Adequate drying and proper storage of flexible endoscopes are essential for maintaining quality in their reprocessing. The aim of the present study was to evaluate the drying stages, storage, and channel conditions of endoscopes through borescope inspection.
Material and methodsThe personnel responsible for endoscope reprocessing were interviewed. Storage conditions at 10 endoscopy facilities were inspected and an internal examination of the channels and ports of the stored equipment was carried out, utilizing a borescope. A total of 74 stored endoscope channels were evaluated.
ResultsOnly 10% of the facilities inspected utilized transport cases for storage and only 10% had rooms exclusively used for storage. Sixty percent of the facilities did not perform any shelf-life control. All the channels evaluated were scratched and fluids were present on 69% of them.
ConclusionsEndoscope reprocessing can be improved through the implementation of drying and storage control and validation tools, as well as the use of borescopes and periodic clinical audits.
El secado y almacenamiento adecuados de los endoscopios flexibles son esenciales para mantener la calidad en su reprocesamiento. El objetivo del presente estudio fue evaluar las etapas de secado y almacenamiento, así como las condiciones de los canales de los endoscopios por medio de inspección con boroscopio.
Materiales y métodosSe entrevistó al personal responsable de reprocesar los endoscopios. Se inspeccionaron las condiciones de almacenamiento en 10 centros endoscópicos y se realizó un examen interno de los canales y puertos del equipo almacenado utilizando un boroscopio. Se evaluó un total de 74 canales de endoscopios.
ResultadosÚnicamente el 10% de los centros inspeccionados utilizaron cajas transportadoras para almacenamiento y sólo 10% tenían cuartos para uso exclusivo de almacenamiento. Sesenta por ciento de los centros no realizó ningún control de vida de anaquel. Todos los canales evaluados presentaron raspaduras y se encontró líquido en el 69% de ellos.
ConclusionesEl reprocesamiento del endoscopio puede ser mejorado por medio de la implementación de controles del secado y almacenamiento, así como mediante herramientas de validación, al igual que por medio de la utilización de boroscopios y auditorías clínicas periódicas.
Gastrointestinal endoscopes are reusable complex medical devices, with multiple long, opaque, and angulated lumens. To ensure safe reuse, endoscopes require a multistep cleaning process, failure of which may culminate in cross-contamination.1 The reprocessing steps include pre-cleaning, leak testing, cleaning, drying, high-level disinfection, final drying, and storage. Cleaning is the most vulnerable step that can fail, resulting in possible outbreaks and health problems.2,3 However, the final steps have also been described as points of weakness that can facilitate the proliferation of microorganisms due to recontamination and biofilm formation, with their involvement in infectious cases reported in several studies.4–8 Drying and storage are crucial steps in maintaining the effectiveness of properly performed reprocessing. However, the guidelines and manufacturers do not provide detailed information on the specific steps that generate differences in processes and failures in execution.7,9 Furthermore, simethicone, a non-water-soluble silicone substance used by endoscopists to enhance images by removing bubbles from mucosal surfaces, can remain adhered to the channel after reprocessing, hindering proper drying and facilitating microbiological growth.10
The permanence of fluids in the channels, often associated with grooves and residues, facilitates the formation of biofilms because microorganisms have tropism for damaged areas and use residues and fluids as substrates for adhesion and proliferation.11 Biofilms are difficult or impossible to remove, are resistant to the action of disinfectant solutions, and their presence may result in reprocessing failures.11–13 Additionally, the final rinse with non-sterile water may allow residual waterborne bacteria to remain in the channels, which, when subjected to insufficient drying, favors microbiological growth, indicating the importance of correct execution, as well as the potential consequences of its failure.13,14
In clinical practice, monitoring and validation of the processing steps have been recommended to improve the process. However, there is limited verification of the internal conditions of endoscope channels, especially regarding the presence of moisture.9
Strategies to validate the drying of endoscopes may involve the use of cobalt blue papers, although they are limited by their inaccuracy in verifying humidity, owing to their sensitivity.15 Another possibility is the use of a borescope - an optical tool designed to visualize the channels of the endoscope.16,17 A borescope can be used to inspect, record, and analyze findings, relating them to possible failures in the reprocessing steps, especially drying.9 Considering the benefits of borescope use, this technology has been recommended by certain guidelines for use at endoscopy facilities.18,19 Previous studies have reported the identification of humidity in endoscope channels using a borescope, which is indicative of important failures in the drying phase.17,20,21 Given the complex structure of gastrointestinal endoscopes, the infection risks involved in reprocessing failures, and the difficulty in validating the reprocessing steps in clinical practice, our aim was to investigate the relationship between the findings of borescope inspection of the internal channels of stored endoscopes and the quality and safety of drying and storing those devices.
In view of the risks involving the permanence of fluids in the endoscope channels and the ability of inadequate storage conditions to facilitate recontamination of ready-to-use endoscopes, we used a borescope to evaluate the drying, storage, and channel conditions of endoscopes at endoscopy facilities.
Material and methodsA cross-sectional study was conducted at endoscopy facilities in the city of Belo Horizonte, Minas Gerais, Brazil. A previous survey determined that 82 endoscopy facilities were registered through the Health Establishment Registry (CNES DATA SUS, the Spanish acronyms). After excluding duplicate facilities and those that did not perform gastrointestinal endoscopy, 51 facilities were contacted. Five attempts were made to contact the facilities via e-mail and telephone. In the event of no response, the service was not considered eligible. Finally, 10 facilities agreed to participate in the study, in which 71 endoscopes were analyzed, totaling 74 inspected channels from 98 endoscopes. Some channels were not examined, as they did not meet the eligibility criteria.
The inclusion criteria were endoscopy facilities that performed upper gastrointestinal endoscopy, colonoscopy, endoscopic retrograde cholangiopancreatography (ERCP) and/or endoscopy in adult patients, and endoscopes with channels > 2.4 mm in diameter. Endoscopes in use at the time of the visit, or those awaiting repair or maintenance were excluded.
The study was conducted at three concurrent moments, beginning with the application of a semi-structured questionnaire based on applicable standards,18,22,23 followed by evaluation of the endoscope channels using variables associated with the reprocessing stages.
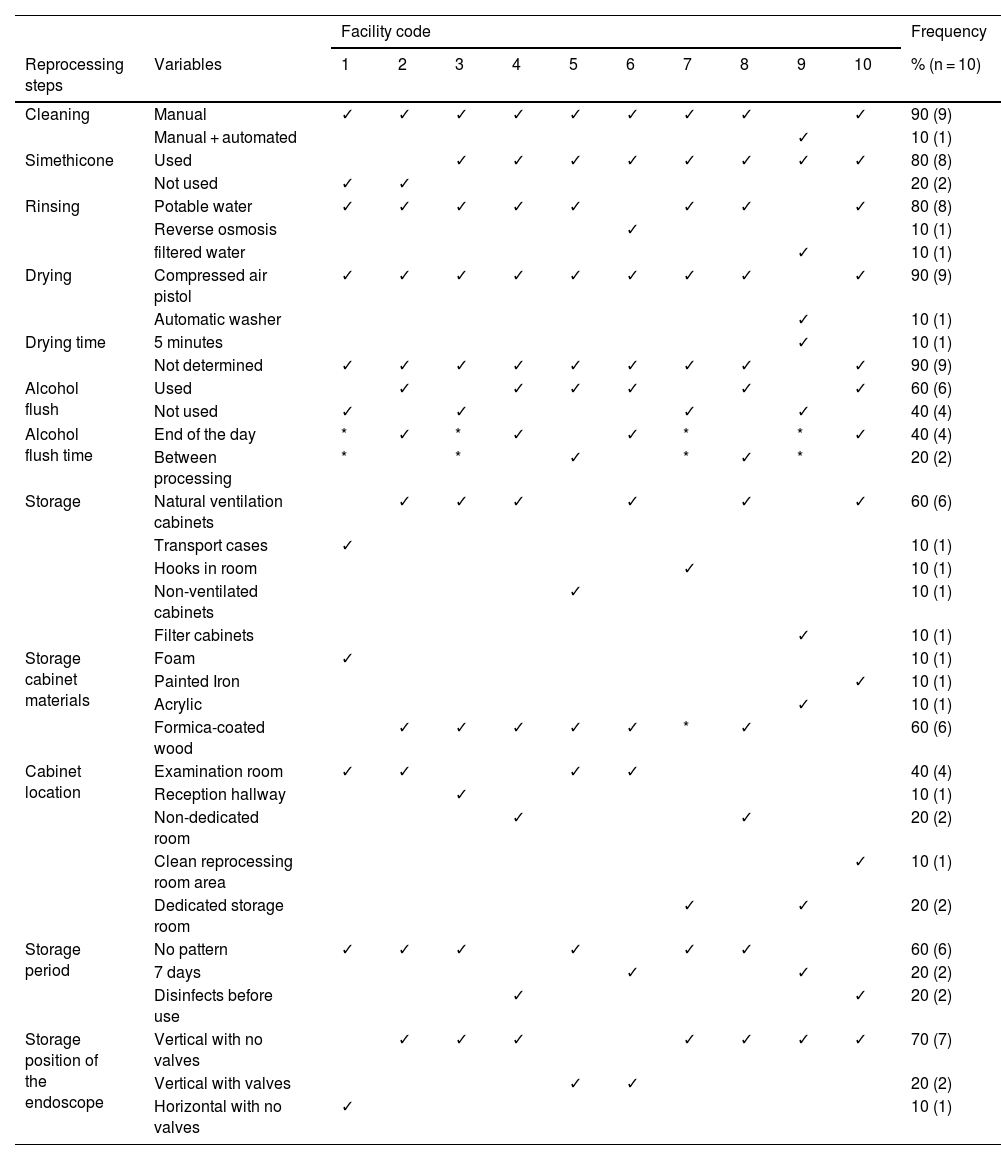
ResultsA total of 10 services were visited, all of which were located inside hospitals. Nevertheless, 30% (3/10) were managed by external clinics and the remaining 70% (7/10) by the corresponding hospital. The number of monthly procedures varied (100-775), with a mean of 264 procedures performed a month per service, with a range of 2-26 endoscopes utilized per service. Table 1 describes the situations of the variables analyzed.
Frequency of adherence to the reprocessing steps at the facilities (n = 10).
| Facility code | Frequency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reprocessing steps | Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | % (n = 10) |
| Cleaning | Manual | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 (9) | |
| Manual + automated | ✓ | 10 (1) | ||||||||||
| Simethicone | Used | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 80 (8) | ||
| Not used | ✓ | ✓ | 20 (2) | |||||||||
| Rinsing | Potable water | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 80 (8) | ||
| Reverse osmosis | ✓ | 10 (1) | ||||||||||
| filtered water | ✓ | 10 (1) | ||||||||||
| Drying | Compressed air pistol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 (9) | |
| Automatic washer | ✓ | 10 (1) | ||||||||||
| Drying time | 5 minutes | ✓ | 10 (1) | |||||||||
| Not determined | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 90 (9) | ||
| Alcohol flush | Used | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 60 (6) | ||||
| Not used | ✓ | ✓ | ✓ | ✓ | 40 (4) | |||||||
| Alcohol flush time | End of the day | * | ✓ | * | ✓ | ✓ | * | * | ✓ | 40 (4) | ||
| Between processing | * | * | ✓ | * | ✓ | * | 20 (2) | |||||
| Storage | Natural ventilation cabinets | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 60 (6) | ||||
| Transport cases | ✓ | 10 (1) | ||||||||||
| Hooks in room | ✓ | 10 (1) | ||||||||||
| Non-ventilated cabinets | ✓ | 10 (1) | ||||||||||
| Filter cabinets | ✓ | 10 (1) | ||||||||||
| Storage cabinet materials | Foam | ✓ | 10 (1) | |||||||||
| Painted Iron | ✓ | 10 (1) | ||||||||||
| Acrylic | ✓ | 10 (1) | ||||||||||
| Formica-coated wood | ✓ | ✓ | ✓ | ✓ | ✓ | * | ✓ | 60 (6) | ||||
| Cabinet location | Examination room | ✓ | ✓ | ✓ | ✓ | 40 (4) | ||||||
| Reception hallway | ✓ | 10 (1) | ||||||||||
| Non-dedicated room | ✓ | ✓ | 20 (2) | |||||||||
| Clean reprocessing room area | ✓ | 10 (1) | ||||||||||
| Dedicated storage room | ✓ | ✓ | 20 (2) | |||||||||
| Storage period | No pattern | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 60 (6) | ||||
| 7 days | ✓ | ✓ | 20 (2) | |||||||||
| Disinfects before use | ✓ | ✓ | 20 (2) | |||||||||
| Storage position of the endoscope | Vertical with no valves | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 70 (7) | |||
| Vertical with valves | ✓ | ✓ | 20 (2) | |||||||||
| Horizontal with no valves | ✓ | 10 (1) | ||||||||||
During the preparation of the patient, 80% (8/10) of the facilities used simethicone; 25% (2/8) of them injected simethicone into the channels during the execution of the endoscopic procedure. During drying, the airflow pressure was controlled in only one facility (at 1.5 atm). The use of tip protectors during storage was observed in 50% (5/10) of the facilities, and in 30% (3/10), the tip protectors were fitted with sponges or materials that did not allow for proper ventilation and cleaning. Non-conformities in the storage area were identified in all of the storage rooms inspected (Fig. 1).
Endoscope storage locations found at the facilities with non-conformities.
1) Presence of rough surface support and storage of other objects next to the endoscopes. 2) Presence of a non-ventilated tip protector on the endoscope and a cardboard box in the cabinet next to the equipment. 3) Equipment leaning against the wall of the storage cabinet. 4) Presence of objects and cardboard boxes in the endoscope storage room. 5) Room dedicated to storage with holes in the walls and incomplete sealing. 6) Wooden storage stand. 7) Presence of objects stored next to the endoscope, cloths for moisture retention, and insufficient height of storage cabinet. 8) Horizontal storage in an unventilated carrying case. 9) Insufficient height of the cabinet, and endoscopes touching the floor of the cabinet. 10) Use of sponge tip protectors, and unventilated area.
Regarding the evaluation of the endoscopes, 71 pieces of equipment were inspected. The endoscopes were stored for an average of 113 h (range: 16-720 h), and only 1.5% (1/71) were in a horizontal position, while the remaining 98.5% (70/71) were hanging vertically. Moreover, 91.5% (65/71) of the inspected endoscopes had no valves positioned in the device, while the remaining 8.5% (6/71) had at least one valve positioned in the channels.
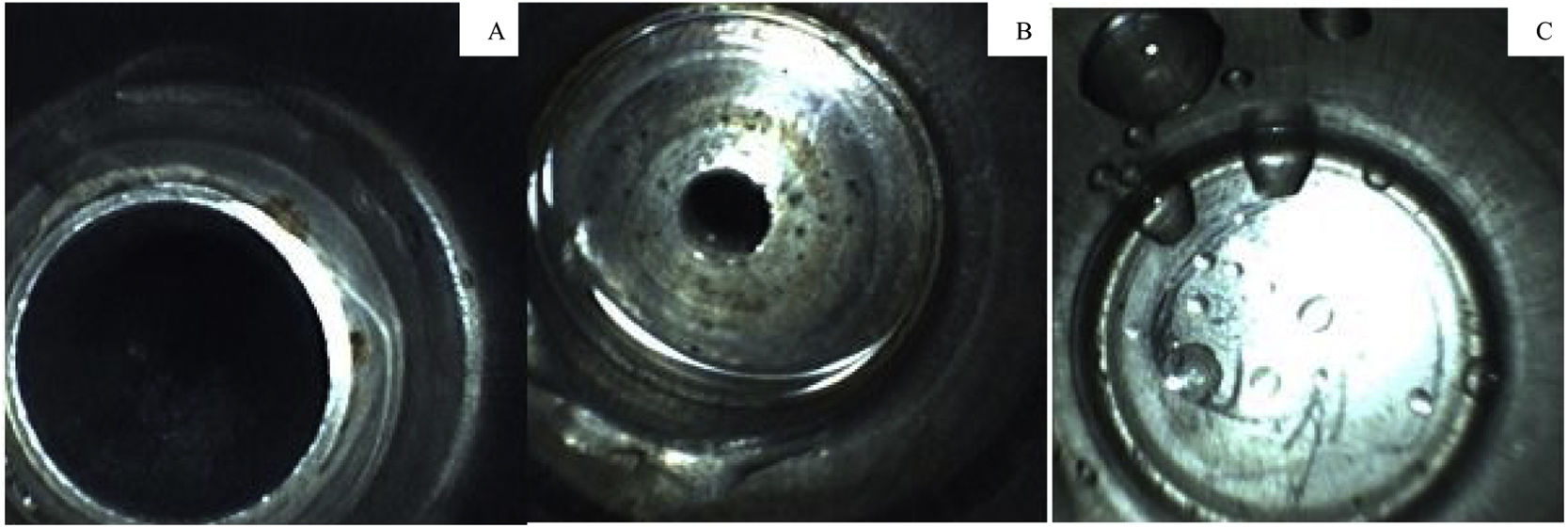
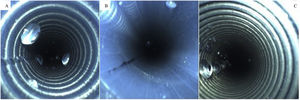
Next, the borescope was used to inspect the suction channel port, and fluid was detected in 3% (3/71) of the endoscopes stored with the valves still in place. Fluid was also detected in the air and water channel port in 6% (4/71) of the endoscopes; of those, 75% (3/4) were stored with the valves still attached. The presence of fluid is shown in Fig. 2.
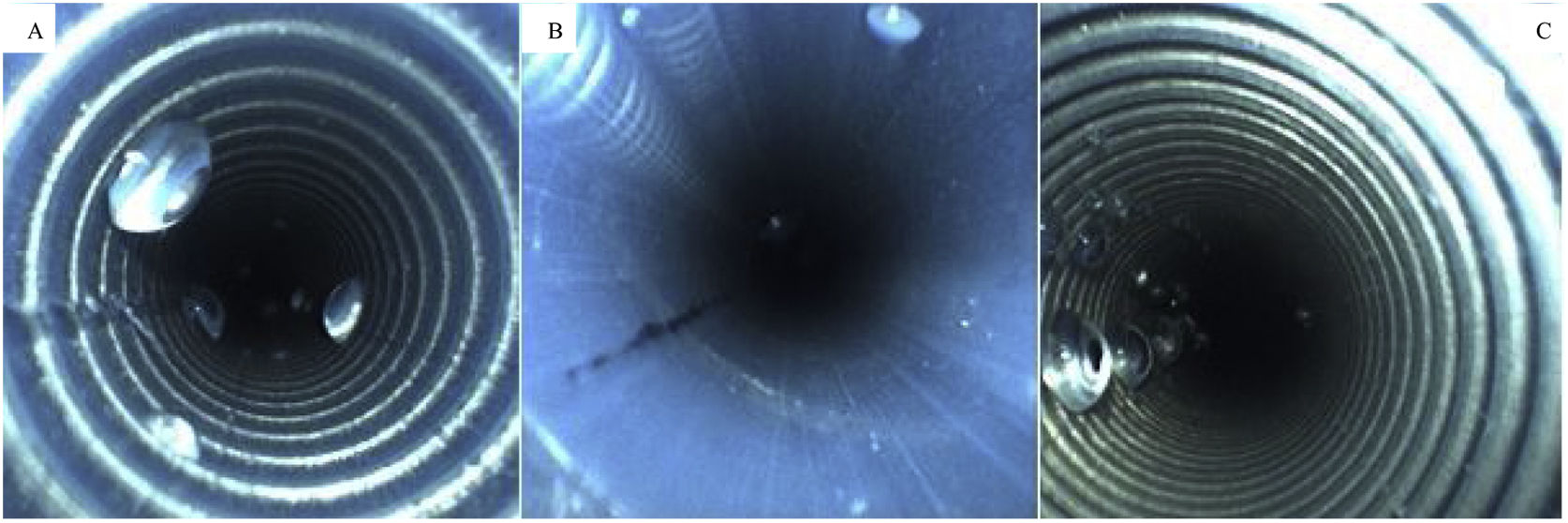
A total of 74 biopsy channels were inspected with the borescope, due to the presence of equipment with double channels. Of the inspected channels, 31% (23/74) had no sign of moisture, 69% (51/74) contained fluid, and 41% (21/51) contained excess moisture as shown in Fig. 3.
All facilities had at least one piece of equipment with fluid/moisture in the channels. Fluid was found in 69% (51/74) of the equipment, distributed as follows: 45.9% (25/51) for gastroscopes, 32.4% (19/51) for colonoscopes, 12.2% (4/51) for duodenoscopes, and 9.5% (7/51) for other endoscopes (p = 0.049). No significant relationships were found between the other variables analyzed.
Discussion and conclusionsOur results highlight a series of failures at endoscopy facilities related to the final stages of reprocessing. The execution of drying and storage has been a concern of several associations and societies, such as the Emergency Care Research Institute (ECRI), which has highlighted the reprocessing of endoscopes as one of the top ten greatest risks regarding the use of health technologies.7,24
Fluid in endoscope channels facilitates the maintenance and multiplication of microorganisms, and consequently, the formation of biofilm.7 Additionally, fluids have the potential to fix non-water-soluble residues, such as simethicone, an oily substance composed of other components (e.g., dyes and sugars), whose removal through the usual cleaning processes at endoscopy facilities is a challenge.10,22,25 As a result, manufacturers have contraindicated the use of simethicone in such processes, but clinics that consider simethicone use beneficial for visualizing images are advised to administer it directly into the biopsy channels because they can be brushed, which better facilitates removal of the substance.26 The use of simethicone in other channels or water pumps increases the possibility of channel obstruction by drug crystallization, as well as the risk of microbiological contamination.10,21,25,26 The quality of the water used to rinse disinfectant residues is another important factor because the water may carry pathogenic microorganisms, such as Pseudomonas sp., from the ducts and reservoirs, increasing infection risks, especially in immunocompromised patients, and allowing recontamination of properly disinfected equipment.27,28 Therefore, guidelines recommend using a purified or sterile water system that is duly monitored and controlled for the presence of microorganisms, especially in view of the difficulties in guaranteeing the drying effects of all channels in clinical practice.2,18,29
When drying failure occurs, residual microorganisms find an ideal environment for proliferation, making effective drying critical.7,14,30 Given the insufficient guidelines and recommendations from manufacturers regarding detailed steps and the difficulty in validating and monitoring the drying phase, it is often performed inadequately in clinical practice, as evidenced by the present analysis and other studies.9,20,30,31 Automated drying processes favor reproducibility and reduce the risk of omissions or human error. Indeed, Barakat20 showed better results with automated processors than with manual drying processes, for carrying out drying for 10 minutes. However, a study by Nerandzic et al.9 suggested that 10 minutes in each channel was insufficient for the drying of all channels and demonstrated the importance of controlling other influencing factors of drying effectiveness, such as airflow pressure, use of alcohol, and channel diameter, in addition to reinforcing the need to validate the process in each available structure.
The use of alcohol as a drying facilitator is controversial among gastroenterology societies and is usually contraindicated by European associations, mainly because of the protein-fixing properties of alcohol and the risk of prion protein contamination.29 In contrast, the American associations take the microbicidal and evaporative properties of alcohol solutions into account and recommend its use.2,18 However, other studies have shown that alcohol use does not influence microbiological reduction or drying time, reinforcing the importance of effective drying.9,14,32 Therefore, the implementation of tools for drying verification, such as the borescope, is essential to guarantee adequate process execution.16,31
Of the tools used to ensure proper drying of endoscope channels, the borescope stands out because it allows internal visualization of the channels, with direct visualization of fluid. Nevertheless, its use is still limited to endoscope channels with diameters larger than those of the borescope.16,31 Even so, borescopes have been shown to be important in detecting failures and in monitoring and verifying drying, which are fundamental for process improvement.1°,16,31
Inadequate storage may also favor the recontamination of ready-to-use endoscopes.24 As a result, there have been clear recommendations aimed at guiding professionals in maintaining reprocessing quality.2,18,29 However, failures in adherence to proper endoscope storage, such as storing them in a horizontal position with valves still attached or in unventilated cabinets, were observed in the present study, corroborating other evidence of compromised reprocessing. Bajolet et al.5 showed that inadequate storage is linked to the potential causality of microorganism transmission, indicating the need for a careful look at that step.
We observed the use of tip protectors on some of the endoscopes evaluated. Their purpose is to protect the distal tip of endoscopes from mechanical damage, but they were made out of adapted materials, such as sponges or foams, which prevents proper cleaning and seals the tips, potentially promoting moisture retention or increasing residual microorganisms and contamination risks.18,33 Guidelines suggest the use of disposable tip protectors that promote tip aeration, do not retain moisture, and have adequate diameters for each endoscope.18
Drying cabinets with airflow in the channels are recommended, as they have shown superior results in maintaining the drying quality of the stored endoscopes after processing.15,17 In some countries, the recommendation for determining a safe storage time should be validated by the manufacturers of those cabinets.29 Other guidelines indicate the gathering of evidence by a multiprofessional team for the safe definition of shelf life duration.2,21 In the present study, none of the facilities inspected had rigorous criteria for defining the shelf life of their endoscopes, nor did they perform any type of validation through microbiological monitoring. Thus, the empirical determination of a maximum storage time could result in reprocessing failures and risks to patients undergoing procedures using such equipment.8
Another point to be highlighted is the frequency of finding fluid inside the biopsy channels and endoscope ports during inspection with a borescope, which indicates fragility in critical steps, such as drying.4 Thus, the use of borescopes in clinical practice may indicate failures and promote improvements in carrying out the drying process, given that the identification of fluid is directly related to the risk of biofilm formation.16,31
The present study has certain limitations that warrant discussion. First, it was not possible to perform a differentiation analysis of the types of fluids identified. Second, we did not perform a microbiological analysis of the equipment evaluated, which would have allowed us to assess the safety of the clinical use of endoscopes. Finally, the sample size was relatively small, owing to the low participation of services in the project, due to either a lack of knowledge about the borescope or restrictions on visits, as a result of the control measures implemented during the COVID-19 pandemic.
Training, education, and ongoing evaluation of the execution of reprocessing steps are recommended to ensure the safety of patients undergoing endoscopic procedures. Training should consider guideline updates, scientific evidence, and the implementation of new technologies to validate the practiced steps. Our findings highlight weaknesses in adherence to the reprocessing steps, especially the final steps, exposing the risk of recontamination of ready-to-use gastrointestinal endoscopes. These results not only highlight the issues to be addressed in team training, but also the improvements to be made in terms of basic resources, and the importance of using the borescope in practice, to improve the drying, storage, and condition of endoscopes.
Future research should seek to improve the validation tools for reprocessing endoscopes, focusing on low cost, ease of implementation and use, and improvements in practice to increase patient safety.
Failures in the final reprocessing steps increase the risk of microbial transmission and should be eliminated to ensure patient safety. The use of validation and monitoring tools, such as the implementation of a borescope and follow-up audits, can generate opportunities for staff training and cycles of continuous improvement, enhancing the safe use and internal conditions of gastrointestinal endoscope channels (Table 1).
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.