New oncologic treatments, particularly immunotherapy (IT), have revolutionized the treatment of advanced-stage malignant tumors. Immune checkpoint inhibitors are the main form of IT and act by increasing T cell activity and the organism’s immune response against neoplastic cells. Targeted therapy is another form of IT that acts by inhibiting oncogenes or inflammation signaling and tumor angiogenesis pathways. However, these mechanisms of tumor destruction can interfere with the host’s immune self-tolerance or with the mechanisms of epithelial tissue repair and predispose to immune system-mediated adverse events that can affect multiple organs, including the digestive tract. The gastrointestinal manifestations of damage caused by IT can range from low-grade mucositis to ulceration, and in some cases, necrosis and perforation. Any part of the gastrointestinal tract can be affected, but there is greater involvement of the small bowel and colon, with a pattern similar to that seen in inflammatory bowel disease. The most common clinical manifestation is chronic diarrhea. The differential diagnosis includes enteropathogenic infections, especially those caused by opportunistic microorganisms; adverse drug reactions; and other inflammatory and malabsorption disorders. Treatment is guided by damage severity. Mild cases can be treated with antidiarrheals and rehydration in the outpatient setting; moderate cases with hospitalization, systemic steroids, and temporary suspension of IT; and severe cases with immunosuppressants or biologic agents and definitive suspension of IT.
Los nuevos tratamientos oncológicos, particularmente la inmunoterapia (IT), han venido a revolucionar el tratamiento de neoplasias malignas en estadios avanzados. Los inhibidores de los puntos de control son la principal forma de IT y actúan aumentando la actividad de las células T y la respuesta inmune del organismo contra las células neoplásicas. La terapia blanco es otra forma de IT que actúa mediante inhibición de oncogenes o vías de inflamación y angiogénesis tumoral. Sin embargo, estos mecanismos de destrucción tumoral pueden interferir con la tolerancia inmune del huésped o con los mecanismos de reparación tisular epitelial, y predisponer a efectos secundarios inmunomediados que pueden afectar múltiples órganos, incluyendo el tracto digestivo. Las manifestaciones gastrointestinales de daño por IT pueden ir desde mucositis de bajo grado hasta ulceración y en algunos casos necrosis y perforación, y pueden afectar cualquier parte del tubo digestivo, con mayor afección del intestino delgado y colon, con un patrón similar al observado en enfermedad inflamatoria intestinal. La manifestación clínica más común es diarrea crónica. El diagnóstico diferencial incluye infecciones enteropatógenas, particularmente por gérmenes oportunistas, efecto secundario de medicamentos, y otros trastornos inflamatorios y malabsortivos. El tratamiento depende de la severidad del daño, y puede incluir desde antidiarreicos y rehidratación en forma externa en casos leves, hospitalización, esteroides sistémicos y suspensión temporal de la IT en casos moderados, e inmunosupresores o agentes biológicos, y suspensión definitiva de la IT en casos severos.
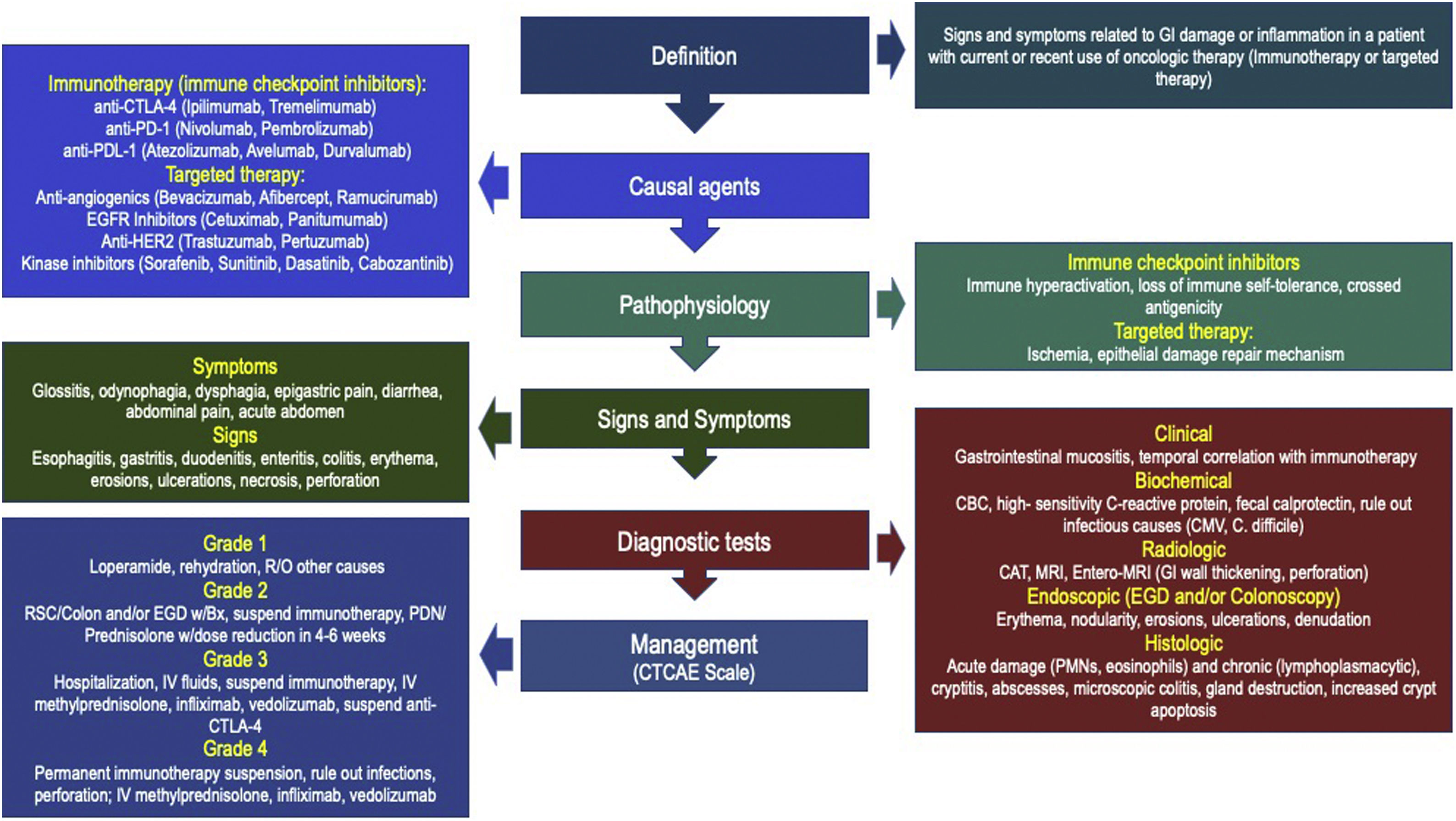
During the last decade oncologic therapy has evolved from traditional treatments such as chemotherapy with cytotoxic drugs, and radiotherapy (RT), alone or in combination, toward medical therapies directed at different oncogenesis-related mechanisms. Among these newer forms of treatment, immunotherapy (IT) and targeted therapy (TT) have revolutionized the way malignant tumors are treated, achieving higher survival rates and even cure rates in some advanced neoplasia.1,2 IT is a form of biologic therapy that acts by increasing immune system capacity to compete and destroy tumors, and includes the checkpoint inhibitors (CPIs), T cell transfer therapy, monoclonal antibodies, and immunomodulators. Among these, CPIs are the most commonly used drugs, and their mechanism of action is to increase the immune response against tumor cells by blocking checkpoints regulating the body’s immunologic mechanisms.3,4 TT is another form of oncologic treatment that works by a number of mechanisms: they attack proteins related to tumor growth control, division, and dissemination, through monoclonal antibodies or small molecules that act on receptors and inflammation pathways associated with aberrant cell growth, and also are able to inhibit oncogenes.4,5 However, several of such mechanisms may interfere with those controlling self-tolerance (IT), or with normal epithelial repair (TT), and are associated with immune-mediated side-effects along different organs, including the gastrointestinal tract.6,7 This review attempts to update the gastroenterologist in this emerging topic whose prevalence is increasing each year and includes clinical practice recommendations published by the main medical associations with expertise in this topic.
Material and methodsA review of published articles was carried out through a crossed search on PubMed Medline and IMBIOMED (from January 2010 to June 2023) of the following terms (in both English and Spanish, including abbreviations): immunotherapy, checkpoint inhibitors, targeted therapy, gastrointestinal damage, toxicity, enteritis, colitis, diarrhea, adverse events, radiology, endoscopy, colonoscopy, histologic findings, immunosuppressive agents, and biologic therapy. More relevant articles were identified, including systematic reviews, meta-analyses, and clinical guidelines both by oncologic and gastroenterology societies. References were ordered and a review was performed divided in the following sections: definitions, epidemiology, pathophysiology, symptoms and signs, diagnostic methods, differential diagnosis, treatment, and as an emerging entity of which there are no available national references, a section of recommendations from international guides was included, according to the following medical societies: the Society for Immunotherapy of Cancer (SITC), the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), the National Comprehensive Cancer Network (NCCN), and the American Gastroenterology Association (AGA). Of a total of 215 articles in the search, 117 were included. Case reports or references previous to the search line were included only if no other publication regarding that specific topic was found. Pediatric articles, those in different languages and those that could not be accessed were excluded from the analysis.
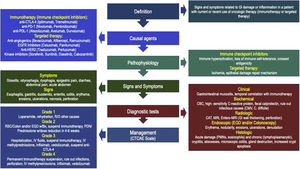
DefinitionsThe term “Oncologic treatment-induced gastrointestinal (GI) Toxicity” has been designed to define the presence of symptoms and signs related to either damage or inflammation in one or more segments of the GI wall, in a patient with current or previous use of any oncologic medication, including chemotherapy, RT, IT, or TT, whereas “Immunotherapy-related GI toxicity” (IGT) is reserved for damage associated with this particular form of therapy.3,4,8–10 Clinical manifestations may vary according to the affected organ, as well as the severity of damage, and range from mild symptoms emerging from one or more organs to severe inflammation associated with the risk of life-threatening complications. Among all forms of IGT, the best understood is “Immunotherapy-related enterocolitis” (ITEC), also known as check-point inhibitor-induced colitis (CPIC).3,10,11 Despite the fact that most patients with mild clinical manifestations of the disease are treated by oncologic teams, gastroenterologists play an important role in the differential diagnosis, risk evaluation, and management of atypical or refractory cases.12
EpidemiologyBetween 60 and 80% (range 40–100%) of patients receiving any kind of oncologic treatment (e.g., chemotherapy, RT, IT, TT or combinations) have been described to develop adverse events at some point of therapy.3,4,8,9,13,14 Unlike RT, in which the range of damage is limited to exposed areas, other forms of treatment can affect different organs and systems at different levels.14–16 In the GI tract, the characteristic damage is mucosal inflammation, a term known as mucositis, that may develop in different grades ranging from erythema, erosions, and isolated or diffuse ulcerations in the most common cases, to transmural inflammation, strictures, necrosis, and perforation in the most severe forms. The most common symptom is diarrhea, occurring in up to 60–80% of cases, and the most affected regions are the small intestine (40–60%), as well as the colon (60–80%), and that is the reason most reviews focused on ITEC/CPIC.3,4,11,12,17,18 The incidence of GI damage is greater with IT versus chemotherapy.19 The risk of mucosal damage increases with the type of IT, higher doses, frequency and length of use, combination therapies, as well as the concomitant use of other medications, particularly nonsteroidal anti-inflammatory drugs (NSAIDs). An independent factor associated with ITEC/CPIC is the histologic type of the primary tumor: patients with hepatocellular carcinoma have been described to have a higher prevalence of immune-mediated adverse events and colonic damage as compared with those with colorectal carcinoma.20,21 Frequency of ITEC/CPIC ranges between 5 and 16% in ipilimumab users for prostate cancer, whereas 21% during treatment for melanoma - including bowel perforation rates up to 6.6%, and between 14 and 35% as therapy for renal cancer.3,11,22–26 Other described risk factors are increasing age, ethnic origin, genetic factors, vitamin D deficiency, and the baseline intestinal microbiota, as well as individual previous illnesses, particularly renal disease and autoimmune disorders. A higher risk of ITEC/CPIC has been described in patients with different autoimmune diseases, including rheumatoid arthritis and psoriasis.27 Several reviews have reported that a previous diagnosis of inflammatory bowel disease (IBD) is an independent risk factor for the development of ITEC/CPIC, and up to 36.8% may develop an exacerbation during therapy with CPIs.28 Although most series of ITEC/CPIC with previous IBD have been excluded from clinical studies, a recent trial reported a prevalence of 41% of any GI damage, and of those, 21% developed severe damage.29 Gastrointestinal toxicity may develop shortly after the first dose and the risk continues up to several months after the last dose, and it seems that the biologic effect may be related not only to drug clearance but also to the particular mechanism of action of the drug, as well as to interactions with the patient’s immune system.4,11
PathophysiologyOncologic agents may damage the GI tract through a series of mechanisms that vary according to the type of drug and may include interaction with DNA synthesis (chemotherapy),13–15 ischemic or oxidation-related damage (RT),13,16 immune-mediated mechanisms (IT),3,14,18 or ischemic damage or alterations in epithelial repair mechanisms (TT),1,5 among others.
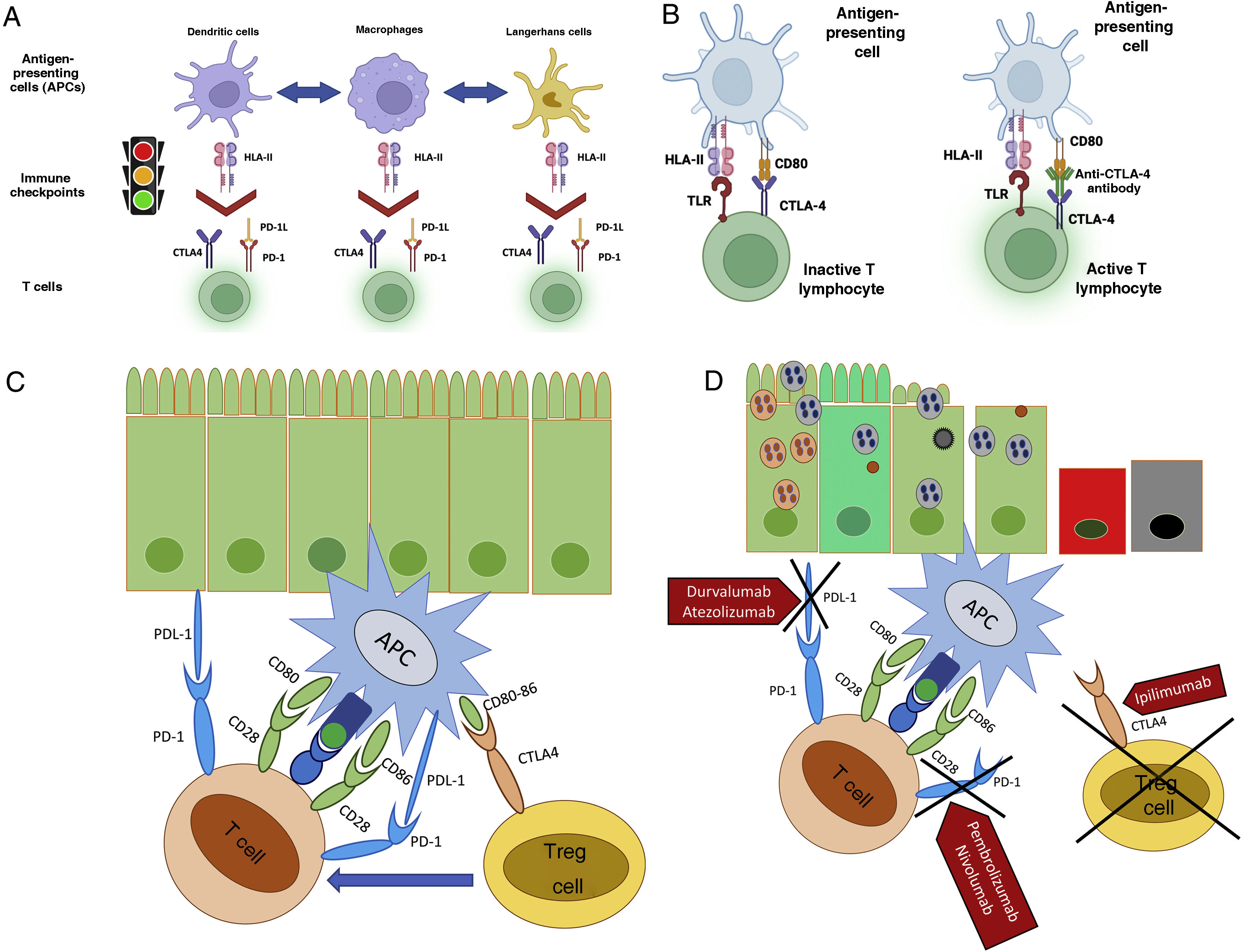
Immune checkpoint inhibitors (CPIs): One of the mechanisms the organism uses to fight neoplasia is the immune system. T cells are stimulated through the engagement between the major histocompatibility complex (MHC) and the antigen-presenting cells (APCs), particularly the dendritic cells (DCs), with the T cell receptors (TCRs), but also an additional signaling through a co-stimulatory or co-inhibitory pathway is required. This signaling is determined by a number of molecules called immune checkpoints (ICPs). The chief mechanism by which neoplastic cells avoid the immune system and survive is to induce a state of T cell hypofunction, achieved mainly by activation of the ICPs that represent the natural break controlling T cell activation by TCRs, APCs, and the MHC, as well as the co-stimulation between the CD28 receptor on the T cell and CD80/86 receptors (also known as B7.1 and B7.2) on the APC surface or its B7 ligand. Once activated, T lymphocytes may then proliferate and produce pro-inflammatory cytokines, and thereafter trigger an inflammatory response.3,4,11,30,31 The antitumor mechanism of action of IT is mainly related to an increased immune response by blocking immune-related negative feedback factors expressed in T cells or in APCs.3,11,30,31
The so-called CPIs are monoclonal antibodies that act by blocking one of several immune checkpoints through 2 different immunoregulatory pathways:
- 1
Cytotoxic T-lymphocyte-associated protein (CTLA-4), also known as CD152 antigen, is a membrane protein expressed on the surface of regulatory T cells (Tregs, also known as CD25), some B cells, and thyme-cells. It shares homology with the T cell CD28 antigen, and acts as a ligand for the CD80 and CD86 receptors.
- 2
Programmed cell death type 1 protein (PD-1), also known as CD279, is a membrane receptor protein expressed under normal circumstances on cytotoxic T cells, B lymphocytes and other immune cells, such as monocytes, natural killer cells (NKs), and DCs. It has 2 ligands: PD-L1, expressed on lymphocytes, vascular endothelial cells, fibroblasts, mesenchymal cells, astrocytes, neurons, and keratinocytes, and PD-L2, expressed on APCs, both members of the B7 family.
Under normal circumstances these membrane proteins act as checkpoint or inhibitory co-factors that decrease the interaction between APCs, DCs, and T cells, promoting cell apoptosis of activated T cells, and reducing the apoptosis of Tregs, allowing a normal immune response, including tumor destruction and a controlled inflammatory status, but avoiding immune overstimulation by downregulating cell and antigen receptors, and preventing the development of autoimmune disease. Several tumor cells may also express one or more of these proteins.1,3,4,11,22,30,31
Two mechanisms are used by CPIs to block these checkpoints:
- 1)
The first mechanism is by blocking engagement of the CTLA-4 protein with the CD80/86 receptor, but allowing engagement with the CD28 receptor, and by blocking Treg engagement with APCs, which prevents inhibitory signaling on cytotoxic T cells, allowing hyperactivation, over-response to luminal antigens, increased proinflammatory interleukin secretion, as well as the loss of Tregs. A secondary mechanism of anti-CTLA-4 agents is the co-stimulatory pathway, competing with the CD28 receptor for ligands on T lymphocytes. These events increase the antitumor immune response but reduce or inhibit tolerance to autoantigens.
- 2)
The second mechanism is to prevent engagement at the PD-1 level or with either of its ligands, PD-L1 and PD-L2. This PD-1-ligand engagement sends an inhibitory signal between APCs and T cells, decreasing the transcriptional activity that regulates proinflammatory cytokine production. Upon blocking this inhibitory signal, it allows uncontrolled activation of both APCs and cytotoxic T cells, with a similar result as that seen with CTLA-4 blockade: an increased potential for tumor destruction, but a decreased protection against loss of immune tolerance, resulting in immune-mediated damage against different organs, including the GI and hepato-biliary tracts (Fig. 1).3,11,22–24
Figure 1.Mechanism of action of checkpoint inhibitors. (A) Regulation of T cell activation by antigen-presenting cells, via immune checkpoints (Image courtesy of Dr. José Antonio Velarde Chávez). (B) Mechanism of T cell activation and inhibition via checkpoint inhibitors (Image courtesy of Dr. José Antonio Velarde Chávez). (C) Loss of immune self-tolerance following checkpoint inhibition, with immuno-mediated associated gastrointestinal damage.
(0.66MB).
Several investigators have proposed at least 4 hypotheses of immune-mediated damage associated with CPIs: (1) Development of T cell infiltration and complement-mediated organ damage by joining cell surface proteins expressed in normal tissues, (2) Promoting recognition and engagement of T cells in antigen-sharing tumor cells, (3) Increased production and release of cytokines in affected organs, and (4) Increased autoantibody levels against target organs or promoting de novo autoantibodies.32
CPIs are classified according to the protein, ligand or immunoregulatory pathway they block, and are divided into 2 groups:
- 1
Anti-CTLA-4 agents (ipilimumab and tremelimumab).
- 2
Anti-PD-1 agents (nivolumab, pembrolizumab, pidilizumab, sintilimab, cemiplimab), and the anti-PD-L1 or PD-L2 (atezolizumab, avelumab and durvalumab), grouped together as a single group (anti-PD-1/PD-L1).
Current approved indications of CPIs include treatment of advanced tumors, without previous response or with recurrence, or associated to microsatellite instability:
- -
Nivolumab (melanoma, colorectal carcinoma (CRC) with microsatellite instability, non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), mesothelioma, gastroesophageal, kidney, and urothelial cancer, Hodgkin’s disease, squamous cell carcinoma of the head and neck)
- -
Pembrolizumab (same indications as nivolumab, plus tumors with microsatellite instability, large B cell lymphoma, cervical carcinoma, gastric carcinoma, Merkel cell carcinoma)
- -
Pidilizumab (diffuse B cell lymphoma)
- -
Cemiplimab (squamous cell skin cancer, basal cell carcinoma, NSCLC)
- -
Atezolizumab (melanoma, NSCLC, small-cell lung carcinoma (SCLC), breast cancer with negative triple markers, urothelial carcinoma).
- -
Avelumab (renal carcinoma, urothelial carcinoma, Merkel cell carcinoma).
- -
Durvalumab (HCC, cholangiocarcinoma, NSCLC, SCLC, urothelial carcinoma).
- -
Ipilimumab (along with nivolumab, in combination with nivolumab, metastatic CRC, HCC, advanced renal carcinoma, mesothelioma, mycosis fungoides, NSCLC, tumors with microsatellite instability, and alone for advanced melanoma).1,30,33,34
Checkpoint inhibitor-associated GI damage resembles that seen in autoimmune IBD, such as ulcerative colitis and Crohn’s disease, with a number of manifestations occurring along the GI tract, ranging from edema and superficial erythema, erosions and/or ulcerations, to more severe damage including strictures, obstruction, and even necrotizing enterocolitis. Many predisposing risk factors involve mechanisms related to inflammatory control. Genetic alterations may be associated with abnormalities in the CTLA-4 protein pathway - an example is crossed antigenicity between antitumor T cells and similar antigens in healthy cells. The patient’s basal microbiome - with an apparent protective factor related to greater abundance of Bacteroidetes and Proteobacteria and lesser abundance of Faecalibacterium prausnitzii - may be associated with either pro-inflammatory or anti-inflammatory changes and may affect CPI expression and effector T cell activation. Other risk factors for ITEC/CPIC are predisposition or the previous presence of autoimmune disease, and the use of potential enterotoxic drugs, such as NSAIDs.4,11,35–39
TT: TT includes a new group of oncologic medications that act by identification and destruction of certain types of cancer cells through either inhibition of abnormal growth-related oncogenes, or by inhibition of selective inflammation or tumor angiogenesis pathways. Several investigators consider that TT is a form of IT, by using immune mechanisms as therapeutic goals.5,39 TT includes 2 main groups (monoclonal antibodies and small molecules) and 4 groups of agents, according to the site of action:
- 1
Anti-angiogenic agents (e.g., bevacizumab, aflibercept, ramucirumab) are monoclonal antibodies with a mechanism of action involving angiogenic stimulation signal inhibition, in order to decrease blood flow toward tumors, through the inhibition of vascular endothelial growth factor (VEGF) inhibitors. Approved indications are NSCLC, CRC, and ovarian, breast and prostate cancer.40,41
- 2
Epidermal growth factor receptor (EGFR) inhibitors (e.g., cetuximab, panitumumab) are monoclonal antibodies that act by downregulating the EGFR receptor signal and inducing antibody-dependent cytotoxicity through NK cell activation. Their main indication is treatment of CCR and head and neck epidermoid carcinoma.42
- 3
Anti-HER2 protein agents (i.e., trastuzumab) are monoclonal antibodies directed at the extra-cellular HER2 (human epidermal growth factor receptor 2) protein portion, which regulates EGFR-associated pathways, and is normally expressed in epithelial cells along the GI tract. HER2 over-expression is associated with more aggressive forms of breast cancer. Anti-HER2 agents induce antibody-dependent cytotoxicity through NK cell activation, similarly to EGFR inhibitors. Current approved indications are positive HER-2 advanced breast cancer, or any neoplasia associated with the expression of this receptor.43,44
- 4
Kinase inhibitors (e.g., sorafenib, sunitinib, erlotinib, dasatinib, axitinib, cabozantinib): most are small molecules that inhibit one or more specific protein kinases and can be classified according to the protein they inhibit: serine/threonine kinase B-raf (BRAF), mitogen-activated proteins (MEK), VEGF receptors, or platelet derived growth factor (PDGFR) receptors. Indications are treatment of HCC and advanced kidney carcinoma (sorafenib, cabozantinib), metastatic renal cancer and gastrointestinal stromal tumors (sunitinib), and chronic granulocytic leukemia and acute lymphoblastic leukemia (dasatinib).41,45,46
The gastrointestinal TT-associated mechanism of damage differs according to group and mechanism of action. Anti-angiogenic agents may induce ischemia, necrosis, and perforation. Anti-EGFR and anti-HER2 agents are associated with interference and disruption with the normal intestinal mucosa repair mechanisms. The kinase inhibitors alter mechanisms associated with proliferation, differentiation, and repair of the GI epithelium, with similar results and degree of damage as seen with CPIs.5,37,39,44,46 In many cases, IT can be combined with TT, in order to get major therapeutic effects in advanced stage tumors, and despite the fact that there is less evidence, a similar degree of damage related to co-administration has been described. An example of combination-associated toxicity has been described recently with the combination of cabozantinib, a drug that acts as both a kinase and VEGFR inhibitor, expressed on small bowel epithelial cells, and when administered along with nivolumab, may be associated with enterocolitis.47
Symptoms and signsSymptoms and signs may differ according to the degree of damage, as well as the affected organ and evolution time.3,5,11,18,38
- -
Oral cavity: a number of manifestations of oral mucositis may be seen, such as erythema, stomatitis, glossitis, oral ulcers, and a wide spectrum of oral dermatosis (i.e., lichenoid reaction, pemphigoid, erythema multiforme), clinically manifested as dysgeusia, oral cavity swelling and pain, tongue redness, pain during swallowing and/or odynophagia/dysphagia.
- -
Esophageal damage: dysphagia, odynophagia, retrosternal pain, nausea, anorexia.
- -
Gastroduodenal damage: dyspeptic symptoms (epigastric pain, nausea, early satiety, bloating), vomiting/oral intolerance, hiccups, black tarry stools.
- -
Enteral and colonic damage: acute and chronic diarrhea, weight loss, anemic syndrome, abdominal pain, bloating, rectal bleeding, tenesmus, urgency, and in most severe cases, symptoms of acute abdomen associated with necrotizing enterocolitis, bowel obstruction or perforation.
The most common clinical presentation is diarrhea (92%), followed by abdominal pain (82%), bloody stools (64%), fever (45%), and vomiting.48
Severity and degree of ITEC/CPIC is classified according to the Common Terminology Criteria for Adverse Effects (CTCAE), that has been designed specifically for colonic damage, and has not yet been validated for damage in other parts of the GI tract. CTCAE is divided into 3 different categories, according to severity of diarrhea, degree of enterocolitis, and presence of peritoneal signs, each one with 5 grades (Grade 1–5) of severity:6,7
- -
Grade 1 CTCAE: mild symptoms, with <3 bowel movements per day, with or without pain, asymptomatic or mild colitis or entero-colitis.
- -
Grade 2 CTCAE: 4–6 liquid bowel movements per day, abdominal pain, and blood or mucus in stool, including nocturnal symptoms.
- -
Grade 3 CTCAE: >6 bowel movements per day, severe abdominal pain, GI bleeding, fever, ileus, and peritoneal signs.
- -
Grade 4 CTCAE: any combination of symptoms plus peritoneal signs, with life-threatening risk.
- -
Grado 5 CTCAE: when the patient dies.
Both CPIs and TT may cause upper as well as lower GI tissue damage, with a wide spectrum of disease, ranging from oral mucositis (30–70%), different degrees of gastropathy (83%), erosive duodenitis (15%), erosive enteritis (jejunitis and/or ileitis) (7–25%), erosive or ulcerative colitis (13–40%), erosive enterocolitis (30%), severe enterocolitis requiring IT withdrawal (2–10%), necrotizing enterocolitis and/or perforation (0.8–5%), which is associated with an overall mortality of 1.1%.3,4,11,18,25,38
Risk of chronic diarrhea development differs, according to the type of CPI or TT. Overall, most studies, systematic reviews, and meta-analyses have reported a higher prevalence of any type of damage when anti-CTLA-4 agents are administered, including early onset of symptoms and ITEC, higher rates of abdominal pain, bloody diarrhea, the most severe degrees of both endoscopic and histologic inflammation, and higher rates of hospitalization and systemic steroid and biologic use, which can be explained by the fact that the CTLA-4 induced-pathway is APC-dependent.49–51 Several systematic reviews with meta-analyses have compared incidence, prevalence, and risk of symptom development, according to CPI sub-group, and the risk is lower with anti-PD-1 agents, such as nivolumab or pembrolizumab (incidence 0.004−0.3%, prevalence 2.9–19%, median 11.5%), intermediate with anti-PD-L1 agents, such as atezolizumab (incidence 0.2–1.2%, prevalence 11.6–23%), and higher with anti-CTLA-4 agents, such as ipilimumab (incidence 4.9–11.2%, prevalence 31–92%, median 54%, 10% grade 3–4 CTCAE: 10%). The prevalence and degree of enteritis, colitis or entero-colitis also differs, according to IT type: anti-PD and PD-L1 agents (global 5−17%, grade 3–4 CTCAE: 1%, IT withdrawal requirement 2–5%), anti-CTLA-4 agents (global 35–40 %, grade 3–4 CTCAE: 5%, IT withdrawal requirement 10%), or combination therapy (global 32–50%, Grade 3–4 CTCAE: 11%, mortality 0.8%).3,5,11,18,34,38,49,50 The most common treatment-related cause of mortality in CPI users is colitis, with 70% of cases.35 A meta-analysis showed global mortality of 5% with overall CPI use, 60% associated with anti-CTLA-4 agents, 26% with anti-PD-1 or anti-PD-L1, and 14% with combination therapies, with early onset of symptoms and GI damage,26 and a time of symptom onset of 14.5 days with combination therapies versus 40 days with monotherapy.35 GI toxicity associated with anti-CTLA-4 agents seems to be dose-dependent, whereas that seen with anti-PD-1 and anti-PD-L1 does not. Use of higher dosage, such as those used for melanoma or renal carcinoma, may increase the risk of enterocolitis up to 35%, and that of perforation to 6%.3,11,24–25 Targeted therapy agents, similar to CPIs, increase the risk of GI damage, with an overall relative risk (RR) for low-grade mucositis of 1.5–4.5.39 A lower risk of damage has been described for bevacizumab (RR 1.8, a number needed to harm (NNH) of 91, perforation rates 1.1%, and 8.8% mortality rate), intermediate risk with erlotinib (RR 3.2, NNH 143) or sorafenib (RR 2.9–3.3), and higher risk with sunitinib (RR 7.7, NNH 111).1,5,41–45 Dasatinib, a second-generation tyrosine-kinase inhibitor used for chronic myeloid leukemia, has been associated with the development of colitis in 17% of users.46 Recently, cabozantinib, another kinase inhibitor, has been approved for treatment of renal carcinoma administered along with nivolumab. The CheckMate study evaluated the presence of adverse effects with each drug, compared with co-administration, and the most common GI adverse events in both groups were diarrhea, with a slightly higher risk in the combination group (64%), CTCAE grade-3 diarrhea (7%), and CTCAE-4 diarrhea (2%), with perforation and mortality rates of 0.9%.47
Diagnostic testsClinical features: ITEC/CPIC should be considered in any patient with new onset of GI symptoms and recent or current use of CPIs or TT. Infectious causes with similar features should be excluded before using any immunosuppressants or biologic agents. Symptoms do not solely correlate with endoscopic or histologic findings, nor predict response to therapy, so definitions and criteria have been proposed by a number of different American and European oncology societies, and most of them agree that ITEC/CPIC should be defined as the presence of symptoms related to the GI target organ, in recent or current IT users, with radiologic or endoscopic evidence of damage.8–10,52–54 There is no consensus definition for TT-related GI toxicity, but overall, the same clinical, endoscopic, and radiologic criteria used for ITEC/CPIC are used.5,39 Median time from onset of diarrhea after first IT infusion ranges from 4 to 8 weeks, although it can be as short as 1 week, particularly in ipilimumab or other anti-CTLA agent users, or as long as several months, even after the last administered dose.34,48 Patients with continued CPI use following adverse effects are at increased risk of developing more serious complications, such as hemorrhage, ischemia, necrosis, toxic megacolon, or bowel perforation in short-term periods, as soon as 5−10 weeks. International guides also describe the diagnosis and management of IT toxicity in other organs and systems, which do not necessarily correlate with the degree of GI damage.7,12,52–54
Biochemical markers: In mild forms, classified as CTCAE grade 1, an extensive work-up is not recommended. For CTCAE grade 2 toxicity, a complete blood cell count, ultrasensitive C-reactive protein and/or erythro-sedimentation rate, serum metabolic analysis, electrolytes, and TSH are suggested, and several medical societies also recommend ruling out celiac disease with serologic tests (i.e., anti-tissue transglutaminase IgA antibodies).2,8–10,12,52–54 Opportunistic infections, such as cytomegalovirus (CMV) and Clostridioides difficile (C. difficile), as well as other diarrhea-associated organisms, should be ruled out with a fecal GI panel performed with polymerase chain reaction (PCR), instead of cultures, which most of the time is reported negative. Two of the newer mucosal colonic biomarkers are calprotectin and lactoferrin, and both are usually increased shortly after a few weeks of IT use, usually at similar values as those seen in IBD. A cut-off value of >150 mcg/g is highly predictive of endoscopic (sensitivity 70%), and histologic (sensitivity 90%) inflammation. Most laboratories use a lower cut-off value of 50 mcg/g to define positivity in qualitative tests. However, this value does not discriminate between erosive and non-erosive conditions, and a quantitative measure is therefore recommended. In cases in which a biologic agent or other forms of immunosuppressive therapy are required, latent infectious diseases should be ruled out with appropriate tests, such as QuantiFERON® (Qiagen) for tuberculosis, PCR for CMV, and serology tests for viral hepatitis B and C.8–10,12,54
Radiologic findings: Imaging studies enable the detection of focal wall thickening at different GI levels, with sensitivity of 79% for computed tomography (CT) scans, 83% for magnetic resonance imaging (MRI), and 74% for positron-emission tomography (PET) scans.55 Newer radiologic technologies, such as entero-CT and/or entero-MRI enable a better evaluation of localization and extent of damage to be made and may help to select an upper or lower endoscopic evaluation. Typical radiologic findings are >4 mm wall thickening with increased enhancement after IV contrast administration. Several patterns of segmental or continuous involvement have been described.56–58 Less common findings are bowel dilation, pericolic fat stranding, and prominent mesenteric vessels.56 Both CT and MRI also enable the identification of complications such as fistula, occlusion, pneumatosis, pneumoperitoneum, megacolon, or bowel perforation, the latter with a prevalence of 0.9–1.7%, and associated with mortality rates of 25% at 60 days.55–58 Enteral and/or large bowel thickening has been described to possibly predict histologic inflammation (Positive Predictive Value or PPV of 96%), as well as steroid requirement for symptomatic response (PPV 92%).59
Endoscopic findings: There are no specific endoscopic findings of oncologic GI toxicity, but esophagogastroduodenoscopy (EGD) and/or colonoscopy enable non-oncologic causes with similar clinical or endoscopic features to be excluded, as well as type, distribution, grade, and length of IT-associated damage to be evaluated, i.e., classified into 4 stages, according to the Mayo Clinic endoscopic score:12
- -
Grade 0: Normal findings.
- -
Grade 1: erythema, loss of vascular pattern, mild fragility.
- -
Grade 2: non-ulcerative inflammation (i.e., diffuse erythema, exudates, nodular aspect, edema, loss of vascular pattern, moderate fragility, and erosions).
- -
Grade 3: Deep or diffuse patchy ulcerations, strictures, denudation areas, and necrosis.
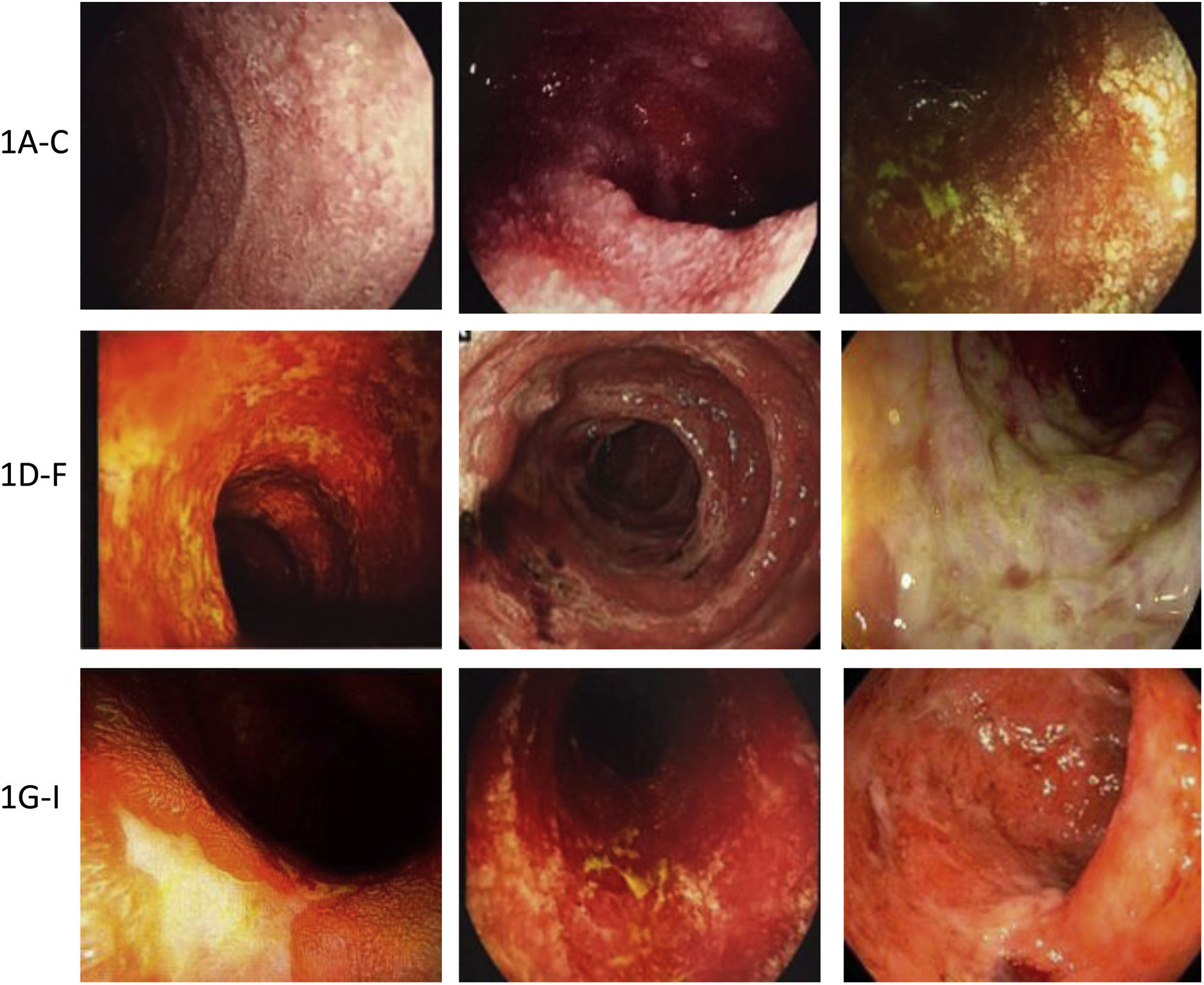
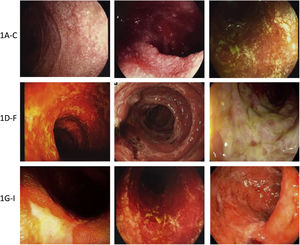
Damage may be limited to a single organ or affect the whole GI tract3,11,17,33,60–62 (Fig. 2).
Examples of immunotherapy-mediated gastrointestinal mucosal damage. (A–C) Examples of duodenal damage (granular mucosa, loss of vascular pattern, atrophy, erosions). (D–F) Examples of colonic damage (ulcerative colitis, necrotc ulceration, necrosis, pseudomembranes). (G–I) Examples of terminal ileum damage (ulcerations, edema, atrophy, friability, necrotic ulcers).
The most common endoscopic findings are:
- 1
Esophagus: erosions, mucosal nodularity or granular mucosa, ulcerations, strictures.
- 2
Stomach and duodenum: erythema, erosions, sub-epithelial hemorrhage, ulcerations, loss of vascular pattern, mucosal frailty/fragility, mucosal denudation, and granular or nodular duodenal aspect.
- 3
Small bowel and colon: erythema, loss of vascular pattern, mucosal fragility, erosions, patchy or diffuse ulcerations, denudation, pseudo-membranes, necrosis, with similar findings to those seen in IBD.
Indications for EGD or colonoscopy follow the same guidelines for general GI symptom evaluation, and terminal ileum evaluation is always recommended during colonoscopy unless severe diagnostic damage is found in the recto-sigmoid region.17,60–65 A retrospective study showed the prevalence of ulcerative disease in 40%, and 42% of non-ulcerative disease among CPI users.62 In 2 of the first published series of endoscopic studies in anti-CTLA-4 users, the most common finding was mucosal ulceration (79%), followed by erosions (13%), with recto-sigmoid involvement in 95–97%, pancolitis in 66%, and patchy distribution damage in 55%. Thus, in the majority of cases, flexible sigmoidoscopy with biopsies may yield a definite diagnosis.63,64 One of the studies showed 60% of erosive damage in the upper GI tract, mainly gastroduodenal, in addition to colonic involvement.63 In another study that included patients with diarrhea and CTCAE grade 3–4 colitis, 27% had ulcerations, 36% non-ulcerative mucosal damage, and 36% had normal findings.60 A study on ipilimumab users reported ulcerative damage in 40% of cases, with left hemicolon involvement in 42%, pancolitis in 40%, ileocolonic damage in 14%, and damage confined to the terminal ileum in 2%.35 The performance of early endoscopic studies fewer than 30 days from symptom onset is associated with a lesser requirement of steroids and biologic agents, as well as fewer hospitalizations,60 and the severity of both endoscopic and histologic damage is a strong predictor for steroid use.66 A multivariate analysis showed that long-term IT use and more severe endoscopic findings predicted the requirement of IFX or VDZ use.60 Another study that evaluated dasatinib-associated toxicity, reported colonic erosion rates of 83.3%, and loss of vascular pattern in 100% of cases.46 There are no comparative studies of endoscopic damage between IT and TT, but most series report higher rates of damage with IT, particularly when anti-CTLA-4 agents are used.35,62
Histopathologic findings and histologic variants: IT-associated histologic findings are not specific and may differ depending on the site of involvement and time of evolution.3,11,61 Even though no specific diagnostic criteria have been described, in general, histologic inflammatory findings can be divided in 5 groups: acute active, chronic active, microscopic lymphocytic or collagenous (similar to graft-versus-host disease), and mixed findings (normal, eosinophil infiltration [similar to findings of ischemic colitis, necrosis, and increased apoptotic activity], and non-specific inflammatory infiltrates).3,62,67 At the gastric level, the most common pattern is mucosal and submucosal inflammation, with intra-epithelial lymphocyte predominance, and micro-abscesses, granulomatous reaction, fibrosis, and increased apoptotic activity in the most severe cases.65,68 Intestinal histopathologic findings include intra-epithelial lymphocytosis, decreased villous height or atrophy, with a pattern similar to that seen in celiac disease, but contrastingly, a mixed pattern with polymorphonuclear cells, erosions, and increased apoptotic activity is also seen. In the large bowel, early findings include neutrophil or eosinophil infiltration, and neutrophilic cryptitis. Following several weeks of IT use, the early histologic pattern becomes both acute (neutrophils, eosinophils) and chronic (lymphoid cells and plasma cells), with diffuse inflammatory infiltrates similar to those seen in microscopic colitis, or with basal plasmacytosis, crypt distortion, and micro-abscesses, similar to those seen in IBD. A study compared histologic findings in a group of patients with ipilimumab-associated colitis and patients with ulcerative colitis and found significant differences: less basal plasmacytosis and crypt distortion but higher apoptotic activity in the IT group.69 In up to 10–12% of ITEC-CPIC, a histologic finding similar to that seen in microscopic colitis has been reported, both lymphocytic (particularly with anti-CTLA-4 agents) and collagenous colitis, mainly with anti-PD-1 and anti-PD-L1 agents.70–72 In mild cases, stratification of lymphocyte infiltrates in fewer than 5, 5−20, and >20 lymphocytes per 100 colon cells is appropriate for the evaluation of microscopic colitis, which seems to behave more like a chronic disease state than an acute flare following an episode of ITEC/CPIC.73–74 In most severe cases, extensive necrotic areas and gland destruction are observed, and increased apoptotic activity may be seen along the crypt epithelium. An international expert panel have proposed that the presence of apoptotic bodies in 10 consecutive crypts is highly suggestive of IT-associated damage.73 Other less common findings, such as Paneth cell metaplasia, bullous pemphigoid, associated reparative reactive changes, and lamina propria fibrosis have also been described.72,75,76 Immunohistochemistry (IHC) enables the type of damage in patients with dual IT use to be differentiated: in anti-PD-1 damage, IHC shows Treg cell predominance over the lamina propria, whereas CD8 + T cell predominance is typical of anti-CTLA-4 mucosal damage.11,72,77,78
Differential diagnosisEvery single inflammatory and non-inflammatory infectious cause with similar clinical features should be included in the differential diagnosis of ITEC/CPIC. Both opportunistic (HIV, Cryptosporidium, Isospora), and non-opportunistic agents (C. difficile, CMV, Cyclospora cayetanensis, Salmonella spp., Campylobacter, Shigella, Yersinia, Escherichia coli O157.H7, giardia, norovirus, rotavirus, enterovirus, and adenovirus) should be excluded. A retrospective cohort of 105 oncologic patients receiving IT with acute diarrhea reported a prevalence of 21.9% of microbiologically documented infection-associated symptoms.79 Drug-associated adverse events, such as those caused by antibiotics, NSAIDs, RT, chemotherapy, or mycophenolate mofetil (MMF), must be considered. NSAIDs may induce GI ulcerative and non-ulcerative damage by a number of mechanisms, including cyclo-oxygenase (COX) inhibition, which is associated with decreased mucosal blood flow, mucus production, and intestinal motility, but also with topical effects, interactions with bacteria and bile acids, as well as overexpression of pro-inflammatory cytokines. The clinical spectrum ranges from microscopic and eosinophilic enterocolitis to erosions, ulcerations with lymphocytic infiltration, and reactive gastropathy; the characteristic finding is the presence of diaphragm-like strictures.15,48 Radiation therapy may induce either acute or chronic damage through several mechanisms, such as endotheliitis, microvascular sclerosis, release of reactive oxygen species (ROS), mitotic cell death, and intestinal wall fibrosis.16 Among the QT agents, taxanes (i.e., paclitaxel, docetaxel) are mitosis inhibitors that alter the polymerization of a group of proteins called tubulins, causing damage to micro-tubules and protofilaments associated with chromosomic movement. Platin adducts (e.g., cisplatin, oxaliplatin) react with DNA bases, RNA, and proteins, inducing deconfiguration and apoptosis. Cytotoxic anti-metabolites (i.e., methotrexate, doxorubicin, 5-fluorouracil, capecitabin, gemcitabine, and irinotecan) cause enterocolonic damage by a number of mechanisms: DNA synthesis interruption, cell division inhibition, and ROS release, which causes the release of several signal factors, such as caspases, β-catenin, and nuclear factor kappa-beta (NF-κβ), leading to permeability alterations, protein-loss associated enteropathy, ischemic damage, and apoptosis, the latter associated with prolonged vasoconstriction or thrombosis, as well as damage to the precursor cell pool. Finally, alkylating agents (e.g., melphalan, chlorambucil, mechlorethamine, ifosfamide, carmustine, mitomycin C, dacarbazine) block cell division through DNA component alkylation.14–15 MMF is an immunosuppressant agent used in autoimmune disorders, as well as in post-transplant organ recipients. Besides dose-dependent diarrhea, this drug may induce erosive colitis associated with neutrophilic cryptitis and enterocyte apoptosis.48 In addition to these drug adverse events, co-existence with other malabsorptive and/or inflammatory conditions, particularly graft-versus-host disease with intestinal involvement, in which apoptotic body development is not uncommon, should be considered.48 Other causes of chronic diarrhea, such as pancreatic insufficiency and endocrinopathies, either primary or secondary to IT, may induce similar symptoms.3,8–10,48,52–54 It is important to consider that IT is frequently associated with systemic adverse events at multiple organs and systems, and that involvement may manifest as digestive symptoms. More recently, agent combination therapies with more than one IT group (e.g., an anti-CTLA-4 agent, such as ipilimumab, plus an anti-PD-1 agent, such as nivolumab), or a CPI plus a TT agent have become more increasingly used for several metastatic types of cancer, such as melanoma, due to resistance to or loss of effect of a single agent. These combination therapies are also associated with an increased rate of secondary effects, either associated with IT itself, or with immunosuppression-associated opportunistic infections.22,47,76,80
TreatmentSeveral aspects must be considered, in order to select the appropriate initial medical therapy, including disease extent and severity, IT type and length of use, previous illnesses, and concomitant drug use. Severity is commonly assessed by using the CTCAE scale, which enables the degree of damage and potential complications to be evaluated, and all guidelines use it as a norm. Nutritional status and degree of hydration must be evaluated and corrected. Signs of systemic toxicity and the presence of peritoneal signs should be properly treated. Antimicrobial agents directed at causal pathogens should be used if an infectious cause is found.3,4,8–10,12,52–54
Most oncology clinical guidelines have established the treatment of ITEC/CPIC, but there is no current consensus for esophageal or gastric damage. However, because of a similar pathophysiologic mechanism, the same therapeutic measures are considered. ITEC/CPIC behaves similarly to IBD, both symptomatically, in pathophysiologic mechanisms, and in endoscopic and histologic features with immune-mediated mucosal damage, so, as in IBD, therapeutic options directed at controlling or stopping this immune over-response should be carried out, either with steroids or with immunosuppressant drugs, to improve outcomes, as soon as possible (Fig. 3):
- -
Systemic steroids: corticosteroids inhibit both the innate and adaptative immune system through a number of mechanisms: they inhibit pro-inflammatory cytokine production, such as interleukin 2 (IL-2) and interferon gamma (IFN-γ), inhibit dendritic cell maturation, induce activated T cell apoptosis, and increase PD-1 expression in CD4+ and CD8 + T cells, suppressing their functions. Glucocorticoids (hydrocortisone, prednisone, prednisolone, methylprednisolone) decrease the number of circulating macrophages, monocytes, T cells, and eosinophils, the expression of major histocompatibility complex class III molecules, adhesion molecules, and vascular permeability, as well as the production of proinflammatory interleukins and prostaglandins. Steroids are considered the first choice of treatment for ITEC/CPIC, particularly intermediate-acting drug activity, such as prednisone or methylprednisolone, at a dosage of 1−2 mg/kg/d, with dose-reduction following a 4 to 6-week period, similar to IBD treatment. As a single measure, they are effective in 59% of cases, and if combined with biologic agents, effectivity increases up to 81% (IFX) and 85% (VDZ).31,81,82 Only one study has evaluated the use of local action steroids, such as budesonide, as a prophylactic measure administered along with IT, but they have not proved to be useful,83 unless the histologic diagnosis is consistent with microscopic colitis.74
- -
5-ASA derivatives: Aminosalicylate derivatives, such as mesalamine and sulfasalazine, display anti-inflammatory effects on the colonic mucosa, by inhibiting both the cyclo-oxygenase and lipo-oxygenase pathways, which is associated with decreased prostaglandin and leukotriene synthesis. The 5-ASAs, as a group, are useful in mild-to-moderate forms of IBD. Though used in ITEC/CPIC, evidence from clinical trials is scarce, yielding conflicting results, and translated results derived from IBD studies have been used.84 A retrospective study reported clinical improvement similar to that of cholestyramine in mild-degree cases of diarrhea and colitis,85 but there are no studies on severe forms of enterocolitis.
- -
Infliximab (IFX): IFX is a chimeric monoclonal antibody that specifically targets tumor necrosis factor alfa (TNF-α) that has been shown to be highly effective for the induction of remission and maintenance of severe cases of both forms of IBD. As an immune-mediated disease, ITEC/CPIC usually responds to corticosteroids, however one out of every 3 patients are refractory to these agents and may deteriorate and progress rapidly to more severe forms, possibly developing life-threatening complications. This anti-TNF agent started to be used as an empiric tool for ITEC/CPIC with partial or incomplete response to steroid therapy, with response rates similar to those seen in IBD, with significant symptomatic improvement following one or 2 infusions. Several case series started to report sustained improvement with more than 2 or with high-dose infusions. Initial IFX studies reported response rates of 81% when combined with steroids. Recently, evidence supporting the use of 3 intravenous doses, to increase histologic remission and decrease the risk of recurrence have emerged, with a success rate between 54 and 100%, and a median of 88%, 7 days after starting the dose. The dose used in clinical trials is the same as in IBD: IFX at an initial dose of 5 mg/k/dose at weeks 0, 2, and 6, increased to 10 mg/k/dose in cases with no response or inadequate response, and every 8 weeks in selected cases.31,82,86–94
- -
Vedolizumab (VDZ): VDZ is an IgG1 monoclonal antibody that specifically targets the α-4β7 integrin subunit in activated T cells, inhibiting their entry through intestinal tissue by blocking the interaction with the mucosal addressin cell adhesion molecule-1(MaDCAM-1), expressed on intestinal endothelial cells. It has been evaluated as an alternate option to IFX, with early evidence coming from case reports,95,96 but recently at least 3 prospective clinical trials and 2 systematic reviews have been published, and the same dose and scheme used for IBD (300 mg IV in IV infusion at weeks 0, 2, and 6, and in persistent or selected cases, every 8 weeks) has achieved clinical remission rates between 86 and 100%, a median of 89%, 86% sustained, as well as endoscopic and histologic remission of 54% and 29%, respectively.50,82,97–99 In 2 recent systematic reviews with meta-analyses, VDZ use was associated with shorter hospital stay lower re-admission rates, and decreased recurrence rates, compared with placebo.50,82 In one review, IFX was superior in terms of the 2-week remission rate (100% vs 83%), but VDZ was associated with higher rates of steroid withdrawal at 4 weeks (50% vs 7.4%).100 Current ASCO and NCCN guidelines recommend it as an alternative to IFX use in refractory cases, treatment failure, adverse effects, or contraindications to anti-TNF agents,8,9 and AGA guideline states that both IFX and VDZ are equivalent and interchangeable options, in cases associated with failure to the previous therapy.12
- -
Combination and maintenance therapies: In cases with suboptimal response following IT withdrawal and conventional or biologic therapy, a change to another immunosuppressive drug or combination therapy with more than one drug may be needed. Evidence is scarce or lacking, but a recent retrospective study showed that the early so-called “selected immunosuppressive therapy”, defined as the co-administration of systemic steroids plus IFX or VDZ, was associated with decreased hospitalization rates and treatment failure or recurrence following steroid withdrawal, and decreased symptom duration. Among patients with severe ITEC/CPIC that have previously responded to a biologic agent and require indefinite IT, an alternative is to continue the biologic agent along with the IT. Two studies have shown this form of therapy is superior, compared with restarting IT without the immunosuppressant drug, with lower grades of colitis recurrence, longer duration of IT treatment, and a higher probability of combination therapy use when 2 CPIs are used or with the CPI + TT combination.101–103 However, safety concerns with the long-term use of these type of agents have emerged, particularly those related to both an increased risk of infections and an increased potential risk of malignancy. At present, in most cases, biologic agents are recommended for remission induction, and once achieved, the dose/frequency is decreased, and the drugs are then withdrawn. In very selected cases, and as an expert opinion because evidence is scarce or lacking, long-term co-therapy may be considered after taking into account the risk-benefit ratio.91,104,105
- -
Other biologic agents: Other agents that have been shown to be effective in IBD, such as ustekinumab, an interleukin 12/23 receptor blocker,106 or tofacitinib, a Janus kinase (JAK) inhibitor107 have also been shown to be effective in steroid-refractory ITEC/CPIC in case reports. Tocilizumab is an anti-IL6 monoclonal antibody used in rheumatoid arthritis (RA), and an emerging treatment for IBD, but there is limited evidence in ITEC/CPIC, and even a case of bowel perforation has been described with its prolonged use, together with steroids, for RA.108 Other agents, such as adalimumab, have not been evaluated for this kind of illness.31,91
- -
Other immunosuppressants: The calcineurin inhibitors, mycophenolate mofetil (MMF), tacrolimus, and cyclosporin, act by binding to calcineurin, forming an intracellular complex, suppressing cytokine release (mainly IL-2, TNF-α, and interferon gamma [IFN-γ]), and inhibiting T cell activation. Evidence in ITEC/CPIC comes from case series, with response rates of 72%,109,110 and both the SITC, as well as the ESMO, consider them alternative therapies to IFX/VDZ.31,52,53 Clinical improvement has been shown with cyclosporine in a case report on IFX-refractory colitis, but there is no further evidence.111 Azathioprine is a purine analog immunosuppressant used as a steroid-sparing drug, that despite being highly effective in IBD, has not been evaluated in ITEC/CPIC.31
- -
Fecal microbiota transplant (FMT): Other research lines in oncologic diseases and their therapies are directed at correcting associated dysbiosis and several trials are ongoing. The microbiota is believed to affect the clinical response to IT and is one of the epidemiologic factors associated with ITEC/CPIC.3–4,11 In 2018, Wang reported 2 cases of refractory colitis successfully treated with FMT,112 whereas another similar case was recently published.113 Preliminary evidence in animal studies or in phase 1 or 2 clinical trials seem to suggest that interventions with microbiota-modifying agents have the potential to affect the 2 inflammatory pathways related to clinical response, or the development of IT-associated adverse events, but none of the current guidelines consider this a form of treatment in acute disease.114
Current recommendations for the treatment of IGT published by the SITC, ASCO, ESMO, NCCN, and AGA vary on certain points and are summarized below8,12,52–54:
CTCAE Grade 1:
- -
Symptomatic management is recommended, oral and or IV rehydration (AGA), non-dairy and low-fiber diet (ESMO).
- -
In general, an extensive diagnostic approach is not necessary (ASCO).
- -
Loperamide 2−4 mg every/6−8 h (VO), maximum dose 16 g/d (AGA, ESMO).
- -
It is safe to continue IT (ESMO).
- -
If diarrhea persists for >14 days, or worsens within 3–5 days, flexible sigmoidoscopy or colonoscopy/EGD + biopsies should be performed, and prednisolone IV 0.5−1 mg/kg/d considered (AGA, ESMO).
- -
In case of IT/CPI-associated gastroduodenal damage, antiemetics are recommended, and if no improvement, EGD should be performed (SITC).
CTCAE Grade 2:
- -
Infection-associated diarrhea should be ruled out prior to any treatment with steroids or other immunosuppressants. Both C. difficile, SARS-COV-2, as well as any opportunistic agents, must be ruled out (AGA, ASCO, ESMO, SITC).
- -
Quantitative fecal calprotectin and/or lactoferrin should be measured in order to stratify the need for endoscopic studies (AGA, ASCO, ESMO, SITC).
- -
Celiac disease should be ruled out (SITC).
- -
RSC/colonoscopy + biopsies or EGD + biopsies should be performed, according to the presence of either upper or lower GI symptoms (ESMO, ASCO, NCCN).
- -
IT should be temporarily withdrawn (AGA, ESMO, ASCO, NCCN).
- -
Endoscopic and histologic ITEC/CPIC findings should be confirmed prior to systemic steroid use (AGA).
- -
Consider abdominal imaging studies in pain-predominant cases or if there is fever or bleeding, but they should not be routinely performed in cases of diarrhea (AGA).
- -
Budesonide and mesalamine are ineffective as both prophylaxis for, or treatment of, ITEC/CPIC, unless a diagnosis of IT-associated microscopic colitis has been made (AGA).
- -
Prednisone (PDN)/prednisolone 0.5−1 mg/kg/d should be started (ESMO).
- -
PDN/prednisolone 1 mg/kg/d as starting dose (ASCO).
- -
PDN/prednisolone 1−2 mg/kg/d as starting dose (NCCN).
- -
PDN 0.5−2 mg/kg or equivalent should be started, with weaning off within 4–6 weeks if there is improvement (AGA).
- -
If there is an adequate response, decrease steroids within 4–6 weeks, temporarily withdraw IT, re-start it once GI inflammation is resolved (AGA, ASCO, SITC, ESMO); consider definite withdrawal of anti-CTLA-4 agents (ESMO, ASCO).
- -
If there is no improvement within 2–3 days, increase steroid dose to 2 mg/kg/d or switch to methylprednisolone 1−2 mg/kg/d (ESMO), or 2 mg/kg/d (ASCO), or to any IV steroid (AGA).
- -
Rule out latent infectious diseases before starting biologic agents (AGA, ASCO, ESMO, SITC).
- -
Apply a single IV dose of IFX or VDZ and re-evaluate; in case of remission, decrease steroid dose and re-start IT; in case of partial improvement continue IFX/VDZ and repeat colonoscopy (AGA).
- -
Give IFX5 mg/kg/dose, and repeat, if necessary, in 2 weeks (ESMO), 5−10 mg/kg/dose at 0, 2, and 6 weeks (ASCO, NCCN), or VDZ 300 mg IV at 0, 2, and 6 weeks if no improvement with high-dose steroid and IFX (NCCN).
CTCAE Grade 3 or worsening:
- -
Patients should be hospitalized in case of dehydration, fever, or systemic symptoms, IV liquids (ASCO, ESMO), contact isolation (ESMO).
- -
Rule out bowel perforation with CT scan, and C. difficile infection (fecal cytotoxin A, B and glutamate dehydrogenase) (AGA, ESMO, ASCO, NCCN, SITC).
- -
Prednisone or equivalent at 1−2 mg/kg/d (ASCO), or hydrocortisone 100 mg IV every 6−8 h, or methylprednisolone 1−2 mg/kg/d IV (NCCN), re-evaluate in 72 h, if improvement to grade 1, decrease steroid within 4–8 weeks (AGA, ASCO, NCCN).
- -
If no improvement within 3–5 days, start IV methylprednisolone 2 mg/kg/d, and consider IFX5 mg/kg/dose (SITC), 5−10 mg/kg/dose (ASCO) at 0, 2, and 6 weeks, or VDZ 300 mg IV at 0, 2, and 6 weeks for refractory cases (failure after 2 doses of IFX), or in cases of IFX contraindication (ASCO, AGA).
- -
Consider other immunosuppressants, such as MMF 500−1000 mg/d, if there is no improvement with steroids (ESMO).
- -
Document endoscopic and histologic healing (presence of ulcerations during steroid therapy, which occurs in up to 56% of patients with ipilimumab, predicting the need for definitive IT withdrawal or use of biologic agents). If there is endoscopic remission, withdraw the biologic agent, or consider co-administration with IT. If there is partial remission, continue the biologic along with the anti-PD/PD-L1 agents (AGA).
- -
Consider definite withdrawal of the anti-CTLA-4 agents (AGA, ASCO, ESMO).
CTCAE Grade 4:
- -
Definitive withdrawal of any form of IT if grade 4 GI toxicity develops (AGA, ASCO, ESMO, SITC).
- -
Every patient must be hospitalized, with IV liquids; rule out bowel perforation, megacolon and/or infections.
- -
Intravenous methylprednisolone at 1−2 mg/kg/day should be started, if there is improvement, switch to oral steroid and decrease dose within 4–8 weeks, and if there is no improvement, start IV IFX at 5 mg/kg/dose (SITC), 5−10 mg/kg/dose (ASCO), at 0, 2, and 6 weeks, or IV VDZ 300 mg IV 0, 2, and 6 weeks for refractory cases (failure after 2 doses of infliximab), or in case of contraindications to IFX (ASCO, SITC).
- -
Consider the combination of a second immunosuppressant (e.g., MMF, tacrolimus, cyclosporine) (ESMO).
- -
Consider prophylaxis against Pneumocystis jirovecii (ESMO).
Clinical worsening during any phase:
- -
Perform a follow-up RSC to document persistent or worsening endoscopic and/or histologic mucosal damage.
- -
Rule out infectious causes, such as CMV colitis, C. difficile-associated pseudomembranous colitis, or TB-associated colitis (ASCO, ESMO, SITC, AGA).
- -
All patients with ITEC/CPIC should be evaluated with liver function tests in order to rule out IT-associated hepatobiliary damage, and repeat tests before re-starting IT, if it was suspended (AGA).
- -
Close follow-up and intensive treatment in case of deterioration with the use of anti-CTLA-4 agents, which may be associated with early deterioration (AGA).
- -
Consider a higher dose of IFX or VDZ (ASCO, ESMO, SITC).
- -
In case of sepsis or perforation at any stage, discontinue any immunosuppressive drug, including steroids, and treat properly (i.e., systemic antibiotics, anti-fungal agents, surgery).
Recurrence: Between 33 and 50% of patients re-starting IT, following an episode of immune-mediated diarrhea or colitis, develop similar symptoms shortly thereafter.115 Risk factors for recurrence are: initial use of anti-PD-1 or PD-L1 agents (RR 3.45), early need for immunosuppressants (RR 3.22), and long-term symptom duration during initial episode (RR 1.01). Average onset of recurrent symptoms is 49 days after re-starting IT. Risk decreases in patients re-starting with only a PD-1 or PD-L1 inhibitor (18–21%). AGA, ASCO and ESMO guidelines recommend definite withdrawal of anti-CTLA-4 agents after grade 3 bowel damage, and any CPI after grade 4 events.8,10,12,52–54
Targeted therapy-associated enterocolitis: All previously published guidelines describe therapy for IT-associated GI damage, but TT-associated GI damage has been evaluated less. When IT and TT are co-administered and the patient develops GI symptoms, the guidelines are the same as for IGT/ITEC, and both therapies are withheld until toxicity disappears and damage improves.47 As a rule of thumb, when TT is administered without IT, the risk of complications and degree of mucosal damage is less than that associated with CPIs, and the recommendation is to withhold medication, rule out infectious and potentially surgical complications, and provide oral or IV rehydration according to severity, electrolyte correction, antidiarrheal medications, and if necessary, broad-spectrum antibiotics, as with IT. However, unlike IT, there is less evidence of improvement with steroids or other immunosuppressive drugs, unless the patient has been receiving combination therapy with IT, has received anti-EGFR or anti-HER2 agents and can have immune-mediated damage, or there are life-threatening complications, such as severe enterocolonic ulcers and no superimposed infection, in which case, a course of steroids similar to that used for ITEC/CPIC may help reduce inflammation and induce tissue damage remission.5,47
Conclusions- -
IT with immune CPIs and TT agents are new forms of oncologic treatment associated with higher survival rates, even in advanced tumors. However, they can both be associated with a number of gastrointestinal adverse effects, particularly mucositis involving any GI segment, with symptom onset shortly after starting therapy and lasting for several weeks after withdrawal.
- -
Mechanisms of IT-associated damage are: immune over-stimulation (CPI), ischemia, and alterations in epithelial repair, and in some cases, immune-mediated damage (TT).
- -
Clinical, radiologic, and endoscopic manifestations differ according to the organ of origin and degree of severity, and may range from mild changes to ulcerations, even to life-threatening complications, such as necrosis and bowel perforation.
- -
Diagnosis requires the temporary association between the use of IT and GI symptom onset, radiologic and endoscopic features, and the ruling out of other conditions, such as infections with similar features. Histopathologic findings are nonspecific and may mimic other conditions, such as microscopic colitis and inflammatory bowel disease. Apoptotic activity in tissue biopsies is considered diagnostic.
- -
Treatment of enteritis and/or colitis associated with IT is based on the degree of damage, and may include oral or IV rehydration, the use of antidiarrheal drugs, oral or systemic steroids, other immunosuppressive drugs, including biologic agents in more severe cases, and temporary or definitive IT withdrawal, particularly anti-CTLA agents. Patients should be closely monitored for potential deterioration, with periodic re-evaluations, modifying management based on improvement or progression.
No sponsorship of any kind was received to carry out this article.
Conflict of interestThe authors declare that they have no conflict of interest.