Irritable bowel syndrome (IBS) is characterized by recurrent abdominal pain, bloating, and changes in bowel habit.
AimsTo determine the clinical effectiveness of the antispasmodic agents available in Mexico for the treatment of IBS.
MethodsWe carried out a systematic review and meta-analysis of randomized controlled clinical trials on antispasmodic agents for IBS treatment. Clinical trials identified from January 1960 to May 2011 were searched for in MEDLINE, the Cochrane Library, and in the ClinicalTrials.gov registry. Treatment response was evaluated by global improvement of symptoms or abdominal pain, abdominal distention/bloating, and frequency of adverse events. The effect of antispasmodics vs placebo was expressed in OR and 95% CI.
ResultsTwenty-seven studies were identified, 23 of which fulfilled inclusion criteria. The studied agents were pinaverium bromide, mebeverine, otilonium, trimebutine, alverine, hyoscine, alverine/simethicone, pinaverium/simethicone, fenoverine, and dicyclomine. A total of 2585 patients were included in the meta-analysis. Global improvement was 1.55 (CI 95%: 1.33 to 1.83). Otilonium and the alverine/simethicone combination produced significant values in global improvement while the pinaverium/simethicone combination showed improvement in bloating. As for pain, 2394 patients were included with an OR of 1.52 (IC 95%: 1.28 a 1.80), favoring antispasmodics.
ConclusionsAntispasmodics were more effective than placebo in IBS, without any significant adverse events. The addition of simethicone improved the properties of the antispasmodic agents, as seen with the alverine/simethicone and pinaverium/simethicone combinations.
El Síndrome de Intestino Irritable (SII) se caracteriza por distensión y dolor abdominal recurrentes, además de cambios en el patrón defecatorio.
ObjetivoDefinir la utilidad clínica de los antiespasmódicos disponibles en México para el tratamiento del SII.
MétodosSe realizó una revisión sistemática y meta-análisis de ensayos clínicos controlados aleatorios de fármacos antiespasmódicos para el tratamiento del SII. Se identificaron los ensayos de enero 1960 a mayo de 2011, para esto se realizó una búsqueda bibliográfica en MEDLINE, the Cochrane Library y en el sitio de registro clinicaltrials.gov. Se tomaron como puntos a evaluar: evaluación global, mejoría de los síntomas, como dolor y distensión abdominal, así como los efectos adversos del tratamiento. El efecto de los fármacos antiespasmódicos vs placebo se expresó como RM e IC 95%.
ResultadosVeintisiete estudios fueron identificados, de los cuales 23 cumplieron los criterios de inclusión. Los medicamentos estudiados fueron pinaverio, mebeverina, otilonio, trimebutina, alverina, hioscina, alverina/simeticona, pinaverio/simeticona, fenoverina y diciclomina. Un total de 2585 pacientes fueron incluidos en el meta-análisis. La mejoría global fue de 1,55 (IC 95%: 1,33 a 1,83). Otilonio y alverina/simeticona tienen resultados que favorecen la mejoría global, la combinación de pinaverio/simeticona mostró mejoría en el alivio de la distensión. Respecto a mejoría del dolor, se incluyeron 2.394 con un OR de 1,52 (IC 95%: 1,28 a 1,80) a favor de los antiespasmódicos en general.
ConclusionesLos antiespasmódicos son más eficaces que el placebo en el SII, sin efectos secundarios significativos. La adición de simeticona parece que mejora las propiedades de los antiespasmodicos, tal es el caso de las combinaciones de alverina/simeticona y pinaverio/simeticona.
Irritable bowel syndrome (IBS) is a frequent gastrointestinal functional disorder in the western world and Mexico is not an exception.1 It is characterized by recurrent abdominal pain, bloating, and defecation disorders.2,3 The pathophysiology of IBS is not yet fully understood4,5; but increased pain sensitivity and altered small bowel and colon motility are main factors contributing to IBS symptoms. When compared with healthy controls, IBS patients demonstrate both visceral hypersensitivity and hyper-reactive motility.6
Antispasmodic agents are believed to reduce pain associated with IBS through the inhibition of contractile pathways in the gut wall and to improve bowel habits by increasing colonic transit time, therefore reducing stool passage frequency. Previous meta-analyses7,8 have proven the usefulness of antispasmodics alone in the treatment of IBS. Nonetheless, antispasmodic availability differs among countries. In the United States, the American College of Gastroenterology review concluded that data were insufficient for making a recommendation as to the effectiveness of the available antispasmodic agents.9 In Europe for example, the utility of the available antispasmodics has been evaluated,10 however, there is no information regarding the effectiveness of those available in Latin America. Therefore, we conducted a systematic review of antispasmodic agents, both alone and in combination, for the treatment of IBS, and carried out a meta-analysis of the data obtained. This was done to determine the clinical effectiveness of the available antispasmodic agents as sole formulations or in combination with simethicone, and to update the current information on IBS treatment in Mexico.
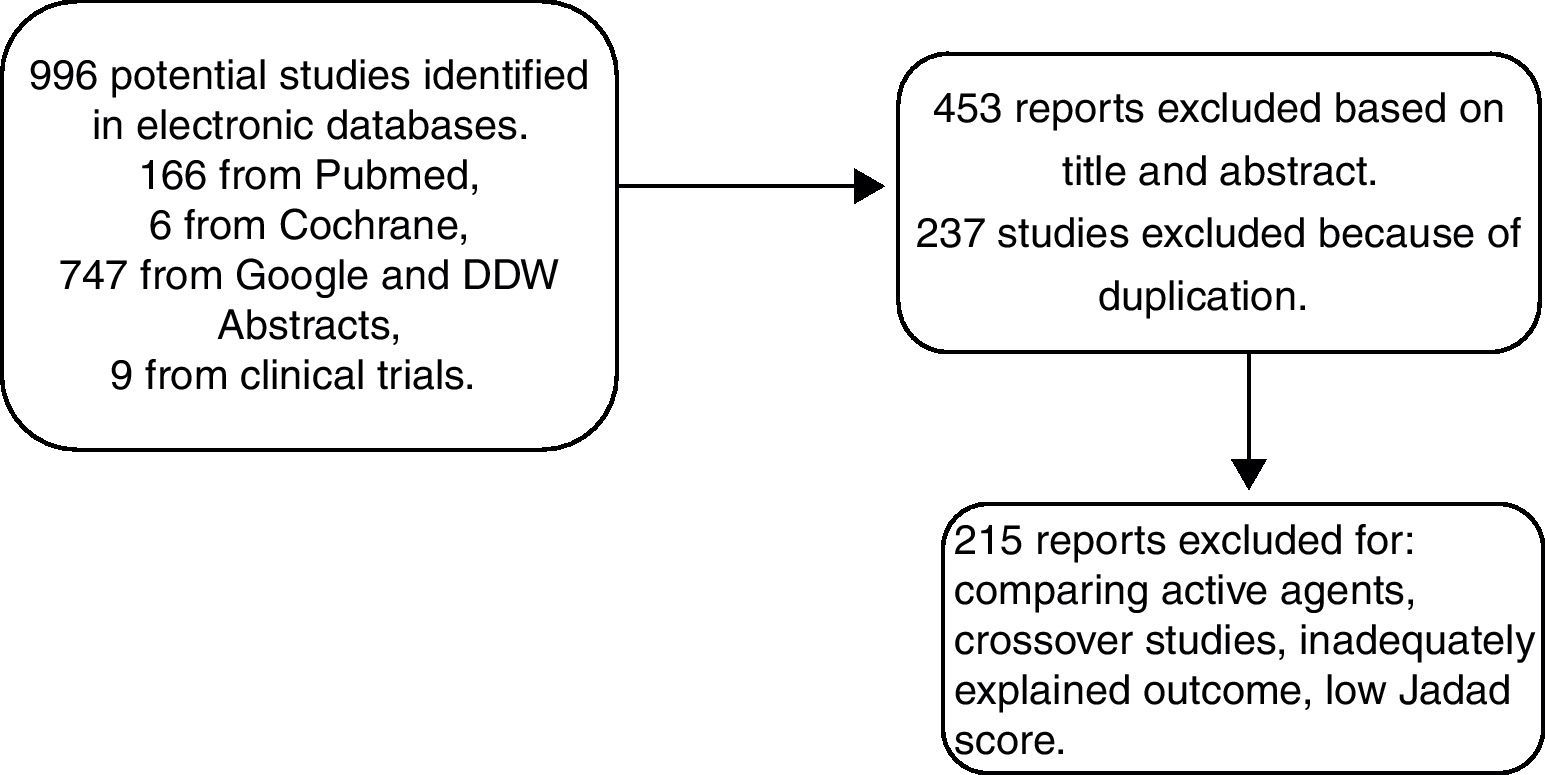
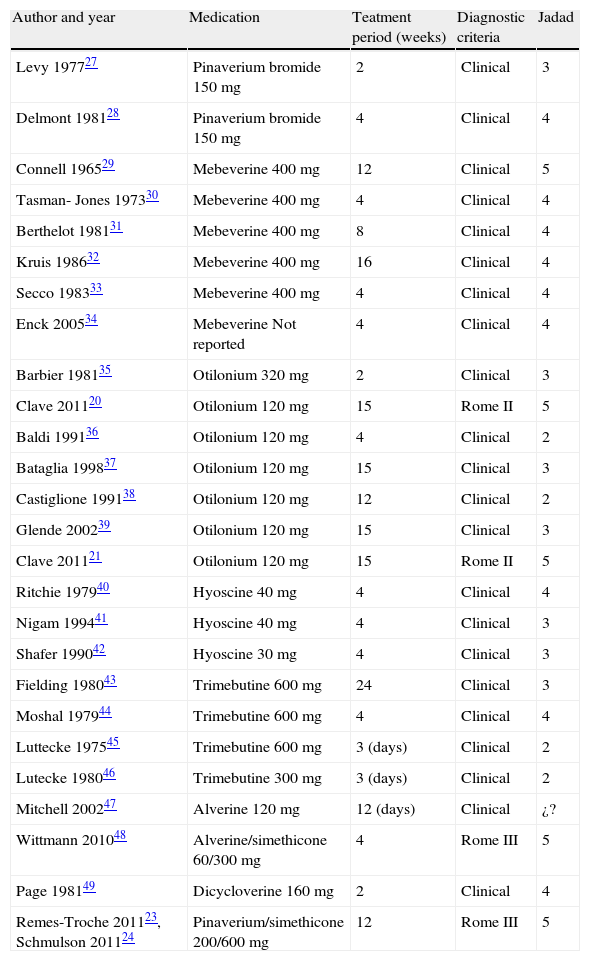
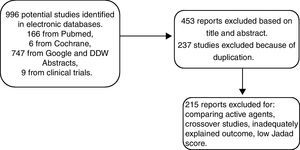
MethodsTo determine the antispasmodic agents that are available in Mexico, we reviewed the therapeutic index of the Dictionary of Medical Specialties (Diccionario de Especialidades Médicas), PLM®, Mexico-2011. We focused the search on section A3 of the index that lists all the agents for functional gastrointestinal disorders. The identified antispasmodics were further searched for in a systematic review conducted in MEDLINE, Cochrane Library, and ClinicalTrials.gov from January 1960 to May 2011 and in abstracts presented at the Digestive Disease Week (DDW) and the Mexican Disease Week (Semana Nacional de Gastroenterología) from 2010-2011. The agents listed in Table 1 were analyzed. Accordingly, the search terms were Irritable Bowel Syndrome and the following antispasmodics: pinaverium bromide, mebeverine, otilonium, trimebutine, alverine, hyoscine, alverine/simethicone, pinaverium bromide/simethicone, alverine/simethicone, fenoverine, and dicyclomine. Two physicians conducted the search, then reviewed the results and resolved the existing discrepancies. Figure 1 explains the selection process for including papers in the meta-analysis. Articles selected for review were those in which the authors employed the same inclusion criteria. Afterwards, the studies were reexamined to confirm that they fulfilled the inclusion criteria. Finally, the meta-analysis was conducted according to predetermined protocols and following the standard recommendations proposed by Sack et al.11 These recommendations consist of a rigorous review which includes the aspects listed in Table 2. When information was lacking, we contacted the authors for its completion.
Clinical trials on antispasmodics that fulfilled inclusion criteria.
| Author and year | Medication | Teatment period (weeks) | Diagnostic criteria | Jadad |
| Levy 197727 | Pinaverium bromide 150 mg | 2 | Clinical | 3 |
| Delmont 198128 | Pinaverium bromide 150 mg | 4 | Clinical | 4 |
| Connell 196529 | Mebeverine 400 mg | 12 | Clinical | 5 |
| Tasman- Jones 197330 | Mebeverine 400 mg | 4 | Clinical | 4 |
| Berthelot 198131 | Mebeverine 400 mg | 8 | Clinical | 4 |
| Kruis 198632 | Mebeverine 400 mg | 16 | Clinical | 4 |
| Secco 198333 | Mebeverine 400 mg | 4 | Clinical | 4 |
| Enck 200534 | Mebeverine Not reported | 4 | Clinical | 4 |
| Barbier 198135 | Otilonium 320 mg | 2 | Clinical | 3 |
| Clave 201120 | Otilonium 120 mg | 15 | Rome II | 5 |
| Baldi 199136 | Otilonium 120 mg | 4 | Clinical | 2 |
| Bataglia 199837 | Otilonium 120 mg | 15 | Clinical | 3 |
| Castiglione 199138 | Otilonium 120 mg | 12 | Clinical | 2 |
| Glende 200239 | Otilonium 120 mg | 15 | Clinical | 3 |
| Clave 201121 | Otilonium 120 mg | 15 | Rome II | 5 |
| Ritchie 197940 | Hyoscine 40 mg | 4 | Clinical | 4 |
| Nigam 199441 | Hyoscine 40 mg | 4 | Clinical | 3 |
| Shafer 199042 | Hyoscine 30 mg | 4 | Clinical | 3 |
| Fielding 198043 | Trimebutine 600 mg | 24 | Clinical | 3 |
| Moshal 197944 | Trimebutine 600 mg | 4 | Clinical | 4 |
| Luttecke 197545 | Trimebutine 600 mg | 3 (days) | Clinical | 2 |
| Lutecke 198046 | Trimebutine 300 mg | 3 (days) | Clinical | 2 |
| Mitchell 200247 | Alverine 120 mg | 12 (days) | Clinical | ¿? |
| Wittmann 201048 | Alverine/simethicone 60/300 mg | 4 | Rome III | 5 |
| Page 198149 | Dicycloverine 160 mg | 2 | Clinical | 4 |
| Remes-Troche 201123, Schmulson 201124 | Pinaverium/simethicone 200/600 mg | 12 | Rome III | 5 |
The table shows all trials initially considered for analysis. Those with a Jadad score below 3 were subsequently eliminated. Total daily dosages are described.
Recommendations by Sacks for conducting a meta-analysis.
| Search of the literature |
| List of trials analyzed |
| Treatment assignment |
| Ranges of patient characteristics, diagnoses and treatments |
| Combinability criteria |
| Measurement |
| Control and Measurement of potential bias |
| Statistical analysis |
| Sensitivity analysis |
| Application of results |
| Remaining problems |
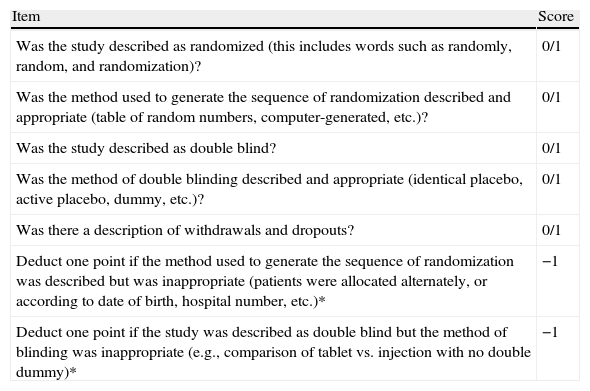
The following criteria were used for selecting the studies: Randomized controlled trials that included subjects over 16 years of age, a diagnosis of IBS based on accepted clinical criteria (Rome I, II or III), or diagnostic criteria supplemented with specific investigations when needed. Antispasmodic agents versus placebo studies were included when there was a minimum 14-day treatment period. Treatment response was evaluated by the global improvement of symptoms or abdominal pain (reported by patients or physicians), abdominal distention/bloating, and frequency of adverse events. Methodological quality was evaluated using the Jadad scale12 (Table 3). This scoring scale evaluates each trial according to the quality of the scientific description of the randomization method. The scale ranges from 0 to 5 points: A score of 2 or less is considered low quality and 3 or higher is considered high quality.12–14 The present review only included studies with a Jadad score of 3 or above.
Jadad Score Items.
| Item | Score |
| Was the study described as randomized (this includes words such as randomly, random, and randomization)? | 0/1 |
| Was the method used to generate the sequence of randomization described and appropriate (table of random numbers, computer-generated, etc.)? | 0/1 |
| Was the study described as double blind? | 0/1 |
| Was the method of double blinding described and appropriate (identical placebo, active placebo, dummy, etc.)? | 0/1 |
| Was there a description of withdrawals and dropouts? | 0/1 |
| Deduct one point if the method used to generate the sequence of randomization was described but was inappropriate (patients were allocated alternately, or according to date of birth, hospital number, etc.)* | −1 |
| Deduct one point if the study was described as double blind but the method of blinding was inappropriate (e.g., comparison of tablet vs. injection with no double dummy)* | −1 |
0=No; 1=Yes; −1=Point deduction*
The Critical Appraisal Skills Program (CASP) was used with Excel for Windows 2000 (Microsoft, USA) for calculating the meta-analysis, and the Comprehensive Meta-Analysis V2© by Biostat, Inc. was also used. Each analysis was run in accordance with standard methodological procedures using the following determinations: a test of heterogeneity15 between active versus control group results. This was considered significant when p<0.10 and/or the value of I2 >25%. Antispasmodic efficacy was defined according to the Peto method.16 In addition, a funnel plot graph17 was used to evaluate publication bias. Finally, the Number Needed to Treat (NNT)18 was determined using the formula NNT=1-TBE (1-OR)/TBE divided by TBE (1-TBE) (1-OR).
ResultsIncluded randomized clinical trialsA total of 450 publications were identified from 1960 to 2011. Twenty-seven studies fulfilled the inclusion criteria and 23 were included in the meta-analysis after the Jadad score was determined. Nine specific agents were tested as monotherapies, plus the alverine/simethicone and pinaverium/simethicone combinations. For the global assessment endpoint, a total of 2585 patients were included; 1297 were allocated to active treatment groups and 1288 to the placebo group. Of these trials, 6 studied mebeverine, 7 otilonium, 3 hyoscine, 2 trimebutine, one alverine plus simethicone (alverine/simethicone), one dicyclomine, 2 pinaverium bromide, and one pinaverium bromide plus simethicone (pinaverium bromide/simethicone). Despite the systematic search for trials with high quality criteria, not all trials reported the effect on all the studied outcomes, i.e. global assessment, pain, abdominal distention/bloating, and adverse events, and therefore a different number of trials was considered for each tested variable.
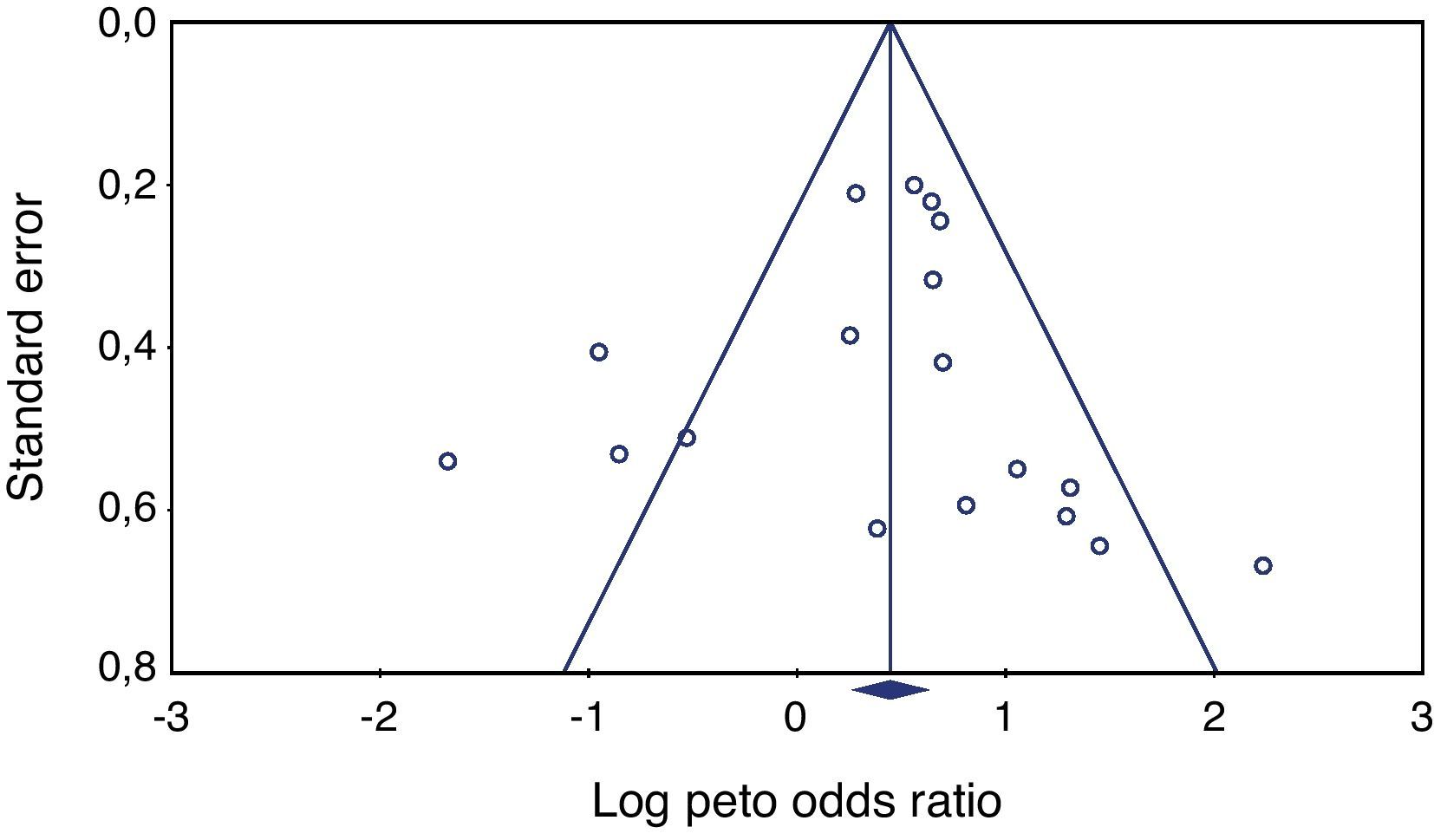
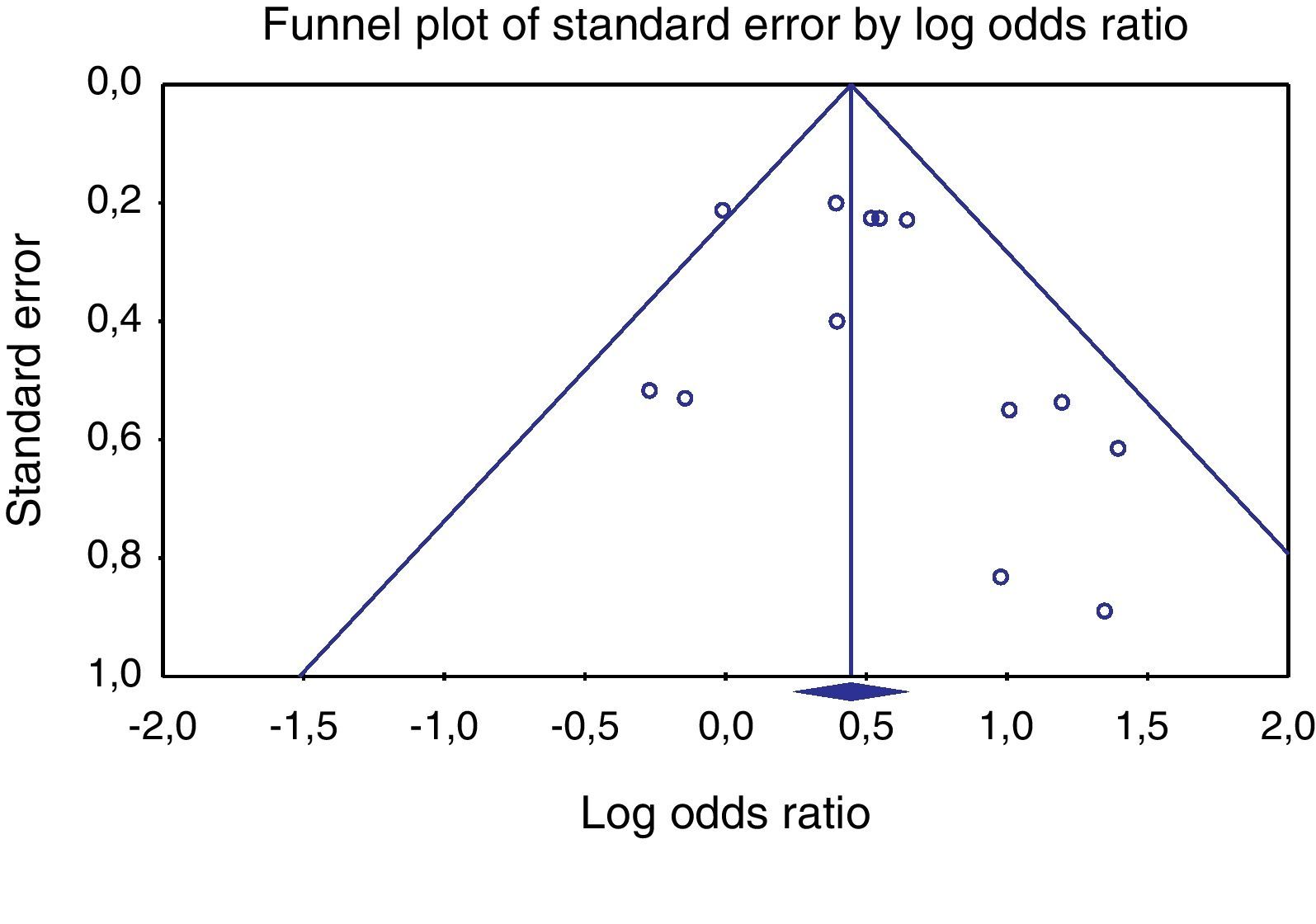
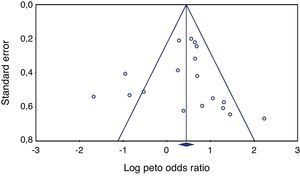
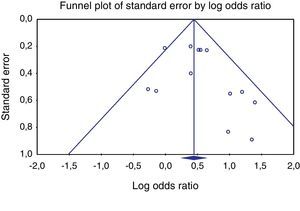
Heterogeneity testing was not significant (p≥0.05), allowing the use of the Peto method and fixed effects. Publishing bias evaluation was tested using the funnel plot shown in Figures 2 and 3.
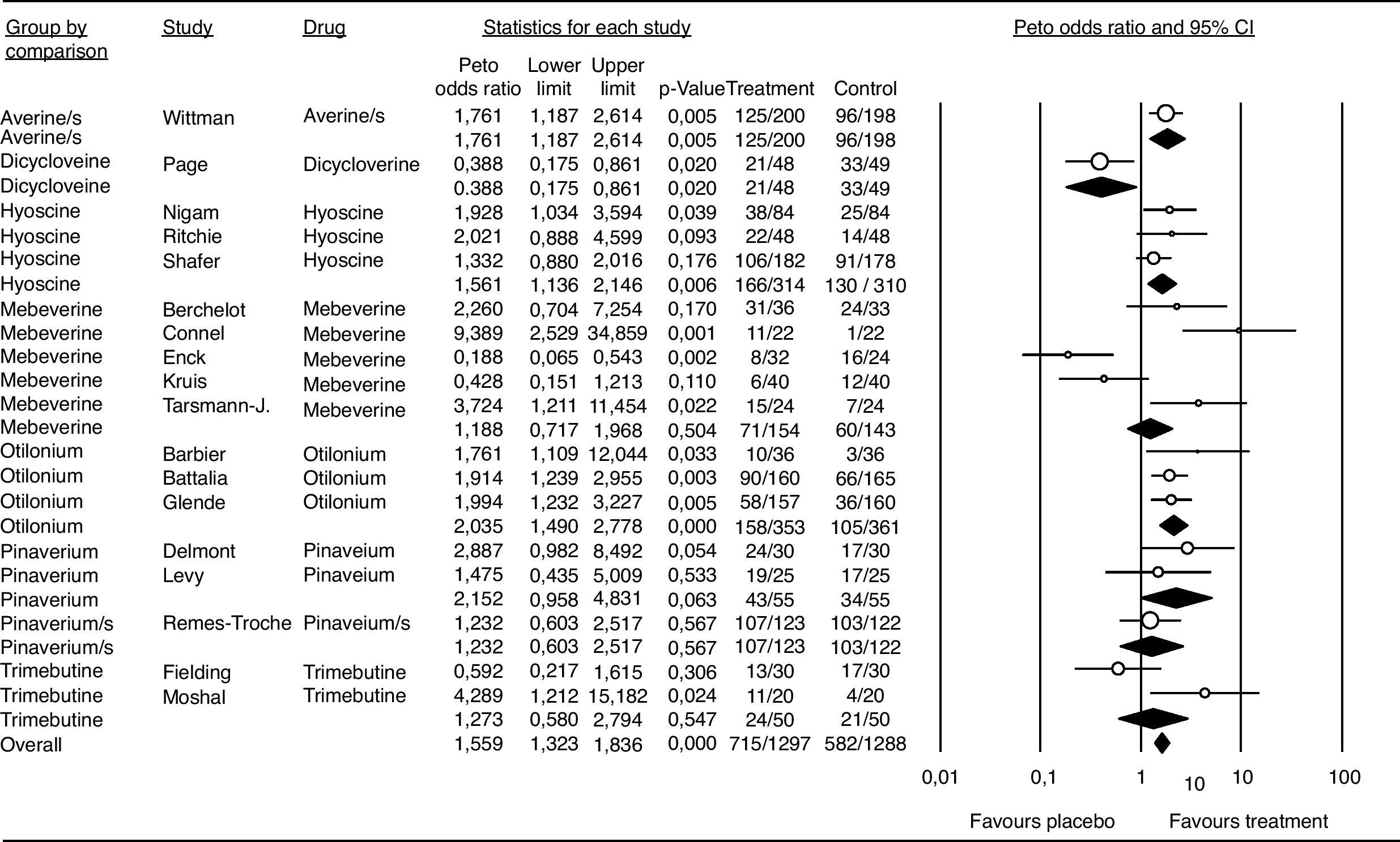
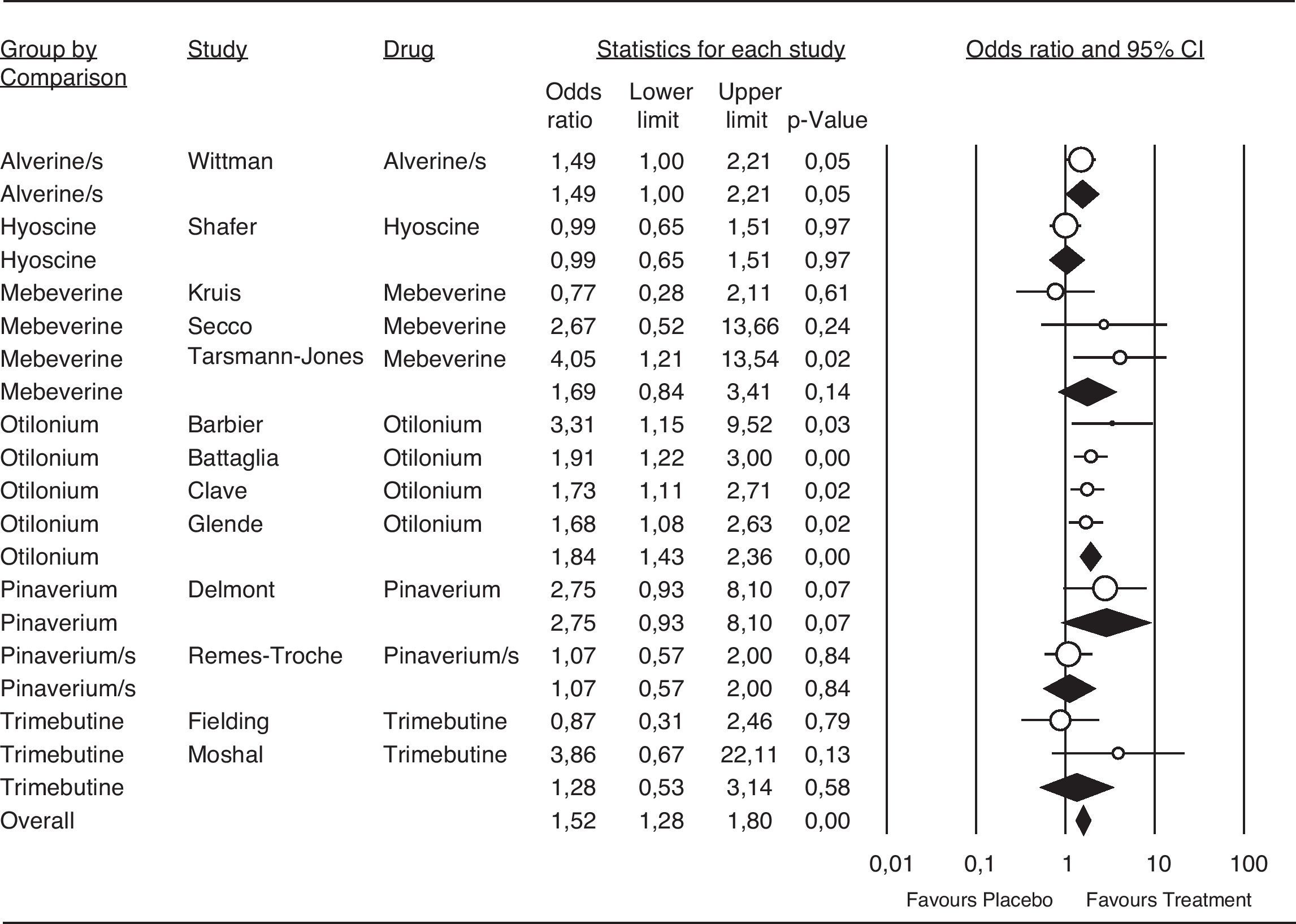
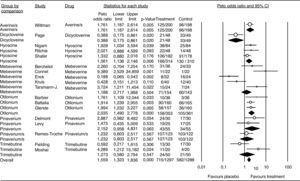
Meta-analysisPatient global assessmentOf the 27 trials included for the global assessment analysis, only 18 had sufficient data for consideration. The total sample included 2585 patients, with 1297 allocated to the treatment group. Global assessment with an OR of 1.55 and a 95%CI of 1.33 to 1.83 was confirmed for all antispasmodics (Fig. 4). Based on the Peto method, a significant difference favoring the alverine/simethicone combination and otilonium was observed. The OR for otilonium was 2.03 (95% CI 1.49-2.77), and was 1,76 (95%CI 1.18-2.61) for the alverine/simethicone combination. The OR for pinaverium bromide was 1.48 (95%CI 0.95-4.63), as shown in Figure 4.
Efficacy of antispasmdics on IBS global assessment. The vertical bars represent the difference in the response rates between antispasmodics (Treatment) and placebo. The white circles represent the OR and the horizontal lines the 95%CI. Overall response of each type of antispasmodic is represented by the black diamonds. Antispasmodics were effective on the global assessment of IBS symptoms (Overall).
Alverine/s: Alverine/simethicone; Pinaverium/s: Pnaverium/simethicone.
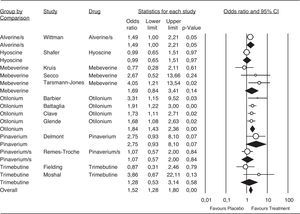
A total of 13 trials contained enough data to evaluate pain relief. They included a total of 2394 patients, 1053 allocated to otilonium and 409 to the alverine/simethicone combination treatment; both providing the highest number of patients for a particular therapy. Antispasmodics tested for abdominal pain relief showed an OR of 1,52 (95%CI 1.28 to 1.80), favoring these agents when compared with placebo. Complete results are shown in Figure 5.
Efficacy of antispasmodics on pain relief. The vertical bars represent the difference in the response rates between antispasmodics (Treatment) and placebo. The white circles represent the OR and the horizontal lines the 95%CI. Overall response of each type of antispasmodic is represented by the black diamonds. Antispasmodics were effective on abdominal pain (Overall). Specifically by type of antispasmodics, only Alverine/s and Otilonium were effective.
Alverine/s: Alverine/simethicone; Pinaverium/s: Pnaverium/simethicone.
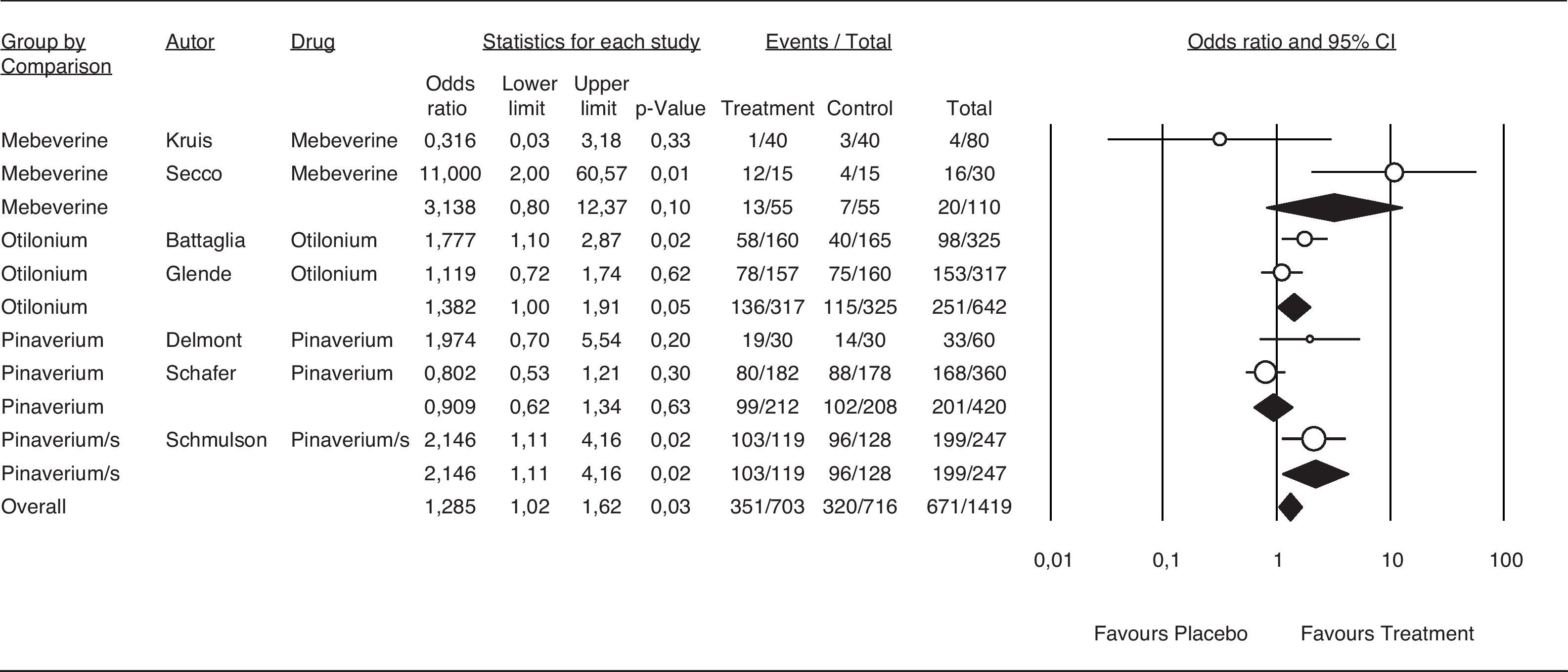
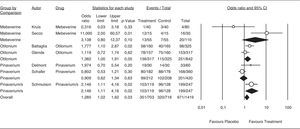
The results for the efficacy analysis of abdominal distention/bloating relief are shown in Figure 6; however, few trials appropriately report this effect. Although efficacy is borderline with an OR of 1.455 (95%CI 1.17-1.81), there is a consistent trend of antispasmodics as a group to relieve abdominal distention/bloating. The combination pinaverium/simethicone showed an OR of 1.455 (95%CI 1.11-9.91).
Efficacy of antispasmodics on abdominal distention/bloating. The vertical bars represent the difference in the response rates between antispasmodics (Treatment) and placebo. The white circles represent the OR and the horizontal lines the 95%CI. Overall response of each type of antispasmodic is represented by the black diamonds. Antispasmodics were effective on abdominal distension/bloating (Overall). Specifically by type of antispasmodics, only the Pinaverium/s (Pinaverium+Simethicone) combination was effective.
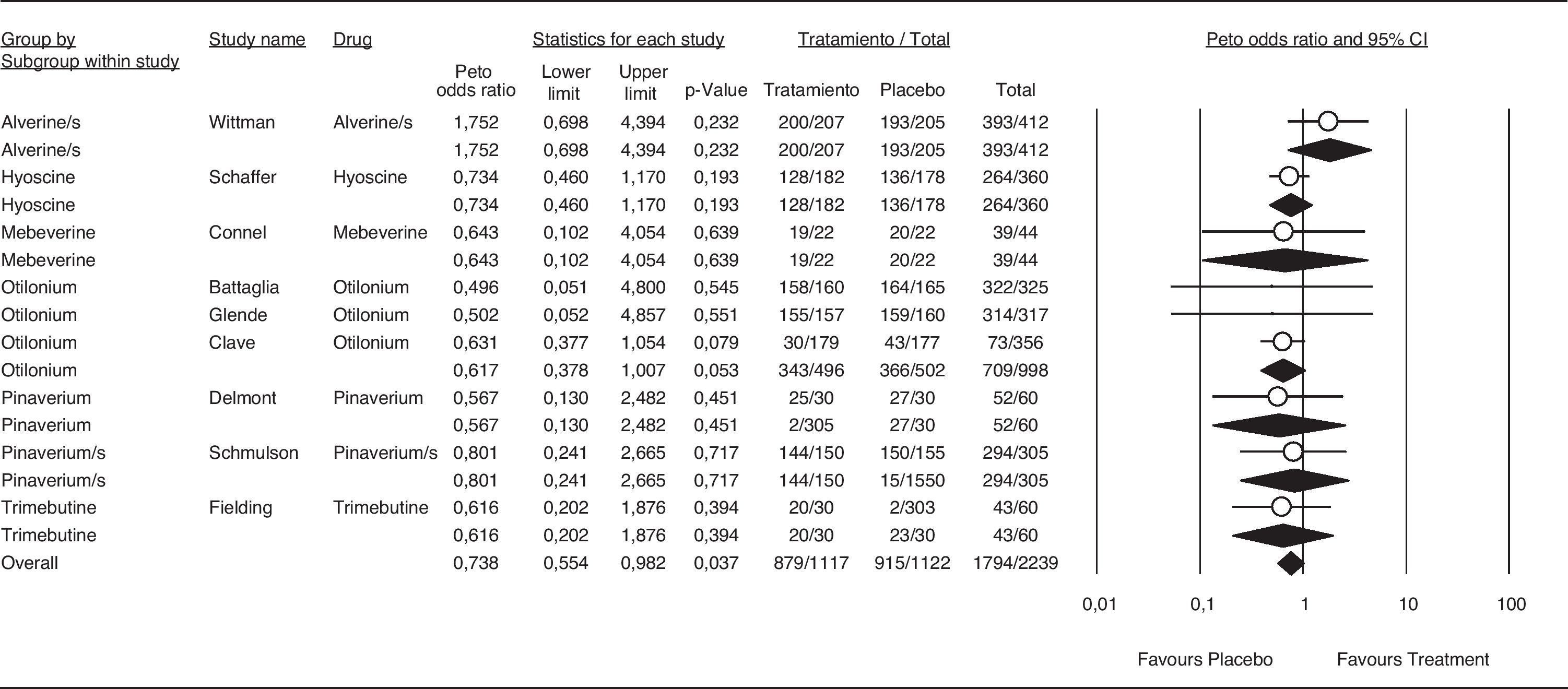
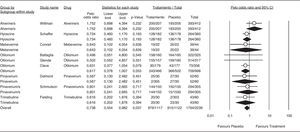
The OR for the antispasmodic treatment group was 0.738 (95% CI 0.54-0.98). Results are shown in Figure 7. Previous meta-analyses7,8,19 have shown antispasmodic treatments to be safe. All trials included in the present meta-analysis consistently showed safety, corroborating the safe profile for these agents demonstrated in the most recent reports.20,21
Number Needed to Treat (NNT)The NNT was calculated only for the antispasmodics showing a significant value of 10 in global assessment (95% CI 6.0- 41.0). The NNT for global improvement was 7 for Otilonium and 8 for Alverine/simethicone and 8 and 11 for pain relief, respectively.
DiscussionDecision-making in medical practice today often requires answers to concrete questions. In 1976 Glass22 proposed a set of different statistical tests in the meta-analysis for quantitative and qualitative analyses based on results from independent trials. Previous studies mention the discrepancy among different trials due to a lack of uniformity in diagnostic criteria. In the present analysis, we decided to remove those trials that had a Jadad score below 3, in other words, of low quality (Table 3). We felt that an analysis of low quality trials could be a significant source of bias for the interpretation of results.
For global assessment, only otilonium 2,035 (95% CI 1.49-2.77) and the alverine/simethicone combination 1.76 (95%CI 1.18-2.61) showed significant values. For pain relief, alverine/simethicone 1.48 (95% CI 1.00-2.19) and otilonium 1.83 (95% CI 1.43-2.34) demonstrated significant values. Recently, two abstracts that studied the combination of pinaverium bromide/simethicone came to interesting conclusions. They reported that pinaverium bromide/simethicone was effective for relieving abdominal pain in patients with active IBS23 and in improving bloating,24 but not visible abdominal distension. These results suggest an effect on visceral perception.24 However, the published abstracts did not contain the data necessary for the current meta-analysis. Therefore, the authors were contacted for the completion of the required information. This is the first meta-analysis to incorporate the combination of antispasmodics with an anti-foaming agent that may constitute a new therapeutic option.
The combination pinaverium/simethicone resulted in an OR of 1.45 (95% CI 1.11-3.91) for bloating. The effect with the addition of simethicone was greater than that of the antispasmodic alone, and was similar to the effect shown by the alverine/simethicone combination. The NNT, calculated from the systematic review or meta-analysis of randomized clinical trials, is a valuable aid in making clinical decisions.18 The NNT was recently included in a meta-analysis of medications to treat IBS.25 Results showed a wide range of NNT values; from 4 to 20 for 5-HT3 antagonists and 5HT4 agonists. Other analyses also included antispasmodic medications21 with a wide NNT range; from 3 to 25 depending on the particular antispasmodic tested. We only calculated the NNT for the global assessment and pain relief in those medications with a significant OR and 95% CI. We found that the antispasmodics with the lowest NNT to achieve global improvement were otilonium and the alverine/simethicone combination; an NNT of 7 for otilonium and 8 for the combination. For pain relief, the NNT was 7 for otilonium and 11 for alverine/simethicone. The NNT from a meta-analysis should be viewed with caution,26 since these data vary according to patient baseline risk and this could be significantly different among the trials included in the analysis.
The weaknesses in this meta-analysis were the variability among the groups of patients across different trials and the insufficiency of data such as treatment adherence and the length of time during which each patient took the medications.
ConclusionsThe lack of methodological coherence in trials published before 1995 makes it difficult to reach final conclusions about the efficacy of certain medications. Publication of the Rome II and III trial design recommendations for functional bowel disorders is an advance in the methodological quality of antispasmodic trials; however, few of them include the recent diagnostic criteria in their design. Antispasmodic agents are better than placebo for treating IBS, with almost no serious adverse events. The alverine/simethicone combination and otilonium showed a NNT of 7 to 11 with significant results for global assessment and pain relief. Pinaverium/simethicone also showed effectiveness in relieving bloating and had better results than pinaverium alone. Future clinical investigations should include the combination of antispasmodics and anti-foaming agents to improve the clinical effect of antispasmodics.
Financial disclosureThis study was supported by the Centro Regional para el Estudio de las Enfermedades Digestivas (CREED), Hospital Universitario Dr. José E. Gonzalez.
Conflict of interestThe authors have no conflicts of interest to declare.
We wish to thank Sergio Lozano Rodriguez, M.D. and Brenda Esqueda M.D. for their help in reviewing the manuscript.