intragastric pH measurement in the proximal esophagus or the hypopharynx is not recommended in the evaluation of GERD patients.25
The Bravo systemThe wireless system (Bravo capsule) of esophageal pH measurement, compared with pH equipment with probes, is better tolerated by the patient and has greater sensitivity for detecting acid reflux and establishing the association of symptoms with reflux episodes. Its limitations are cost and availability, chest pain, and the fact that it does not detect non-acid reflux.26–28
Esophageal impedance-pH monitoringThe ambulatory 24h esophageal impedance-pH measurement is indicated in cases of refractory GERD to identify the role of non-acid reflux in persistent symptoms that do not respond to PPIs. It can detect excessive supragastric burping and rule out rumination when combined with high resolution manometry, but it cannot diagnose biliary reflux.12,29,30
The indication for measuring esophageal reflux with or without PPI depends on the pre-test probability of presenting with GERD:
- a.
Patients with a low pre-test probability of presenting with GERD, that is, those patients with PPI-refractory symptoms, extra-esophageal manifestations, negative endoscopy, or those patients that are candidates for anti-reflux surgery can be evaluated through conventional pH study, impedance-pH test, or the Bravo capsule without PPI treatment.31
- b.
Patients with a high pre-test probability of presenting with GERD, that is, those patients with typical symptoms, endoscopy with hiatal hernia, or with PPI response should be evaluated through an impedance-pH test with PPI treatment.
The indices for the association of symptoms with reflux episodes (symptom index and symptom association probability) are useful for classifying patients with GERD according to the Rome criteria.
Because their precision depends on the adequate recording of symptoms by the patient and on the percentage of time with reflux, these indices should not be used as the only criteria for indicating anti-reflux surgery.32,33
BilitecThe ambulatory measuring of duodenogastroesophageal reflux with Bilitec 2000 is of little clinical usefulness and is currently unavailable. Its use is limited to research studies.34–35
Esophageal manometryEsophageal manometry is not useful for diagnosing GERD. It should be routinely performed in the preoperative evaluation of patients that are candidates for anti-reflux surgery to rule out severe alterations in esophageal motility (achalasia, scleroderma). It is indicated for locating the LES and the proper placement of pH electrodes.32,36
High resolution and impedance manometryHigh resolution esophageal manometry is superior to conventional manometry in relation to diagnostic performance because it utilizes a standardized, objective measuring system that enables the simultaneous visualization of the contractility of the entire esophagus, and as a result, contractility patterns are more easily recognized and have greater reproducibility. It is very useful for evaluating dysphagia after anti-reflux surgery. When combined with an impedance study, it can distinguish rumination from GERD-associated regurgitation and it detects excessive supragastric burps associated with GERD.37,38
Other technologies for diagnosing gastroesophageal reflux diseaseThe following are new techniques whose clinical usefulness in the diagnostic evaluation of patients with GERD is still being studied:
- a.
Impedance measuring of the esophageal mucosa distinguishes patients with GERD from those without GERD, with achalasia, and with eosinophilic esophagitis.39
- b.
The determination of pepsin in saliva through monoclonal antibodies is a noninvasive method that distinguishes patients with GERD from those with functional heartburn and identifies patients with laryngopharyngeal reflux. Its diagnostic sensitivity and specificity depends on the levels of pepsin and the number of saliva samples analyzed and when they were taken. Two samples in the postprandial period are recommended.40
- c.
The pharyngeal pH measuring system (Restech) is a new system for detecting aerosolized and liquid acid in the hypopharynx during 24h. It is poorly correlated with impedance-pH monitoring and its role as a predictor of surgical treatment response is controversial.41
- d.
Hypopharyngeal impedance-pH monitoring is a novel technique specifically designed for detecting reflux episodes in the proximal esophagus and hypopharynx. It has shown advantages in the detection of laryngopharyngeal reflux in patients with bronchopulmonary disorders, but its usefulness as a predictor of response to surgical treatment is not yet clear.42
- e.
Impedance planimetry utilizing an endoscopic functional luminal imaging probe (EndoFLIP) measures esophageal distensibility and the esophagogastric junction. Its usefulness in GERD diagnosis and in the calibration of fundoplication during anti-reflux surgery is still being evaluated.43,44
The treatment of GERD patients should be individualized and oriented towards the clinical presentation of the disease and symptom intensity.
The aim in relation to the nonerosive variant with typical symptoms is symptom control, and in the erosive variant, is erosion cicatrization and the prevention of complications.
In patients with BE, the aim is to prevent progression to dysplasia and adenocarcinoma.
And in patients with atypical GERD (cough, asthma, laryngitis, etc.), the aim is to control the symptoms and prevent complications, as long as there is evidence associating laryngeal symptoms with GERD (see Diagnosis section).
Nonpharmacologic treatmentLifestyle modifications
Lifestyle modifications and dietary recommendations should be individualized for each patient.45,46 Evidence shows that it is recommendable to:
- •
Lose weight, in overweight and obese subjects47
- •
Stop smoking45,46
- •
Reduce alcohol consumption45
- •
Raise the head of the bed48,49
- •
Sleep in the left lateral decubitus position
- •
Avoid abundant food intake at least 2h before going to bed at night, especially if the subject has nocturnal symptoms50
There is no evidence for a general recommendation to eliminate foods that can apparently trigger reflux symptoms, such as: spicy food, citrus fruit, foods with a high fat content, products with caffeine, and carbonated beverages.45 If the patient finds that any of these foods are associated with his or her symptoms, eliminating them from the diet can be beneficial.
Pharmacologic treatmentMedications used in GERD treatment are: antacids, alginates, sucralfate, histamine2-receptor antagonists (H2RAs), prokinetics, PPIs, and TLESR inhibitors.
Antacids and alginatesAntacids and alginates are recommended for symptom relief and do not contribute to erosion cicatrization or prevent the development of complications.51,52 There is no evidence supporting their chronic use.
Histamine2-receptor antagonistsH2RAs should not be used as first-line treatment and are auxiliaries in PPI management.53 They can be used in cases of GERD with typical and sporadic symptoms. They can also be used as treatment in cases of NERD if they produce symptom relief and in cases of nocturnal GERD (together with a PPI in the morning), but they are recommended for short periods since tachyphylaxis is produced in 7 days.54,55 They are also indicated in GERD in the context of PPI hypersensitivity or side effects.
SucralfateThere is no evidence for its recommendation.12
ProkineticsThese medications should not be used as the sole treatment in GERD management. When symptoms suggest gastric emptying alterations (e.g. overlap with dyspepsia), prokinetics can be used in combination with a PPI.2,56,57
It is important to be aware that prokinetics can have relevant side effects that should be watched for, such as hyperprolactinemia, late dyskinesia, diarrhea, and headache.
Proton pump inhibitorsPPIs are first-choice medications for the treatment of GERD in all its clinical forms, given that they provide greater and faster symptom relief, as well as higher cicatrization percentages, compared with placebo, antacids, and H2RAs.51–55,58
There are currently various PPIs (Table 3) and all of them, when adequately prescribed, are efficacious.2,12,53 Even though studies show varying rates in intragastric pH control and symptom response, meta-analyses have shown that effectiveness among the different PPIs is similar.59
Proton pump inhibitor regimens and types of doses.
| Proton pump inhibitor | Standard dose | Double dose | Divided dosea |
|---|---|---|---|
| Conventional | |||
| Omeprazole | 20mg 30min before breakfast | 20mg 30min before breakfast and before dinner | 10mg 30min before breakfast and before dinner |
| Lansoprazole | 15mg 30min before breakfast | 15mg 30min before breakfast and before dinner | – |
| Rabeprazole | 20mg 30min before breakfast | 20mg 30min before breakfast and before dinner | 10mg 30min before breakfast and before dinner |
| Pantoprazole | 40mg 30min before breakfast | 40mg 30min before breakfast and before dinner | 20mg 30min before breakfast and before dinner |
| Esomeprazole | 40mg 30min before breakfast | 40mg 30min before breakfast and before dinner | 20mg 30min before breakfast and before dinner |
| Dual delayed-release | |||
| Dexlansoprazole | 30mg in the morning, regardless of food intake | 60mg in the morning, regardless of food intake | – |
| Immediate release | |||
| Omeprazole+HCO3 | 20 mg+1,100mg 30min before breakfast | 40 mg+1,100mg 30min before breakfast | – |
It is important to instruct patients that “conventional” PPIs should be taken at least 30min before breakfast, because that is the time of day in which there is a greater quantity of active pumps in the parietal cells.12 A dual delayed-release PPI, dexlansoprazole, has recently become available. In pharmacokinetic studies, it has been shown to maintain adequate therapeutic levels, regardless of food intake, which facilitates treatment adherence.60 Some studies have even recommended dexlansoprazole use as management therapy in patients with NERD that have achieved symptom control with double doses of conventional PPIs.61 However, more studies are needed comparing dexlansoprazole with the other PPIs. A combination of omeprazole and bicarbonate with no enteric coating layer is also available in Mexico and is considered a drug of immediate release, which apparently has a faster effect. Nevertheless, more studies with respect to this are required.
Long-term safety of proton pump inhibitorsIn general, PPIs are considered safe and adverse effects are rare (< 2%), the most common of which are: nausea, diarrhea, headache, insomnia, and anaphylaxis.62
In regard to long-term safety, some adverse effects have been described in the last few years: osteoporosis,63,64 vitamin deficiencies,65 hyperplastic polyps,66 interaction with medications (clopidogrel),67,68 bacterial overgrowth, and Clostridium difficile infection.69–71
Due to this, numerous meta-analyses have been conducted with controversial results. Nevertheless, evidence allows the following recommendations to be made:
- •
Chronic PPI use, especially in subjects over 65 years of age, is a risk factor for C. difficile and other enteric infections, such as Salmonella and Campylobacter.69
- •
Chronic PPI use should not be considered the sole risk factor for osteoporosis.64
- •
Even though short-term PPI use is associated with the development of community-acquired pneumonia in a high-risk population, it is no greater than that associated with H2RA use.70,71
- •
Concomitant PPI use with clopidogrel does not increase the risk for cardiovascular events.67,68
At present, baclofen has shown greater usefulness for reducing TLESRs. This drug is not available in Mexico, but there is moderate evidence that the use of this GABA-β agonist reduces transient sphincter relaxations, thus reducing reflux episodes (acid and non-acid).72,73 It is recommended as an adjuvant to PPIs in patients with failed response to these, although its high incidence of adverse effects must be taken into account, such as headache, dizziness, and constipation, limiting its clinical use.
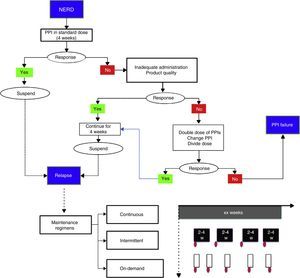
Treatment strategies and durationNonerosive reflux disease treatmentThe standard PPI dose is recommended in these cases (Table 3) for a period of 4 weeks (fig. 3).2,12,58 If symptom control is achieved, the medication should be suspended and reinitiated in case of relapse.74,75
Nonerosive reflux disease management. In the proposed maintenance regimens, the red ovals indicate symptom reappearance. For example, in the on-demand regimen, once symptoms appear, treatment is given for the length of time the patient has symptoms (white rectangles); in the intermittent regimen, treatment duration can vary from 2 to 4 weeks, even when symptoms are no longer present.
PPI: proton pump inhibitor.
If there is recurrence, the following maintenance regimens with PPIs can be employed:
- a.
Continuous: uninterruptedly use the minimum PPI dose that provides symptom control.73
- b.
On-demand: use the standard dose every time the patient presents with symptoms and suspend it when they have been controlled.72
- c.
Intermittent: use the standard dose for defined periods of at least 2-4 weeks every time there is symptom recurrence.73
Another maintenance option is to switch from a PPI to a H2RA, using the latter for short periods and only in the case of mild or intermittent symptoms.76
If response is partial or nonexistent with the initial PPI dose, the following situations should be investigated (fig. 3):77
- •
adequate drug prescription and administration
- •
product quality
- •
treatment adherence
If none of the abovementioned applies, then doubling the dose (see Table 3), changing the PPI, or dividing the dose during the day (if there are nocturnal symptoms) are strategies that should be tried.78–82 If there is no symptom control despite taking these measures, the patient should be re-evaluated (see Diagnosis) and categorized as having PPI failure or refractory GERD.
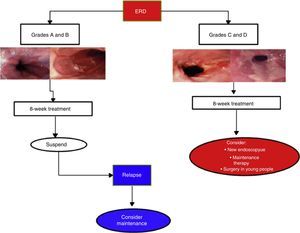
Erosive reflux disease treatmentIn cases of ERD, 8-week PPI treatment achieves symptom control and endoscopic cure of lesions in more than 80% of the cases.2,12,51 Duration depends on lesion severity (Fig. 4).
In cases of esophagitis C and D, experts recommend repeat endoscopy study at 8 to 12 weeks from the beginning of treatment to rule out BE that has been hidden by inflammation. Due to the fact that in C and D cases recurrence is nearly 100% at 6 months, endoscopic evaluation is recommended upon treatment completion.77,83 Maintenance treatment should be individualized in those cases and evaluated in accordance with patient expectations and his or her environment. These are options that can include continuous PPI treatment and even the possibility of surgical treatment (see Surgical treatment section).
Treatment of atypical gastroesophageal reflux diseaseExtraesophageal manifestations with typical symptoms: in patients with extra-esophageal symptoms (laryngitis, cough, and asthma) that also suffer from typical symptoms (heartburn and/or regurgitation), beginning therapy with a PPI trial (double dose for 8 to 12 weeks) is indicated.12 In these cases the response to atypical symptom is unpredictable. If there is good response, treatment should be prolonged and evidence suggests that the double dose should be prescribed for periods of up to 2-3 months.84 If there is no improvement in 3 months, the symptoms are most likely secondary to another cause and diagnosis should be reconsidered.
No evidence supports the use of prokinetics in patients with laryngopharyngeal reflux, whether combined with a PPI or as monotherapy. A recent systematic review, including only 4 high quality studies to be analyzed, determined that there was a high risk for bias due to the heterogeneity with which laryngopharyngeal reflux was defined.
In patients that present only with supposed atypical manifestations of GERD, a monitoring test, such as an impedance-pH study, is recommendable before administering PPI treatment.85 If there is no abnormal esophageal exposure to acid or positive symptom association, GERD is ruled out as the cause of extra-esophageal symptoms and the patient should be re-evaluated.
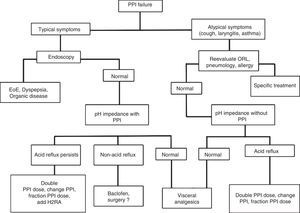
Treatment of erosive reflux disease in cases of proton pump inhibitor failure (refractory gastroesophageal reflux disease)Patients categorized as having PPI failure or refractory GERD according to Figure 3 should be re-evaluated to rule out other causes that could explain their symptoms.
If the patients persist with typical symptoms, endoscopy is recommendable to rule out entities that include eosinophilic esophagitis and organic dyspepsia, among others, and thus reconsider treatment.12,86 If endoscopy is negative, manometry should be carried out and then an esophageal impedance-pH monitoring study while the patient is taking a PPI.12,85 Based on this study, there are 3 possibilities (Fig. 5):
- 1.
Persistence of abnormal esophageal exposure to acid: in these cases a change of PPI, dividing or increasing the dose, or adding a nocturnal dose of H2RA can be recommended.
- 2.
Esophageal exposure to normal acid, but positive symptom association with non-acid reflux: baclofen (not available in Mexico) is indicated in these cases, and although still controversial, some studies suggest surgical treatment could be beneficial.72,73,87,88
- 3.
Negative study (with no evidence of acid or non-acid reflux): GERD is ruled out as the cause of symptoms in these cases and they are classified as functional heartburn. Visceral analgesics and other neuromodulators, such as amitriptyline, desipramine, citalopram, and venlafaxine are indicated.89
If the patients persist with atypical symptoms and there is no improvement, they should be reevaluated by a specialist in otorhinolaryngology, pneumology, allergology, or psychiatry, depending on the case. If a pH study was not carried out during the initial evaluation of the case, it is important to perform one without treatment, and depending on the result, reconsider the treatment. If the pH study is negative, GERD can be ruled out as the cause of the symptoms, but if the study is positive, then the PPI should be changed or the dose divided or increased.
Treatment of special casesBarrett's esophagusDefinition of Barrett's esophagusBE is a premalignant lesion of the esophagus that is defined as the replacement of the squamous epithelium of the distal portion of the esophagus with columnar epithelium of any length, suspected by its endoscopic appearance and corroborated by a histopathology study reporting complete intestinal metaplasia.90,91
Prevalence of and risk factors for Barrett's esophagusGERD is the most important pathogenic factor for the development of BE and its prevalence in GERD patients varies from 10 to 15%. Additional factors such as race, age, male sex, smoking, obesity, and hiatal hernia, among others, also play a significant role.92
The intentional search for BE through endoscopy is justified in subjects that have various risk factors: men above 50 years of age, a history of GER symptoms of long progression (more than 5 years), especially if the patient presents with obesity or overweight.91,93
Just having symptoms of GER is not sufficient justification for performing endoscopy in the search for BE. Despite the fact that a considerable number of the adult population complains of reflux symptoms, only a small number of them develop BE. The prevalence of BE reported in the general population varies from 1.2 to 1.6%.94,95
Treatment and surveillance of Barrett's esophagusIdeally, cases of BE should be treated by a specialist and preferably at a referral center. Despite the fact that at present there is not enough evidence to support medical or surgical treatment as a strategy for preventing the neoplastic progression of BE, PPIs should be used, preferably in a continuous regimen, to control reflux symptoms.
Endoscopic surveillance at established intervals in accordance with the presence and grade of dysplasia is recommended in all patients with BE. The diagnosis of dysplasia should be confirmed by a second pathologist or, if possible, the sample should be sent to an expert pathologist. Intense PPI treatment between follow-up endoscopies is highly recommendable.
The recommended strategies for endoscopic vigilance are (Table 4):
- •
EB with no dysplasia: endoscopic surveillance with biopsy is recommended in the first year. If the patient continues with no dysplasia, endoscopy with biopsy should be performed every 5 years.
- •
BE with low-grade dysplasia: endoscopy with biopsy directed at the site of dysplasia every 6 months. After 2 consecutive years with no dysplasia, the patient can return to the endoscopy regimen for no dysplasia.
- •
BE with high-grade dysplasia: endoscopic or surgical resection based on the availability of resources and trained endoscopists or expert surgeons. If the patient is not a candidate for or does not accept resection, endoscopy with biopsy should be performed every 3 months.
Recommendations for Barrett's esophagus surveillance.
| Society | High-grade dysplasia | Low-grade dysplasia | No dysplasia |
|---|---|---|---|
| AGA94 | Definitive treatment EMR/RFA or surveillance every 3months | Repeat EGD in 6 months. If there is dysplasia: RFA or annual surveillance | EGD every 3-5 years. Select high-risk patients (RFA) |
| ASGEa | Definitive treatment | EGD every 6 months for 2 years, then every year | 2 EGDs during first year; then every 3 years |
| ACG98 | Definitive treatment | Repeat EGD at 6 months; then every year until regression | 2 EGDs during first year; then every 3 years |
EGD: esophagogastroduodenoscopy; EMR: endoscopic mucosal resection; RFA: radiofrequency ablation.
The current endoscopic BE treatment recommendation is endoscopic mucosal resection of the visible lesions and/or radiofrequency ablation of the residual BE to prevent metachronic lesions or recurrent neoplasia, as well as patient follow-up with endoscopic surveillance.
Endoscopic ablation therapy with liquid nitrogen in spray (cryotherapy) has been shown to be efficacious in small studies and has been proposed as alternative therapy for treating high-grade dysplasia in BE patients, but is not presently recommended as first-line therapy.
Endoscopic management of gastroesophageal reflux diseaseEndoscopic management of uncomplicated gastroesophageal reflux diseaseToday, the 2 clinically available methods for managing uncomplicated GERD are radiofrequency ablation (Stretta) and endoluminal fundoplication with EsophyX. The effectiveness of these methods has not been well confirmed and they should not be recommended in all patients.
Endoscopic management of complicated gastroesophageal reflux disease (esophageal stricture)- a.
The initial treatment of choice for managing esophageal strictures due to reflux is endoscopic dilation with plugs or balloon.
- b.
There is no evidence of a clear advantage in using plug dilation or balloon dilation for the management of esophageal strictures caused by reflux.
- c.
The management of refractory or complex strictures can include the use of fully covered self-expandable metal stents or endoscopic radial incision of the stricture.
- d.
Patients that receive endoscopic treatment of esophageal stricture due to reflux should also receive double dose, continuous PPI treatment.
Before considering surgery, it is indispensible to have objective documentation of GERD, whether demonstrating mucosal damage through endoscopy (esophagitis of at least grade B of the Los Angeles Classification, stricture or BE equal to or greater than 3cm in length) or through 24h esophageal pH study in the absence of epithelial alterations. Therefore, all patients that are candidates for surgery should undergo preoperative endoscopy and, in some cases, a pH study to confirm the diagnosis. It may be necessary to carry out an esophagogram in certain patients and ideally all should undergo a preoperative manometry test (see Diagnosis section).
Indications for surgical treatment of gastroesophageal reflux diseasePatients with an objective diagnosis of GERD can be considered for surgical treatment in the following cases:
- a.
Patients that present with a reduced quality of life, persistent symptoms that cause problems, and/or disease progression despite adequate PPI therapy.96,97
- b.
Patients with good PPI response, PPI-dependent, and with acceptable quality of life can be considered for surgery if they wish to have it. The patient must be informed as to the potential side effects and possible risks of the surgery.98,99
- c.
Anti-reflux surgery can improve quality of life in certain patients with NERD.100,101
- d.
Patients with extra-esophageal symptoms that have positive symptom correlation and good PPI response can benefit from anti-reflux surgery.102–105
- e.
Patients with GERD and obesity can benefit more from bariatric surgery, such as gastric bypass, than from anti-reflux surgery. Indications are based on body mass index and bariatric surgery criteria.106,107
- f.
Patients with large hiatal hernia (> 5cm).
- g.
Patients with aspiration pneumonia
Novel devices, such as the LINX and EndoStim, are currently being evaluated and have shown promising results. However, more comparative, long-term studies are required to determine their place in GERD treatment.108–111
Treatment of gastroesophageal reflux disease during pregnancyHeartburn is a frequent symptom during pregnancy and its main etiologic factor is the hormonal effect of progesterone. Complications of GERD during pregnancy are not common and therefore the routine performance of diagnostic studies, such as endoscopy, is not indicated.
Treatment should be carried out in ascending order, beginning with lifestyle and dietary modifications. First-line drug management should be based on antacids and sucralfate. If symptoms persist, an H2RA can be included. However, nizatidine should not be used, given that there have been reports of spontaneous abortions and congenital malformations in laboratory animals, leaving PPI use for untreatable or complicated symptoms and preferably from the second trimester of gestation. The FDA considers all PPIs class B drugs, except omeprazole, which is considered class C.112–114 In general, prokinetics are not recommended for GERD treatment, and so their use during pregnancy should be oriented toward the management of difficult-to-control nausea and vomiting, specifically with metoclopramide, categorized as a class B drug by the FDA.115
Treatment of gastroesophageal reflux in the elderlyThe aims of GERD treatment in persons above 65 years of age are essentially the same as those in patients below that age. Prevalence is difficult to establish, given that there are few studies specifically conducted on this age group and the concomitant diseases and other treatments hinder both the diagnosis and treatment of these patients. In general, frequency and symptom intensity in relation to heartburn and regurgitation are lower in patients above the age of 65, but complications of GERD, such as bleeding, stricture, or BE as primary manifestations of the disease are frequently found.
PPIs are the first-line treatment in any of the clinical presentations of GERD in this group of patients. The recommended doses are the same and it should be kept in mind that treatment will very likely be continuous. Special care must be taken in relation to PPIs that compete with other medications that have hepatic metabolism at the P450 cytochrome level, such as warfarin, phenytoin, theophylline, benzodiazepines, and calcium channel blockers. It appears that omeprazole and esomeprazole are the PPIs that compete the most for the hepatic metabolism site and so they should be used as little as possible.116
Financial disclosureThe authors and coauthors wish to thank the Asociación Mexicana de Gastroenterología for funding this work.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors and coauthors wish to thank Dr. Francisco Bosques-Padilla, President of the Asociación Mexicana de Gastroenterología for extending them his confidence in relation to this work.
They also wish to give special thanks to the Asociación Mexicana de Gastroenterología for their trust in this working group and the support and funding provided for carrying out this important project.
2015 GERD Study Group:
Carmona-Sánchez, Ramón. San Luis Potosí, San Luis Potosí, Mexico.
Noble-Lugo, Alejandra. Mexico City, Mexico.
Nogueira-de Rojas, José Ramón. Irapuato, Guanajuato, Mexico.
Soto-Pérez, Julio. Mexico City, Mexico.
Gómez-Escudero, Octavio. Puebla, Puebla, Mexico.
González-Martínez Marina. Mexico City, Mexico.
Torres-Barrera, Gustavo. Monterrey, Nuevo León, Mexico.
Abreu y Abreu, Ana Teresa. Mexico City, Mexico.
Uscanga-Domínguez, Luis. Mexico City, Mexico.
Icaza-Chávez, María Eugenia. Mérida, Yucatán, Mexico.
Bernal-Reyes, Raúl. Pachuca, Hidalgo, Mexico.
Hernández-Guerrero, Angélica. Mexico City, Mexico.
López-Colombo, Aurelio. Puebla, Puebla, Mexico.
Coss-Martínez, Enrique. Mexico City, Mexico.
Torres-Villalobos, Gonzalo. Mexico City, Mexico.
Please cite this article as: Huerta-Iga F, Bielsa-Fernández MV, Remes-Troche JM, Valdovinos-Díaz MA, Tamayo-de la Cuesta JL, en representación del Grupo para el estudio de la ERGE 2015. Diagnóstico y tratamiento de la enfermedad por reflujo gastroesofágico: recomendaciones de la Asociación Mexicana de Gastroenterología. Revista de Gastroenterología de México. 2016;81:208–222.
The names of the 2015 GERD Study Group members are listed in the Appendix.
The recommendations for the diagnosis and treatment of GERD published in this article represent the final result of national experts on the theme after a complete and detailed review of the relevant and high quality scientific literature available. While it does not possess the methodological rigor of a clinical guideline or a consensus, the principal aim of this document is to offer the physician a practical path to making an accurate diagnosis and deciding on the best therapeutic option for the GERD patient through the responsible use of human and technologic resources, and at the same time, to increase treatment efficacy and lower its cost.