Since the 1960s, several studies have shown the effect of aging on esophageal motility, with inconsistent results.
The aim of the present study was to evaluate the manometric results in older adult patients (≥ 60 years of age) with an esophageal disorder and compare them with adults under 60 years of age.
Materials and methodsA cross-sectional, retrospective study was conducted that included a sample of 1,175 patients (936 older adults and 239 non-older adults). The patients were evaluated and compared with respect to (i) sex, (ii) main complaint for which esophageal manometry was indicated, (iii) comorbidities, (iv) current medications, (v) smoking, and (vi) manometry results.
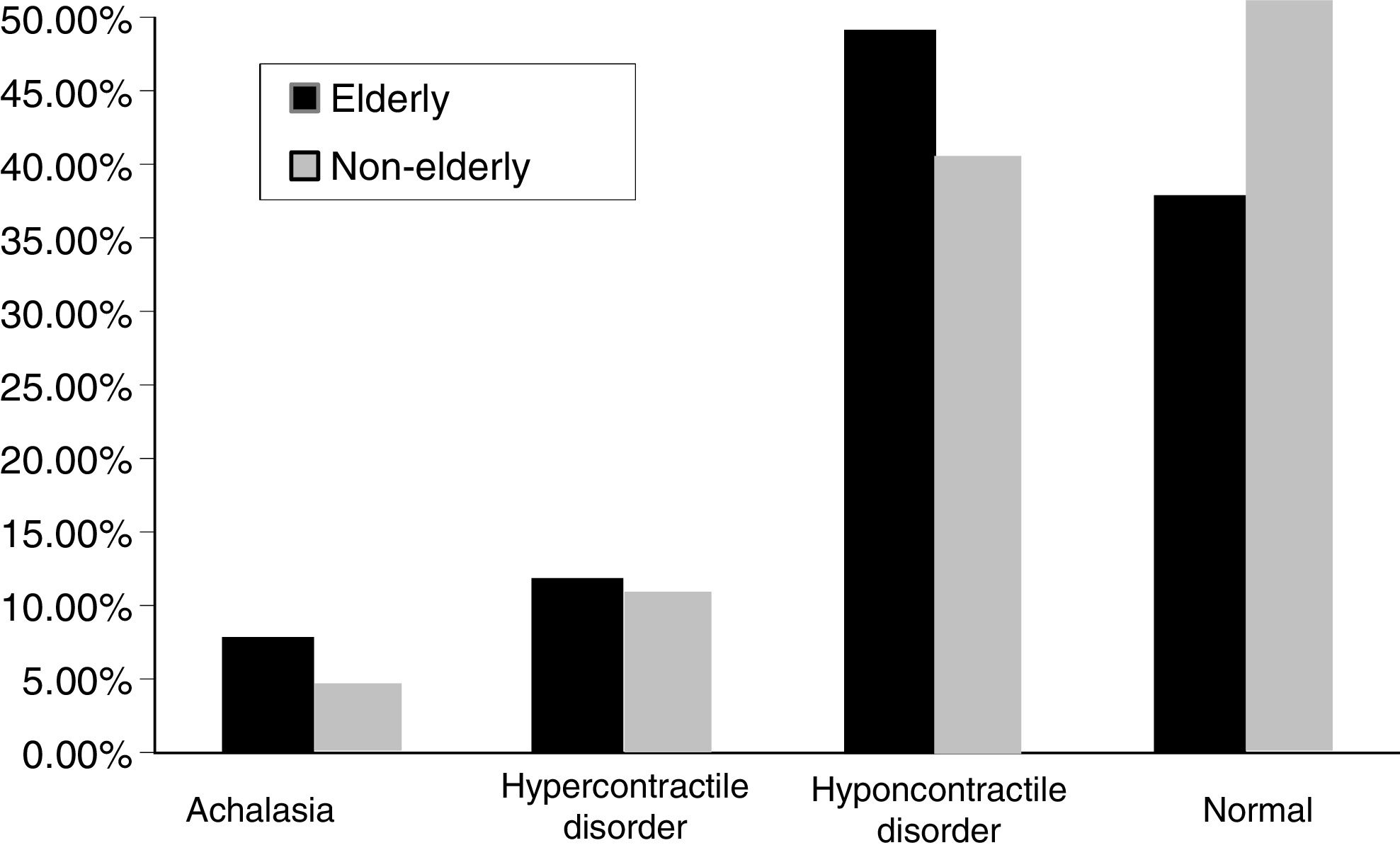
ResultsPatient age ranged from 19 to 92 years (women made up 76.5% of the older adults and 72.8% of the non-older adults). Normal lower esophageal sphincter relaxation and normal peristalsis were more frequent in the non-older patient group (91.1% vs. 84.8% and 87.4% vs. 76%, respectively). The manometry results for the non-older adults vs. the older adults, respectively, were: achalasia (2.9% vs. 5.9%); hypercontractile disorder (9.2% vs. 10.4%); hypocontractile disorder (38.5% vs. 47.6%); and normal values (49.4% vs. 36.1%). After excluding the variables that could change esophageal motility, the results revealed significant differences between the two study groups.
ConclusionsEsophageal manometry demonstrated statistically significant differences between the older adult and non-older adult study population evaluated.
Desde los años 60 s varios estudios han mostrado el efecto del envejecimiento sobre la motilidad esofágica, con resultados inconsistentes.
El objetivo del presente estudio fue evaluar los resultados manométricos en pacientes adultos mayores (≥ 60 años) con una enfermedad del esófago y compararlos con los de adultos menores de 60 años.
Materiales y métodosSe realizó un estudio retrospectivo transversal que incluyó una muestra de 1, 175 pacientes (936 adultos mayores y 239 adultos no mayores). Los pacientes fueron evaluados y comparados por (i) sexo, (ii) queja principal por la que se indicó la manometría esofágica, (iii) comorbilidades, (iv) medicación al momento, (v) tabaquismo y (vi) resultados de manometría.
ResultadosLa edad de los pacientes tuvo un rango de 19 a 92 años (76.5% de los adultos mayores y 72.8% de los adultos no mayores fueron mujeres). La relajación de esfínter esofágico bajo normal y la peristálsis normal fueron más frecuentes en los adultos no mayores que en los adultos mayores (91.1% vs 84.8% y 87.4% vs 76%, respectivamente). Los resultados para la manometría de adultos mayores vs adultos no mayores respectivamente fueron: acalasia (2.9% vs 5.9%); esófago hipercontráctil (9.2% vs 10.4%); esófago hipocontráctil (38.5% vs 47.6%). Después de excluir las variables que podrían cambiar la motilidad esofágica, los resultados revelaron diferencias significativas entre los dos grupos de estudio.
ConclusionesLa manometría esofágica demostró diferencias estadísticamente significativas entre la población de adultos mayores y de adultos no mayores del estudio.
Manometric and radiologic studies performed in the 1960s showed that a number of esophageal dysfunctions appeared with aging, but patients with systemic diseases, such as diabetes mellitus or neurologic disorders, were not excluded from those reports.1–2 In the following decade, Hollins and Castell conducted studies on men 70 to 87 years of age, excluding systemic diseases (neurologic pathologies and diabetes mellitus) and described a slight reduction in the amplitude of peristaltic contractions. However, the speed and duration of contractions in primary peristalsis were considered to be within normal values.3 In the 1990s, Ribeiro et al.4 reported that lower esophageal sphincter (LES) residual pressure was lower in older adult patients with achalasia, but the resting pressure was similar to that found in younger patients. In addition, there was a lower percentage of patients with normal motility in the older adults and a greater probability of achalasia and diffuse esophageal spasm.
In 2003, Robson and Glick5 assessed motility in individuals ≥ 65 years of age and compared the results with younger individuals between 18 and 45 years of age, finding no significant difference between the 2 groups. Andrews et al. restricted the older adult group to only include patients 80 years of age and compared them with younger adults with dysphagia. The manometric findings remained similar.6 However, those same authors7 assessed the effect of age and sex in another study and reported that aging increased the chances of not having normal motor function. They also stated that nonspecific motor disorders, including ineffective hypotensive peristalsis and achalasia-like conditions, were more common.
In 2014, Besanko et al.8 analyzed the effect of aging on esophageal motility in healthy older adults (81 ± 1.7 years of age) and young adults (23 ± 1.7 years of age) through high-resolution manometry and found lower baseline LES pressure in the older adults, as well as a decrease in its complete relaxation. However, as the authors themselves remarked in the Discussion section, the inclusion of only 10 individuals in each group was a potential limitation of their study.
Shim et al.9 reported the effects of aging on 62 patients > 65 years of age, using esophageal impedance manometry. They described a reduced baseline pressure and a lower distal contractile integral at the upper esophageal sphincter (UES), but no significant difference in impedance values, compared with controls.
O’Rourke et al.10 compared the usefulness of esophagram versus high-resolution manometry in detecting esophageal dysmotility. They found that the esophagram was useful in the assessment of structural and anatomic abnormalities but was a poor screening exam for the detection of esophageal dysmotility. Patients with suspected esophageal dysphagia should be referred for high-resolution manometry to evaluate motility disorders, regardless of esophagram results.10
There is a worldwide trend toward a demographic increase in the population > 60 years of age, given that older adults represented 7.3% of the population in 1991. That percentage is estimated to increase to 22% (2 billion individuals) by 2050.11 Thus, the present-day definition of degenerative changes that occur in old age may actually be physiologic aging processes, and not, for instance, specific esophageal motility disorders, and so re-evaluation may be warranted.12–14
The aim of the present study was to assess the manometric results in a population of older adult patients (≥ 60 years of age) and compare them with the findings in individuals < 60 years of age, to determine possible differences between the 2 groups. All the patients had clinical indications for the manometric examination.
Materials and methodsIn a retrospective, cross-sectional study conducted at the Hospital do Servidor Público Estadual in São Paulo, Brazil, the medical records of patients that underwent esophageal manometry, within a time frame of 8 years, were evaluated, and the patients were divided into the following 2 groups:
- (1)
Older adults (≥ 60 years of age)
- (2)
Controls: younger adults (19-59 years of age).
The age criterion for distinguishing the older adult from the younger adult was based on age categories used by the World Health Organization.12 The inclusion criteria were patients above 18 years of age in whom esophageal manometry was indicated.
Exclusion criteria. Patients that had previously undergone gastrointestinal tract surgery (gastrectomy, esophagectomy, esophageal myotomy, fundoplication) or endoscopic procedures (esophageal dilation, botulinum toxin injection) and patients that did not have a previous upper gastrointestinal endoscopy performed up to one year before the procedure were excluded.
The study variables evaluated were sex, the main complaint for which esophageal manometry was indicated, comorbidities, medications used (diuretics, angiotensin inhibitors, nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, hypoglycemics, beta blockers, calcium channel blockers, lipid-lowering agents, bisphosphonates, calcium), smoking, and manometry results. Patient data were assessed through the medical records.
The manometry results were characterized as follows15–17:
― Normal values used to categorize the manometric features
― LES station analysis (wet swallows):
- •
Resting pressure: 10-45 mm Hg.
― Esophageal body motility analysis (wet swallows):
- •
Peristaltic performance as % of normal swallows: > 90%.
- •
Mean amplitude (distal): 30-180 mm Hg.
Manometric classification of esophageal motility abnormalities (adapted from Castell15)
― Achalasia
- •
Absent distal peristalsis. (Required for diagnosis)
- •
Elevated resting LES pressure (> 45 mm Hg). (May be seen, not required)
- •
Incomplete LES relaxation (residual pressure > 8 mm Hg). (May be seen, not required)
- •
Elevated baseline esophageal pressure. (May be seen, not required)
― Diffuse esophageal spasm
- •
Simultaneous contractions (> 20% wet swallows). (Required for diagnosis)
- •
Intermittent normal peristalsis. (Required for diagnosis)
- •
Repetitive contractions (> 3 peaks). (May be seen, not required)
- •
Prolonged duration contractions (> 6 s). (May be seen, not required)
- •
Retrograde contractions. (May be seen, not required)
- •
Isolated incomplete LES relaxation (> 8 mm Hg). (May be seen, not required)
― Nutcracker esophagus
- •
Increased distal peristaltic amplitude (>180 mm Hg). (Required for diagnosis)
- •
Increased distal peristaltic duration (> 6 s). (May be seen, not required)
― Hypertensive LES
- •
Resting LES pressure > 45 mm Hg. (Required for diagnosis)
- •
Incomplete LES relaxation (residual pressure > 8 mm Hg). (May be seen, not required)
― Hypocontracting esophagus (can be secondary to gastroesophageal reflux disease)
- •
Increased non-transmitted peristalsis ( > 30%). (Any or all may be seen)
- •
Low distal peristaltic amplitude (< 30 mm Hg). (Any or all may be seen)
- •
Hypotensive LES (resting LES pressure < 10 mm Hg). (Any or all may be seen)
- •
Ineffective esophageal motility (Any or all may be seen)
― Scleroderma esophagus
- •
Low LES pressure. (Required for diagnosis)
- •
Weak or absent distal peristalsis. (Required for diagnosis)
- •
Normal upper esophagus and upper esophageal sphincter. (Required for diagnosis)
- a)
Normal
- b)
Hypocontractile disorder (LES hypotony, inefficient esophageal motility, hypocontractility or atony of the esophageal body)
- c)
Hypercontractile disorder (LES hypertonia, diffuse esophageal spasm, nutcracker esophagus, and achalasia)
The 2 study groups underwent conventional esophageal manometry examinations, with Alacer® Multiplex II (Alacer Biomedica®, São Paulo, Brazil) equipment, performed by the same examiner who followed the methodology established in previously published papers.18,19 The manometry protocol utilized 10 wet swallows at 30 s intervals with the station pull-through technique and probe traction every 1 cm. An 8-channel polyvinyl flexible probe was used, with a 4.5 mm external diameter and a 0.8 mm internal diameter. The 4 distal channels were set radially at the same level and the proximal channels were set with a 5 cm space between them (Alacer Biomedica®, São Paulo, Brazil). Each was perfused using a constant infusion system with a 0.6 ml/min/channel flow. Those channels were connected to external pressure transducers (Alacer Biomedica®, São Paulo, Brazil). The recorded pressures were picked up by a polygraph (Alacer Biomedica®, São Paulo, Brazil), and after being converted into digital images, were transferred to a microcomputer in real time.
A total of 1,098 manometries were carried out in the older adult group, over a period of 8 years. Given that there was no previous study with comparable features to calculate the control samples, a 4:1 (older adults: younger adults) examination proportion was chosen, resulting in a total number of 271 patients in the control group.
The statistical power of the sample was then calculated in relation to the outcome of the manometry examinations. The total number of manometries performed within the same period on individuals that could be controls was 2,356. The control group patients were randomly selected, using the Excel program (sampling tool). Once the exclusion criteria were applied (162 patients excluded in the older adult group and 32 in the younger adult group), the study sample was composed of 1,175 patients, 936 of whom were older adults and 239 younger adults. The sample had 93% power to detect a difference between groups, in relation to manometry outcome (effect size = 0.114852), considering a chi-square test with a level of significance (α) of 5% (0.05).
Statistical analysisThe categorical variables were expressed as frequency and percentage in each category. The statistical significance for the different categorical variables was verified using the Pearson’s chi-square test, the likelihood-ratio test, or the Fisher’s exact test. The multiple comparisons were carried out using the chi-square test, the Fisher’s exact test, and the Student’s t test. Conclusions regarding multiple comparisons were associated with the Bonferroni method. The significance level was set at 5% (α = 0.05) and the SPSS version 19 software was employed.
To calculate the sample power for detecting a difference between the groups in relation to the manometry results, the chi-square test was used to compare the proportions between the 2 groups, and a 5% significance level was used (α = 0.05).
Ethics Committee Review- •
Informed consent was requested from all the patients in the study.
- •
The study was approved and documented by the Ethics Committee of the Faculty of Medicine of the Universidade de São Paulo and the Hospital do Servidor Público Estadual in São Paulo, the institutions involved in the data collection and analysis.
- •
The authors declare that the present study contains no personal information enabling patient identification.
The total number of patients was 1,369, as described below:
- a)
The older adult group: 1,098 patients; after the exclusion criteria were applied: 936.
- b)
The control group: 271 patients; after the exclusion criteria were applied: 239.
The exclusions in the older adult group were due to previous esophageal surgery (n = 3), endoscopic dilation (n = 14), fundoplication (n = 65), gastrectomy (n = 28), myotomy (n = 11), incomplete records (n = 24), and no endoscopy (n = 17).
The control group exclusions were due to previous esophageal surgery (n = 2), endoscopic dilation (n = 1), fundoplication (n = 15), gastrectomy (n = 2), gastroplasty (n = 3), myotomy (n = 2), incomplete records (n = 1), and no endoscopy (n = 6).
In the older adult group, the following number of patients had no information on different study variables: sex (n = 1), smoking (n = 32), disease history (n = 3), current medication use (n = 4), LES relaxation assessment in the esophageal manometry exam (n = 11); and in the younger adult control group: smoking (n = 6), LES relaxation assessment in the esophageal manometry exam (n = 2 ), assessment of the average esophageal body pressure in the esophageal manometry examination (n = 2).
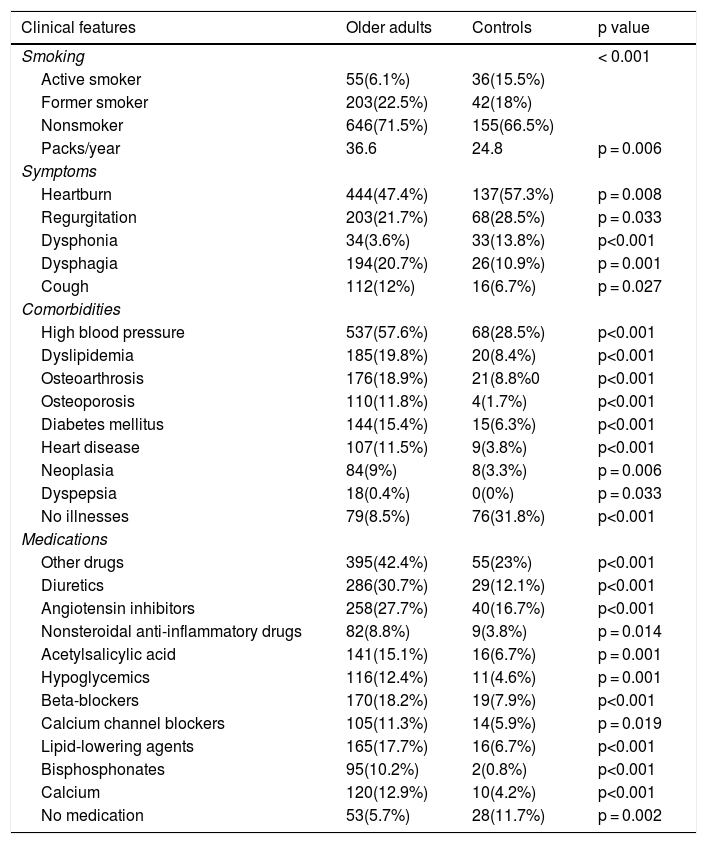
Patient age varied from 19 to 92 years. A total of 715 (76.5%) patients in the older adult group and 174 (72.8%) patients in the control group were females. The groups were not statistically different with regard to sex (p = 0.273) (Table 1).
Clinical features of the older adult group and the control group.
| Clinical features | Older adults | Controls | p value |
|---|---|---|---|
| Smoking | < 0.001 | ||
| Active smoker | 55(6.1%) | 36(15.5%) | |
| Former smoker | 203(22.5%) | 42(18%) | |
| Nonsmoker | 646(71.5%) | 155(66.5%) | |
| Packs/year | 36.6 | 24.8 | p = 0.006 |
| Symptoms | |||
| Heartburn | 444(47.4%) | 137(57.3%) | p = 0.008 |
| Regurgitation | 203(21.7%) | 68(28.5%) | p = 0.033 |
| Dysphonia | 34(3.6%) | 33(13.8%) | p<0.001 |
| Dysphagia | 194(20.7%) | 26(10.9%) | p = 0.001 |
| Cough | 112(12%) | 16(6.7%) | p = 0.027 |
| Comorbidities | |||
| High blood pressure | 537(57.6%) | 68(28.5%) | p<0.001 |
| Dyslipidemia | 185(19.8%) | 20(8.4%) | p<0.001 |
| Osteoarthrosis | 176(18.9%) | 21(8.8%0 | p<0.001 |
| Osteoporosis | 110(11.8%) | 4(1.7%) | p<0.001 |
| Diabetes mellitus | 144(15.4%) | 15(6.3%) | p<0.001 |
| Heart disease | 107(11.5%) | 9(3.8%) | p<0.001 |
| Neoplasia | 84(9%) | 8(3.3%) | p = 0.006 |
| Dyspepsia | 18(0.4%) | 0(0%) | p = 0.033 |
| No illnesses | 79(8.5%) | 76(31.8%) | p<0.001 |
| Medications | |||
| Other drugs | 395(42.4%) | 55(23%) | p<0.001 |
| Diuretics | 286(30.7%) | 29(12.1%) | p<0.001 |
| Angiotensin inhibitors | 258(27.7%) | 40(16.7%) | p<0.001 |
| Nonsteroidal anti-inflammatory drugs | 82(8.8%) | 9(3.8%) | p = 0.014 |
| Acetylsalicylic acid | 141(15.1%) | 16(6.7%) | p = 0.001 |
| Hypoglycemics | 116(12.4%) | 11(4.6%) | p = 0.001 |
| Beta-blockers | 170(18.2%) | 19(7.9%) | p<0.001 |
| Calcium channel blockers | 105(11.3%) | 14(5.9%) | p = 0.019 |
| Lipid-lowering agents | 165(17.7%) | 16(6.7%) | p<0.001 |
| Bisphosphonates | 95(10.2%) | 2(0.8%) | p<0.001 |
| Calcium | 120(12.9%) | 10(4.2%) | p<0.001 |
| No medication | 53(5.7%) | 28(11.7%) | p = 0.002 |
Chi-square test. Likelihood-ratio test.
The manometry examination results of normal LES relaxation (91.1% vs. 84.8%) and normal peristalsis (87.4% vs. 76%) were significantly more frequent in the control group, compared with the older adult group (p = 0.018 and p < 0.001, respectively).
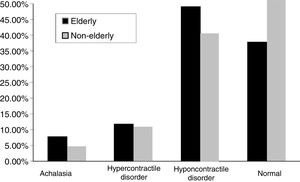
The other manometric results were: achalasia, n = 7 in the younger adult group (2.9%) and n = 55 in the older adult group (5.9%), hypercontractile disorder, n = 22 in the younger adult group (9.2%) and n = 97 in the older adult group (10.4%), hypocontractile disorder, n = 92 in the younger adult group (38.5%) and n = 446 in the older adult group (47.6%), and normal results, n = 118 in the younger adult group (49.4%) and n = 338 in the older adult group (36.1%). The difference was statistically significant between the groups with respect to hypocontractile disorders and normal results (p = 0.001) (Fig. 1).
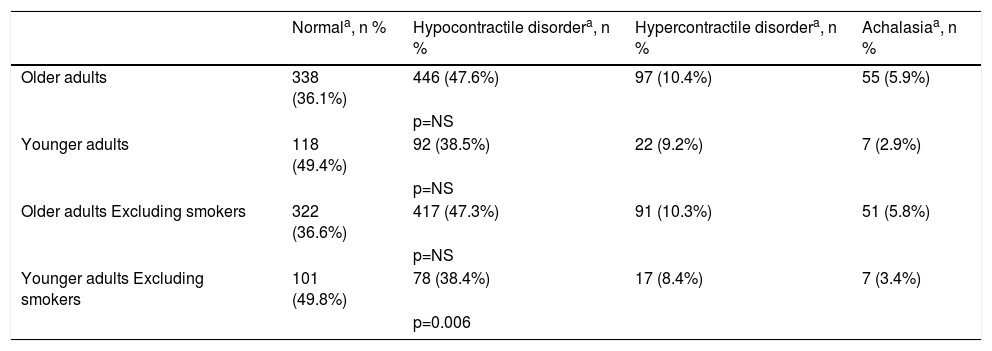
When active smokers were excluded, the younger adult group was significantly different from the older adult group (p = 0.006), with regard to normal manometry and hypocontractile results (Table 2).
Esophageal manometry results in the 2 patient groups, with and without the exclusion of smoking.
| Normala, n % | Hypocontractile disordera, n % | Hypercontractile disordera, n % | Achalasiaa, n % | |
|---|---|---|---|---|
| Older adults | 338 (36.1%) | 446 (47.6%) | 97 (10.4%) | 55 (5.9%) |
| p=NS | ||||
| Younger adults | 118 (49.4%) | 92 (38.5%) | 22 (9.2%) | 7 (2.9%) |
| p=NS | ||||
| Older adults Excluding smokers | 322 (36.6%) | 417 (47.3%) | 91 (10.3%) | 51 (5.8%) |
| p=NS | ||||
| Younger adults Excluding smokers | 101 (49.8%) | 78 (38.4%) | 17 (8.4%) | 7 (3.4%) |
| p=0.006 |
Chi-square test. Likelihood-ratio test.
Hypercontractile disorder: hypertonic lower esophageal sphincter, diffuse esophageal spasm, and nutcracker esophagus; Hypocontractile disorder: hypotonic lower esophageal sphincter, ineffective esophageal motility, hypocontractility or atony of the esophageal body; NS: not significant.
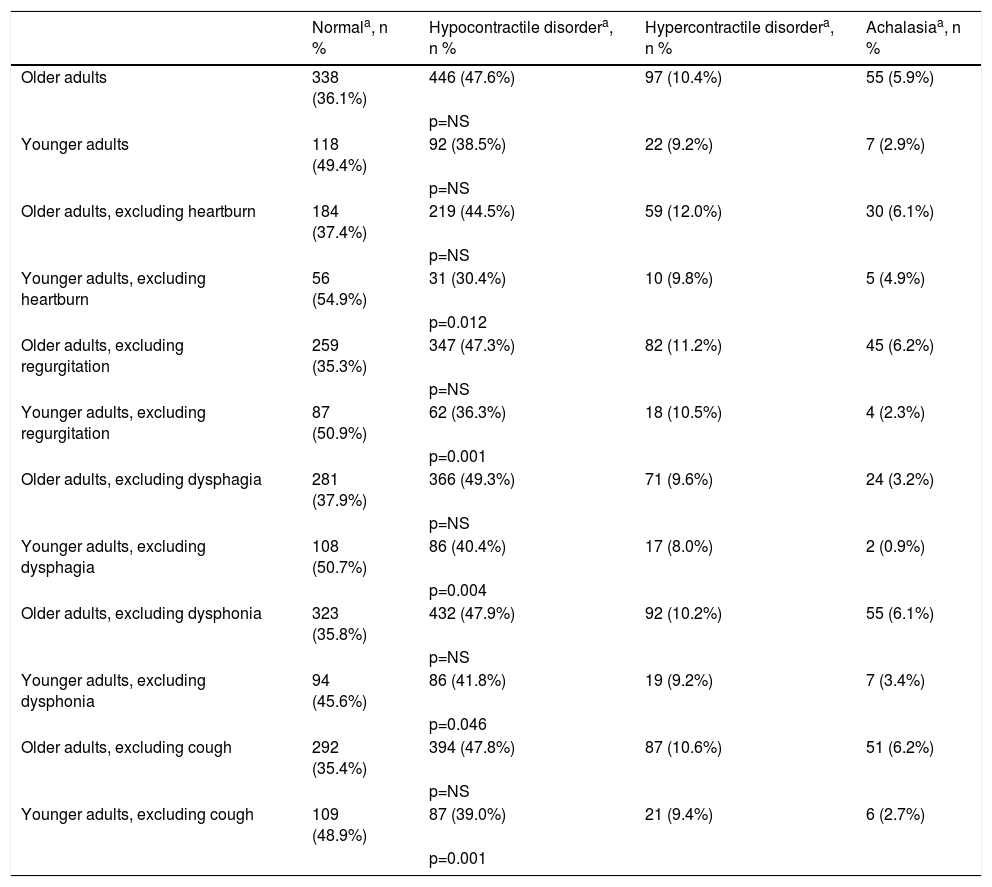
When the main symptoms were excluded, including heartburn (p = 0.012), regurgitation (p = 0.001), dysphagia (p = 0.004), dysphonia (p = 0.046), and cough (p = 0.001), the younger adult group was significantly different from the older adult group in relation to normal manometry and hypocontractile disorder (Table 3).
Esophageal manometry results in the 2 patient groups, with and without the exclusion of the main symptoms included in the table.
| Normala, n % | Hypocontractile disordera, n % | Hypercontractile disordera, n % | Achalasiaa, n % | |
|---|---|---|---|---|
| Older adults | 338 (36.1%) | 446 (47.6%) | 97 (10.4%) | 55 (5.9%) |
| p=NS | ||||
| Younger adults | 118 (49.4%) | 92 (38.5%) | 22 (9.2%) | 7 (2.9%) |
| p=NS | ||||
| Older adults, excluding heartburn | 184 (37.4%) | 219 (44.5%) | 59 (12.0%) | 30 (6.1%) |
| p=NS | ||||
| Younger adults, excluding heartburn | 56 (54.9%) | 31 (30.4%) | 10 (9.8%) | 5 (4.9%) |
| p=0.012 | ||||
| Older adults, excluding regurgitation | 259 (35.3%) | 347 (47.3%) | 82 (11.2%) | 45 (6.2%) |
| p=NS | ||||
| Younger adults, excluding regurgitation | 87 (50.9%) | 62 (36.3%) | 18 (10.5%) | 4 (2.3%) |
| p=0.001 | ||||
| Older adults, excluding dysphagia | 281 (37.9%) | 366 (49.3%) | 71 (9.6%) | 24 (3.2%) |
| p=NS | ||||
| Younger adults, excluding dysphagia | 108 (50.7%) | 86 (40.4%) | 17 (8.0%) | 2 (0.9%) |
| p=0.004 | ||||
| Older adults, excluding dysphonia | 323 (35.8%) | 432 (47.9%) | 92 (10.2%) | 55 (6.1%) |
| p=NS | ||||
| Younger adults, excluding dysphonia | 94 (45.6%) | 86 (41.8%) | 19 (9.2%) | 7 (3.4%) |
| p=0.046 | ||||
| Older adults, excluding cough | 292 (35.4%) | 394 (47.8%) | 87 (10.6%) | 51 (6.2%) |
| p=NS | ||||
| Younger adults, excluding cough | 109 (48.9%) | 87 (39.0%) | 21 (9.4%) | 6 (2.7%) |
| p=0.001 |
Chi-square test. Likelihood-ratio test.
Hypercontractile disorder: hypertonic lower esophageal sphincter, diffuse esophageal spasm, and nutcracker esophagus; Hypocontractile disorder: hypotonic lower esophageal sphincter, ineffective esophageal motility, hypocontractility or atony of the esophageal body; NS: not significant.
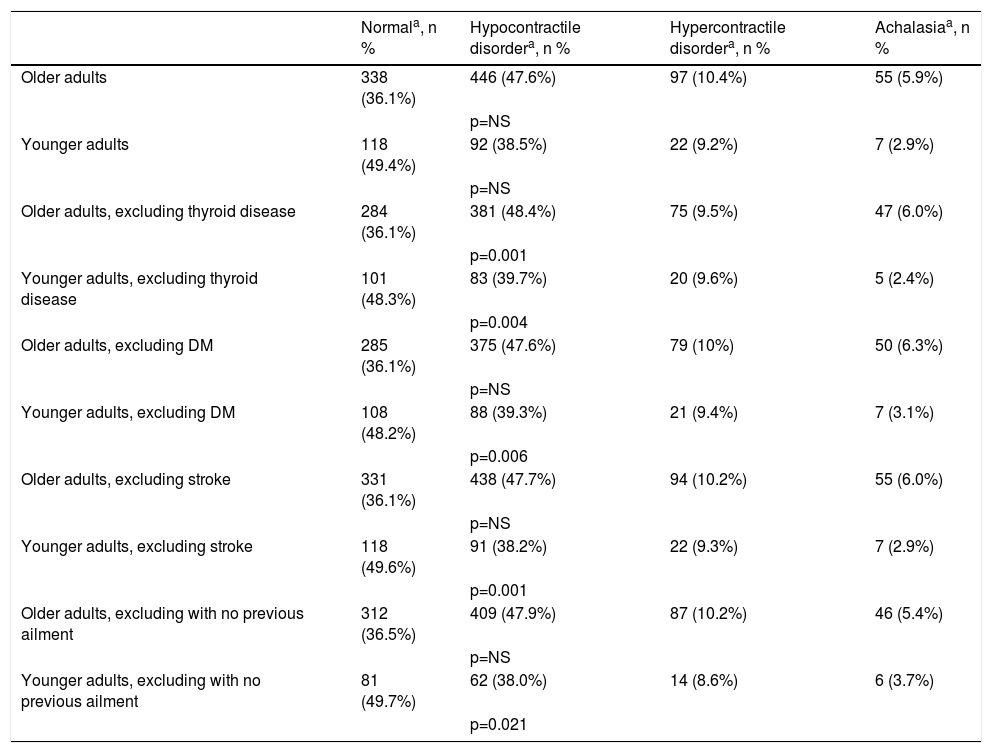
When the pathologies, such as thyroid diseases (p = 0.004), diabetes mellitus (p = 0.006), previous stroke (p = 0.001), and patients without any ailment (p = 0.021) were excluded, the younger adult group became significantly different from the unfiltered older adult group, with respect to normal manometry and the hypocontractile disorder results (Table 4).
Esophageal manometry results in the 2 patient groups, with and without the exclusion of the comorbidities that change esophageal motility.
| Normala, n % | Hypocontractile disordera, n % | Hypercontractile disordera, n % | Achalasiaa, n % | |
|---|---|---|---|---|
| Older adults | 338 (36.1%) | 446 (47.6%) | 97 (10.4%) | 55 (5.9%) |
| p=NS | ||||
| Younger adults | 118 (49.4%) | 92 (38.5%) | 22 (9.2%) | 7 (2.9%) |
| p=NS | ||||
| Older adults, excluding thyroid disease | 284 (36.1%) | 381 (48.4%) | 75 (9.5%) | 47 (6.0%) |
| p=0.001 | ||||
| Younger adults, excluding thyroid disease | 101 (48.3%) | 83 (39.7%) | 20 (9.6%) | 5 (2.4%) |
| p=0.004 | ||||
| Older adults, excluding DM | 285 (36.1%) | 375 (47.6%) | 79 (10%) | 50 (6.3%) |
| p=NS | ||||
| Younger adults, excluding DM | 108 (48.2%) | 88 (39.3%) | 21 (9.4%) | 7 (3.1%) |
| p=0.006 | ||||
| Older adults, excluding stroke | 331 (36.1%) | 438 (47.7%) | 94 (10.2%) | 55 (6.0%) |
| p=NS | ||||
| Younger adults, excluding stroke | 118 (49.6%) | 91 (38.2%) | 22 (9.3%) | 7 (2.9%) |
| p=0.001 | ||||
| Older adults, excluding with no previous ailment | 312 (36.5%) | 409 (47.9%) | 87 (10.2%) | 46 (5.4%) |
| p=NS | ||||
| Younger adults, excluding with no previous ailment | 81 (49.7%) | 62 (38.0%) | 14 (8.6%) | 6 (3.7%) |
| p=0.021 |
Chi-square test. Likelihood-ratio test.
DM: diabetes mellitus; Hypercontractile disorder: hypertonic lower esophageal sphincter, diffuse esophageal spasm, and nutcracker esophagus; Hypocontractile disorder: hypotonic lower esophageal sphincter, ineffective esophageal motility, hypocontractility or atony of the esophageal body.
NS: not significant.
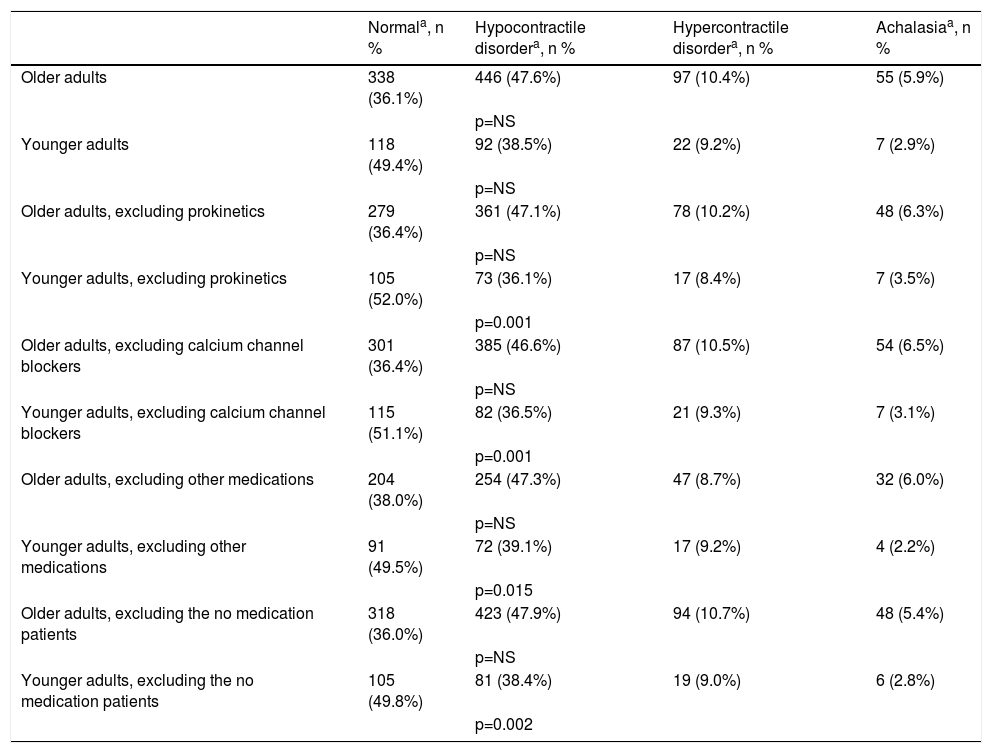
In the present study, 883 (94.3%) older adult patients and 211 (88.3%) younger adult patients were taking medications. When the no medication patients, prokinetic drugs, calcium channel blockers, and other drugs (such as nitrate, baclofen, and sildenafil) were excluded, a new pattern was produced: the younger adult group became significantly different from the older adult group, regarding the normal manometry and hypocontractile disorder results, respectively (p = 0.002; p = 0.001; p = 0.001, and p = 0.015) (Table 5).
Esophageal manometry in the 2 patient groups, with and without the drugs that change esophageal motility.
| Normala, n % | Hypocontractile disordera, n % | Hypercontractile disordera, n % | Achalasiaa, n % | |
|---|---|---|---|---|
| Older adults | 338 (36.1%) | 446 (47.6%) | 97 (10.4%) | 55 (5.9%) |
| p=NS | ||||
| Younger adults | 118 (49.4%) | 92 (38.5%) | 22 (9.2%) | 7 (2.9%) |
| p=NS | ||||
| Older adults, excluding prokinetics | 279 (36.4%) | 361 (47.1%) | 78 (10.2%) | 48 (6.3%) |
| p=NS | ||||
| Younger adults, excluding prokinetics | 105 (52.0%) | 73 (36.1%) | 17 (8.4%) | 7 (3.5%) |
| p=0.001 | ||||
| Older adults, excluding calcium channel blockers | 301 (36.4%) | 385 (46.6%) | 87 (10.5%) | 54 (6.5%) |
| p=NS | ||||
| Younger adults, excluding calcium channel blockers | 115 (51.1%) | 82 (36.5%) | 21 (9.3%) | 7 (3.1%) |
| p=0.001 | ||||
| Older adults, excluding other medications | 204 (38.0%) | 254 (47.3%) | 47 (8.7%) | 32 (6.0%) |
| p=NS | ||||
| Younger adults, excluding other medications | 91 (49.5%) | 72 (39.1%) | 17 (9.2%) | 4 (2.2%) |
| p=0.015 | ||||
| Older adults, excluding the no medication patients | 318 (36.0%) | 423 (47.9%) | 94 (10.7%) | 48 (5.4%) |
| p=NS | ||||
| Younger adults, excluding the no medication patients | 105 (49.8%) | 81 (38.4%) | 19 (9.0%) | 6 (2.8%) |
| p=0.002 |
Chi-square test. Likelihood-ratio test.
Hypercontractile disorder: hypertonic lower esophageal sphincter, diffuse esophageal spasm, and nutcracker esophagus; Hypocontractile disorder: hypotonic lower esophageal sphincter, ineffective esophageal motility, hypocontractility or atony of the esophageal body; NS: not significant.
Since the 1960s, several studies have attempted to demonstrate the effect of aging on esophageal motility, with inconsistent results.1–8 For example, the populations studied were composed of healthy older adult individuals, but with different age cutoff points for defining the term, older adult,3–8,20–23 and they also included patients with diseases (e.g., achalasia)24 or specific symptoms (e.g., dysphagia).6 The results varied, from studies showing important changes in the amplitude of esophageal body contraction and LES tone in the older adult, compared with younger adults,2–4,7,8,20,21,23,24 to studies that found no such differences between the 2 groups.
The most frequent symptoms in the younger adult patients were heartburn (more than one-half of the cases) and regurgitation (more than one-fourth of the cases). According to the report by Richter, in spite of frequent gastroesophageal reflux disease in the older adult, heartburn was usually less severe or more infrequent, compared with the younger patients.25 Mold and Rankin26 also suggested that gastroesophageal reflux disease is underdiagnosed in the older adult because the refluxed material can be less acidic than in younger patients, the intensity of heartburn may be reduced due to changes in pain perception, and older adults may underreport reflux symptoms. Consequently, dysphagia was reported as more frequent in just over one-fifth of those patients. In 2015, Kawani et al.27 studied the effects of aging and acid reflux on esophageal motility, during high-resolution manometry in 40 healthy young subjects (< 45 years of age), 40 healthy older adults (> 65 years of age), and 40 older adults (> 65 years of age) with mild esophagitis. They reported that aging may cause a decrease in the success rate of secondary peristalsis, and acid reflux may be the cause of a decrease in the distal contractile integral in primary peristalsis and secondary peristalsis.8,21,22 In 2005, Achem and DeVault28 reported that gastroesophageal reflux was less frequent in older adults due to the greater prevalence of pathologies that involve dysphagia (e.g., Zenker’s diverticulum, Parkinson’s disease, stroke). In our study, the source disease could not be identified, but dysphagia symptoms tended to occur in similar proportions. Most certainly, other factors are inherent to physiologic esophageal aging. Different authors have reported a greater-than-expected probability of achalasia, achalasia-like conditions, and a reduced frequency of complete LES relaxation in the older adult.4,7,8,29
More frequent normal peristalsis in the younger adult group in our study was a finding similar to that of Csendes et al.,20 who reported a small reduction in the frequency of primary peristalsis in older adults. Similarly, Adamek et al.21 demonstrated a greater percentage of aperistalsis in the older adult, as did Nishimura et al.22 Our results of a higher number of normal manometry results in the younger adult group were consistent with the higher percentage of normal motility in younger adult patients described by Ribeiro et al. and Andrews et al.4,6
In contrast, hypocontractile disorders were more frequent in the older adult group than in the younger adult group. The studies by Andrews et al. and Shim et al.7,9 had similar results, demonstrating a greater likelihood of ineffective/hypotensive peristalsis in older adults. In a systematic review of 16 articles on esophageal manometry in healthy or dysphagic older persons (> 60 years of age), the authors stated that an age-related loss of central and/or enteric nervous system functions and esophageal compliance modifications, such as those related to the loss of elastic tissues, might explain the reduced peristalsis amplitude and greater likelihood of peristaltic failure.30 An experimental study on rats supports the hypothesis that age-related cell loss occurs exclusively in the cholinergic neurons in the myenteric plexus. However, those authors concluded that nitrergic neurons are not completely spared from the effects of age, either.31
Mei et al.32 demonstrated that UES and esophageal body pressure responses to low-volume ultra-slow reflux and associated post-reflux residue were reduced in older adult individuals, compared with young patients. Said deterioration could have negative effects on airway protection for people in that age group. The effect of aging on a number of airway protective reflexes, such as the pharyngo-UES contractile reflex, laryngo-UES contractile reflex, and the reflexive pharyngeal swallow, causes an increase in the threshold for stimulation, suggesting desensitization of those reflexes in the older adult. A decrease in the number of tension-sensitive receptors could contribute to diminishing secondary peristalsis and UES pressure in the older adult, as well.32,33
Significant statistical differences were identified between our study groups, but we were aware that some of the variables could affect motility and, therefore, we decided to exclude them and verify whether the differences between the manometry results were maintained.
Since the 1960s, studies have reported manometric changes in patients with diabetes mellitus, with and without peripheral neuropathies, including changes in esophageal body motility and the LES.34,35 More recently, utilizing high-resolution manometry, George et al.36 found a higher incidence of primary esophageal gastric junction outflow obstruction in diabetic patients, compared with non-diabetics. In contrast, data on thyroid diseases are scarce, but esophageal changes have been described in Graves’s disease, thyrotoxic myopathy, myxedema, hyperthyroidism, and hypothyroidism. They appear to be abnormalities that can be reversed through treatment, but the mechanisms are not totally understood.35,37,38 With regard to stroke, abnormal LES function has been reported, as well as an increase in aperistalsis and incomplete LES relaxation.39
Medications can affect esophageal function. Calcium channel blockers may cause a reduction in LES pressure and in the amplitude of esophageal body contraction. The same effect can be observed with nitrates.40 A few prokinetic drugs, such as mosapride and metoclopramide,41,42 can cause an increase in LES pressure and in the amplitude of esophageal body contraction. Baclofen can cause a reduction in transitory LES relaxation and an increase in LES pressure.43 And finally, trials on sildenafil have shown a reduction in LES pressure and in the amplitude of esophageal contraction.44
After excluding smoking, diabetes mellitus, thyroid diseases, stroke, and drugs that interfere with esophageal motility, we still found a significantly greater percentage of hypocontractile disorder in the older adults and a normal result in the younger adults. As stated above, those findings are consistent with the results described in the trials reported by Ribeiro et al. and Andrews et al.4,7
Recently, Cock et al. studied bolus clearance in asymptomatic older adults during high-resolution impedance-manometry in 45 healthy volunteers (30 young controls and 15 older subjects) and observed that impaired bolus clearance occurred more frequently in asymptomatic older subjects than in the young controls. That study also showed impaired oropharyngeal and esophageal function in the same older individuals and could represent a similar pathophysiologic process, with an abnormal swallowing mechanism, with vagal activation, and/or a loss of sensory modulation of swallowing.45
One of the limitations of our study was its cross-sectional and retrospective design, with the characteristic missing information that can cause outcome measurement error (patients with lost information are different from those with no information loss). Another problem was that no prior comparable study was available from which to calculate a representative control sample. However, our calculation of a 93% sample power and a randomized control sample were most likely sufficient for limiting selection error.
Another study limitation was the use of conventional manometry rather than high-resolution manometry. Although the latter method is more costly, it has the advantages of greater recognition of anatomic landmarks and hiatal hernias, of enabling the classification of achalasia into 3 different types, and of better treatment orientation. In addition, impedance combined with high-resolution manometry provides valuable information regarding bolus transit, reflux episodes, rumination syndrome, and belching disorders. In a comparison of high-resolution esophageal manometry with conventional manometry, normal esophageal motility was the most frequent finding (47% and 36%, respectively; p = 0.054). Hypotensive LES was the most common motility disorder identified by conventional manometry (27.3%), whereas ineffective esophageal motility was the most common esophageal motor disorder identified by high-resolution manometry (25.3%).46
Therefore, we believe that high-resolution esophageal manometry has great advantages over conventional manometry. However, the proportion of possible diagnostic errors or correctness were inherent in our 2 groups, which does not invalidate our results and emphasizes the importance of using the same criteria in the entire study population. High-resolution manometry has only been available to us this past year, but we intend to carry out studies in the near future using only that new technology.
Because our study was conducted at a tertiary care hospital with symptomatic patients, the outcome could be extended to a population with such characteristics. If we associate the fact of having used a representative sample, we could also extrapolate it to patients with similar characteristics from either a primary care or a secondary care hospital.
Nevertheless, we believe the results cannot be validated for healthy individuals, and further studies on that population are required. Therefore, we conclude that there was a statistically significant difference in the manometric results between the older adult group and the younger adult group, even when the variables that could affect esophageal motility were excluded.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Kunen LCB, Fontes LHS, Moraes-Filho JP, Assirati FS, Navarro-Rodriguez T. Los patrones de motilidad esofágica están alterados en pacientes adultos mayores. Revista de Gastroenterología de México. 2020;85:264–274.