Helicobacter pylori (H. pylori) infection remains the leading cause of several gastroduodenal diseases. Despite the fact that multiple antibiotic regimens have been used to change its associated morbidity and mortality, the prevalence of this bacterial infection continues to be disproportionately high worldwide, mainly due to antibiotic resistance. To assess the noninferiority efficacy and safety of 210-day triple regimens on H. pylori eradication, we evaluated clarithromycin 500mg, lansoprazole 30mg, and amoxicillin 1g, all bid (standard triple therapy or CLA, Group 1) vs. pantoprazole 80mg, levofloxacin 500mg and azithromycin 500mg, all od (PLA, Group 2). Both regimens were compared in treatment-naïve patients.
Materials and methodsAn open label phase IIIb randomized and noninferiority trial comparing CLA vs. PLA was carried out for a 10-day period, within the time frame of June 2012 and March 2014. Eradication was verified with 13C-urea breath testing. Gastric biopsies were tested for fluorescence in situ hybridization (FISH)-clarithromycin resistance prior to any antibiotic administration. Efficacy and safety results were analyzed according to the noninferiority methodological approach.
ResultsFrom the 227 H. pylori positive subjects that were randomized, 194 were finally analyzed as per-protocol. The group 2 eradication rate was 63% and was noninferior to the group 1 eradication rate of 58.5% (upper limit 95% CI: 0.11608; below the noninferiority margin: 0.1200). FISH clarithromycin-resistance was found in 28.2% of the cases. Adverse events, all minor and self-limited, were significantly higher in group 1 than in group 2 (86 vs. 65.4%; p=0.001).
ConclusionsFirst-line H. pylori eradication with pantoprazole/levofloxacin/azithromycin combination therapy is as effective as the standard triple therapy, with better tolerability and easier dosing. Clarithromycin resistance should be considered when selecting antibiotics in Helicobacter pylori eradication treatments.
ClinicalTrials.gov identifier NCT02726269.
La infección por Helicobacter pylori (H. pylori) representa el factor etiológico más importante en numerosas enfermedades gastroduodenales. A pesar de haberse utilizado múltiples esquemas antibióticos para modificar su morbimortalidad asociada, la prevalencia de esta infección bacteriana continúa siendo desproporcionadamente alta en todo el mundo, debido principalmente a la resistencia antibiótica. A los efectos de evaluar la eficacia no inferior y la seguridad de 2regímenes de triple terapia durante 10 días para la erradicación de H. pylori comparamos claritromicina 500mg, lansoprazol 30mg y amoxicilina 1g, 2 veces al día (terapia triple estándar o CLA, grupo 1) vs. pantoprazol 80mg, levofloxacina 500mg y 500mg de azitromicina, 1 vez al día (PLA, grupo 2). Los 2regímenes fueron comparados en pacientes previamente no tratados.
Material y métodosEntre junio del 2012 y marzo del 2014 se llevó a cabo un estudio abierto de fase iiib aleatorizado y de no inferioridad que comparó CLA versus PLA en un periodo de 10 días. La erradicación se verificó con pruebas de aliento con 13C-urea. Las biopsias gástricas se analizaron para resistencia a claritromicina por hibridación fluorescente in situ, anterior a la administración de cualquier antibiótico. Los resultados de eficacia y seguridad fueron analizados siguiendo la metodología de no inferioridad.
ResultadosDe los 227 sujetos positivos a H. pylori que fueron aleatorizados, finalmente se analizaron 194 por protocolo solamente. La tasa de erradicación del grupo 2 fue del 63%, no inferior a la tasa de erradicación del grupo 1 del 58.5% (límite superior IC 95%=0.11608; margen de no inferioridad=0.1200). La resistencia a la claritromicina evaluada fue del 28.2%. Los eventos adversos fueron significativamente mayores en el grupo 1 que en el grupo 2 (86% vs. 65.4%, p=0.001).
ConclusionesLa erradicación de H. pylori en primera línea con terapia combinada de pantoprazol/levofloxacina/azitromicina resulta tan efectiva como con la terapia triple estándar, aunque con mejor tolerabilidad y una dosificación más fácil. La resistencia a la claritromicina debe considerarse en los tratamientos de erradicación del H. pylori.
ClinicalTrials.gov identificación: NCT02726269.
Helicobacter pylori (H. pylori) gastric infection is the main cause of gastritis, duodenal ulcer, and gastric cancer. In Mexico, the national mean H. pylori seroprevalence reported in the general population is 66%.1,2 In addition, the rate of gastric cancer mortality was 4.5 per 100,000 in 1980 and has increased to 6.5 per 100,000 over a 10-year period.2
Recommendations for H. pylori eradication schemes are widely available.3 Best results have been obtained with the so-called triple therapy, which includes 2 antibiotics and a proton pump inhibitor (PPI). In Mexico, the combination of clarithromycin, amoxicillin, and a PPI has been the recommended option, as metronidazole resistance (up to 80%) is among the highest in the world.4 In a recent study that compared sequential and traditional schemes in 7 Latin American countries (Chile, Colombia, Costa Rica, Honduras, Nicaragua, and Mexico [2 sites]), eradication rates ranged from 66 to 77% for the Mexican subjects.5 The IV Maastricht Consensus states that antibiotic combination must be selected according to the reported resistance.3 However, in Mexico there is little current information regarding identified resistance, with only a few recent studies suggesting that clarithromycin resistance may be on the rise.6 However, those studies emphasize that the resistance of H. pylori to clarithromycin ranges between 17.8 and 24%, according to the case series analyzed.4,7 Thus, the search for therapeutic alternatives is necessary to improve those unacceptable levels of antibiotic resistance, highly correlated with important therapeutic failures in H. pylori eradication.
Levofloxacin, a broad-spectrum quinolone, has been used as a second-line treatment to eradicate H. pylori and in patients with penicillin allergy.3,8 Data suggest that levofloxacin plus a macrolide could be as effective as the traditional triple therapy. Clinical trials performed with triple therapies based on quinolones have reported eradication rates of 80 to 90.4% after 10 days, depending on the therapy evaluated.9 Although it is true that there is also a growing trend toward resistance to quinolones in Latin America, that trend has not yet reached the level of resistance to macrolides, especially clarithromycin.10,11 Therefore, quinolone-based schemes continue to represent a promising scenario for the eradication of H. pylori in Mexico.12
The aims of the present study were to compare the triple scheme with the alternative scheme of levofloxacin+azithromycin+a PPI and to determine the frequency of clarithromycin resistance in the population of metropolitan areas in Mexico.
Materials and methodsSubjectsThe present phase IIIb study was a prospective, open-label, randomized, parallel-group, noninferiority controlled trial. It was carried out from June 2012 to January 2014 at 4 outpatient clinics in two cities located on the Mexican plateau: Mexico City and the city of Toluca.
Two hundred and thirty treatment-naïve subjects aged 18 to 65 years, with H. pylori infection proven by endoscopic biopsy, were included in the study and signed statements of informed consent. Exclusion criteria were severe comorbidity, pregnancy, lactation, and study drug allergy. Patients were randomized through a permuted-block randomization procedure to ensure balance in the number of patients assigned to each clinical site. The procedure used computer-generated random numbers and was carried out by the clinical research organization (CRO) responsible for running the study.1 Because patients were recruited in a competitive manner, as many permuted blocks as necessary were assigned to each clinical site until the previously calculated sample size was completed.
Treatment regimenPatients were randomly allocated into one of 2 groups: Group 1 received clarithromycin 500mg twice daily (bid), lansoprazole 30mg bid, and amoxicillin 1g bid (Pylopac®, Medix SA de CV, Mexico); group 2 received pantoprazole 80mg once daily (Zoltum®, Laboratorio Monte Verde SA, Argentina), levofloxacin 500mg (Laboratorios Asofarma de México SA de CV, Mexico), and azithromycin 500mg (Truxa®, Laboratorios Monte Verde SA, Argentina. Both groups received the treatment for 10 days. Antibiotics were prescribed after meals, whereas the PPI was taken under fasting conditions. No other medication was allowed until the end of the treatment. Medications were kindly provided by ASOFARMA de México.
AssessmentsBlood samples for the safety analysis were collected in a central laboratory after the statements of informed consent were signed and before treatment. Patients were evaluated using the 13C-urea breath test (13C-UBT) 4 weeks after H. pylori eradication treatment. An additional blood sample was drawn for comparison with the initial results. Eradication of H. pylori was defined as a negative 13C-UBT.
Patient compliance and treatment-related adverse events (AEs) were assessed at the end of the treatment. To evaluate compliance to the allocated treatment, all patients received a printed diary to record medication intake (date, time of administration, and daily frequency) and AEs. AEs were graded as mild if they did not interfere with daily activities, moderate if they interfered with daily activities to some extent, and severe if either suspension of treatment or hospitalization were required, or if the AE resulted in death of the patient. Since the target population for the primary efficacy analysis was per-protocol, only those patients that complied 100% with the administration of the assigned treatment were considered valid for said analysis, in accordance with the data reported in the printed diary.
Histologic and microbiologic evaluationAll biopsies were reviewed by a central pathologist (LB). Endoscopic biopsies were immediately fixed in 10% buffer formalin, embedded in paraffin, sectioned (4-mm slice thickness), and dehydrated in a series of increasing ethanol/xylol concentration. Each section was stained with hematoxylin and eosin (H&E). The diagnosis of gastritis was established in accordance with the updated Sydney system.13
Identification of H. pylori and determination of macrolide resistance using fluorescence in situ hybridization
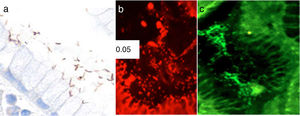
Identification of H. pylori was performed on H&E stains. In cases with morphologic features suggestive of H. pylori infection not identified by H&E stains, immunohistochemistry for H. pylori (anti-Helicobacter pylori; 1:500; Thermo Scientific, Fremont, CA) was performed. Fluorescence in situ hybridization (FISH) was carried out according to a previously described protocol.14 Briefly, formalin-fixed paraffin-embedded 4-mm tissue sections were spotted onto slides coated with poly-L-lysine and processed by hexane and ethanol. Hybridization was done using the commercially available BACTfish H pylori combi kit (Izinta Trading Co. Ltd., Hungary). The probe for H. pylori identification (Hpy 1) (5′CACACCTGACTGACTATCCCG-3′) was labeled with fluorescein isothiocyanate (FITC), which provides a green signal, and the probes for detecting the 3 most prevalent clarithromycin-resistance mutations ClaR1 (A2143G) 5′CGGGGTCTTCCCGTCTt-3′, ClaR2 (A2144G) 5′CGGGGCTCTCCGTCTT-3′, and ClaR3 (A21443C) 5-CGGGGTCTTGCCGTCTT-3′, were labeled with red fluorochrome (Cy3) (fig. 1). Following hybridization for 90min at 46° C, sections were washed with wash buffer twice at 46° C for 15min.14 Air-dried sections were stained with 4′, 6′ diamino-2-phenylindole (DAPI). Slides were evaluated using a fluorescence Nikon Eclipse 80i microscope. Images were taken with a Nikon DS-Fi1 camera and processed with NIS-Elements 2.1 software (Nikon Corporation, Shinagawa Intercity Tower, Tokyo, Japan).
Photomicrographs of gastric mucosa. a) Identification of H. pylori through immunohistochemistry. b) Positive FISH (resistant H. pylori strain): presence of resistance to clarithromycin mutations (red dots). c) Negative FISH (sensitive H. pylori strain): no resistance to clarithromycin mutations.
According to current statistical guidelines on noninferiority trial statistical analysis, non-intention-to-treat (non-ITT) analysis, such as the on-treatment or per-protocol (PP) approach, is more desirable than the intention-to-treat analyses, given that in noninferiority trials, an intention-to-treat analysis will often increase the risk of falsely claiming noninferiority (type I error).15 Thus, the efficacy analysis was based on the H. pylori eradication rate in subjects that finished treatment as PP. The modified intention-to-treat (mITT) approach, in which patients received at least one dose of the allocated treatment and completed the 13C-UBT, was undertaken for further supportive reasons and safety analysis.
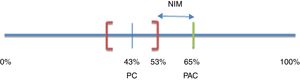
Noninferiority margin calculationConsidering a noninferiority approach,16 a pre-established margin of noninferiority (δ) for the treatment effect in the primary efficacy outcome was defined. Bochenek et al.17 compared the efficacy of 2 triple treatment regimens versus 2 dual therapy regimens for H. pylori eradication in patients with short-term peptic ulcer (7 days). An eradication percentage of 65% was obtained for one of the triple combinations (pantoprazole+amoxicillin+clarithromycin or PAC) and 43% for one of the two double combinations (pantoprazole+clarithromycin or PC). Because they were superiority designs, results were analyzed in intention-to-treat populations. The 95% confidence intervals (95% CI) were 56-73% and 36-53% for PAC and PC, respectively.
The noninferiority margin (δ) for the present study was estimated considering that any treatment with 3 active ingredients (PAC) should be superior to any other treatment with only 2 active ingredients (PC). Thus, the percentage of H. pylori eradication with PAC was expected to be greater than the upper limit of the 95% CI calculated for the eradication rate observed with PC (53%). The maximum admissible decrease in efficacy in relation to that observed for the triple treatment (PAC) was then defined as δ. Therefore, δ was the difference between the percentage of eradication of H. pylori achieved with the triple treatment (PAC) and the upper limit of the CI calculated for the double treatment (PC): δ=65-53%=12%15,16 (fig. 2).
Noninferiority margin of efficacy
NIM: noninferiority margin; PAC: pantoprazole+amoxicillin+clarithromycin; PC: pantoprazole+clarithromycin. Based on Bochenek et al.17.
Under that premise, in our study group 2 (PLA) was considered noninferior to group 1 (CLA), when the upper limit of the 95% CI for the difference in the eradication rate between both groups was lower than the pre-established noninferiority margin (δ=0.12 or 12%).
Patients that were lost to follow-up or could not complete the treatment course because of severe AEs were considered treatment failures and excluded from the per-protocol analysis. Continuous variables were described using the mean and standard deviation. The chi-square test and Student's t test were used to compare the differences between the 2 study groups regarding baseline data and AEs; a p value <0.05 was considered significant. The paired Student's t test was used to compare the biochemical results for the safety analysis. The IBM SPSS 21 program was used to perform the statistical analysis.
The study was approved by an independent ethics committee2 and by the COFEPRIS (Mexican Health authority) and was conducted in accordance with the Helsinki Declaration for the protection of human subjects, adhering to the Good Clinical Practice guidelines.
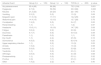
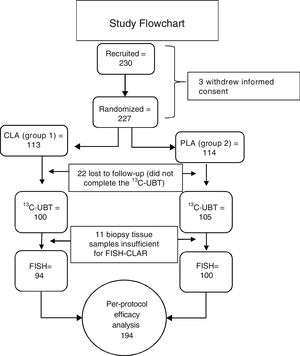
ResultsGroup characteristicsTwo hundred and thirty subjects were included in the study. Three subjects withdrew their informed consent before starting treatment. Twenty-two of the 227 randomized subjects were lost to follow-up and did not complete the 13C-UBT. Because the biopsies from 11 subjects did not have sufficient tissue for the clarithromycin resistance test to be performed, of the 205 initially eligible patients, only 194 subjects were included in the PP primary efficacy analysis (fig. 3). No differences in sex, age, weight, height, or endoscopic diagnosis were found among the 205 eligible participants (Table 1). Non-steroidal anti-inflammatory drug (NSAID) intake and family history of gastric cancer showed no significant difference. The main comorbidity was cardiovascular disease (3.9%), followed by degenerative osteoarthropathy (2%). One subject had type 2 diabetes mellitus, one subject was positive for human immunodeficiency virus, and one subject had compensated cirrhosis (0.5% each). For the purposes of the PP analysis (primary noninferiority efficacy), only those patients that underwent all of the procedures included in the study protocol (per-protocol population, 194 subjects with 100% adherence) were considered.
Demographic characteristics of the subjects by treatment group.
| Baseline variable | Group 2 (n=105) | Group 1 (n=100) | p value |
|---|---|---|---|
| Age (years) | 42.64±11.33 | 42.47±11.9 | 0.91 |
| Women | 57.1% (60) | 68% (68) | 0.10 |
| Weight (kg) | 69.7±15 | 70.10±13 | 0.87 |
| Height (cm) | 163±10 | 161±18 | 0.41 |
| History of NSAID use | 18.1% (19) | 12% (12) | 0.22 |
| Gastric cancer family history | 7.6% (8) | 8% (8) | 0.85 |
| Diagnosis Peptic ulcer | 9.5% (10) | 7% (7) | 0.51 |
| Dyspepsia | 86.7% (91) | 79% (79) | 0.14 |
| Hiatal hernia | 25.7% (27) | 39% (39) | 0.10 |
| Esophagitis | 23.8% (25) | 32% (32) | 0.59 |
| Duodenitis | 10.5% (11) | 14% (14) | 0.59 |
NSAID: nonsteroidal anti-inflammatory drug
The eradication rate was 59% (55/94) in group 1 and 63% (63/100) in group 2. In the PP population (n=194), the upper limit of the 95% CI for the difference in the eradication rates between the group 1 and group 2 therapies was lower than the pre-established noninferiority margin of 0.12 (95% CI: -0.18043, 0.11608; p <0.0001). Thus, the success rate of H. pylori eradication with both treatments resulted statistically comparable, supporting the noninferiority of the PLA group in relation to standard therapy. Both the primary analysis carried out in the PP population and the secondary analysis undertaken in the modified intention-to-treat population supported the same conclusion of noninferiority between the treatments tested (Table 2).
Two-sample test for equality of proportions with continuity correctiona
| X2 | df | p value | Ha | 95%CI | Sample estimates | ||
|---|---|---|---|---|---|---|---|
| 0.1804259; | Prop1 | Prop 2 | |||||
| PPb | 0.0956 | 1 | 0.7572 | 2-sided | 0.1160781 | 0.5978261 | 0.6300000 |
| mITTc | 0.0974 | 1 | 0.6852 | 0.1813621; | 0.5600000 | 0.6000000 | |
| 0.1170143 | |||||||
CI: confidence interval; df: degrees of freedom; mITT: modified intention-to-treat; PP: per-protocol.
Clarithromycin resistance was found in 28.9% (56/194) of the study PP population subjects (Table 3), randomly distributed between both groups and at all clinical sites. A sub-analysis by site (Table 3) showed that H. pylori resistance was higher in Toluca than in Mexico City: 50% (19/37) vs. 23.5% (37/157), respectively, (p=0.002 in the global comparison). Those results suggest that there may be regional differences in the pattern of resistance within the country.
Clarithromycin resistance diagnosed by FISH (PP population=194).
| Site | Patients recruited | FISH (+) | % CLAR | p value |
|---|---|---|---|---|
| Toluca (1) | 37 | 19 | 50% | 0.002a |
| Mexico City (3) | 157 | 37 | 23.5% | |
| TOTAL | 194 | 56 | 28.9% |
Toluca: 1 site; Mexico City: 3 sites.
CLAR: Clarithromycin resistance; FISH: Fluorescence in situ hybridization
Table 4 shows the results of bivariate and multivariate logistic regression analyses to identify independent influencing factors for a successful H. pylori eradication rate, with persistence of H. pylori as the dependent variable. When comparing subjects with or without persistent H. pylori, the bivariate analysis showed that the odds of having a successful H. pylori eradication rate were influenced by the variables of sex, dyspepsia, and absence of clarithromycin resistance. However, in the multivariate analysis, only the absence of clarithromycin resistance was significantly associated with a successful treatment outcome.
Odds ratios estimated by logistic regression analysis in the per-protocol population (=194). Comparison of subjects with or without persistent H. pylori.
| Variable | Bivariate analysis (95% CI) | Multivariate analysis (95% CI)a | ||||
|---|---|---|---|---|---|---|
| Odds ratio 95% CI p | Odds ratio 95% CI p | |||||
| Treatment (group 2) | 0.944 | 0.530 - 1.683 | 0.96 | |||
| FISH resistance negative | 0.280 | 0.143 - 0.550 | 0.000 | 0.256 | 0.123- 0.532 | <0.000 |
| Male sex | 5.78 | 1.35 - 24.72 | 0.013 | |||
| Dyspepsia | 2.64 | 1.20- 5.84 | 0.026 | |||
Finally, positive 13C-UBT results in patients with negative FISH resistance were obtained in 17.2% of the subjects, regardless of the treatment they had received, which might suggest resistance to amoxicillin or levofloxacin.
SafetyThe 205 subjects randomized to treatment were included in the safety analysis (Table 5). AEs were reported by 86% of the subjects in group 1 and 65.4% of those in group 2 (p=0.001). The most frequent AEs were dysgeusia (33.3%), loose stools (24.3%), nausea (22.1%), headache (14.7%), epigastric pain (14.2%), abdominal discomfort (13.7%), somnolence (6.9%), dizziness (5.9%), arthralgia (3.9%), and insomnia (3.9%). Dysgeusia was reported by 59% of the subjects in group 1 and by only 8.7% in group 2 (p <0.000). There was no difference between treatment groups regarding the frequency of any of the other AEs. There were no differences in the biochemical parameters evaluated before and after each treatment, nor were there differences between the treatments assigned (p=0.89).
Adverse events by treatment.
| Adverse Event | Group 2 (n=105) | Group 1 (n=100) | TOTAL (n=205) | p value |
|---|---|---|---|---|
| Any adverse event | 65.4 (68) | 86 (86) | 75.5 (154) | 0.001 |
| Dysgeusia | 8.7 (9) | 59 (59) | 33.3 (68) | 0.000 |
| Nausea | 21.2 (22) | 23 (23) | 22.1 (45) | 0.75 |
| Vomiting | 2.5 (3) | 0 (0) | 1.5 (3) | 0.08 |
| Epigastric pain | 11.5 (12) | 17 (17) | 14.2 (29) | 0.26 |
| Abdominal pain | 14.4 (15) | 13 (13) | 13.7 (28) | 0.76 |
| Constipation | 1.9 (2) | 5 (5) | 3.4 (7) | 0.22 |
| Rectal bleeding | 1 (1) | 1 (1) | 1 (2) | 0.97 |
| Headache | 10.6 (11) | 19 (19) | 14.7 (30) | 0.08 |
| Fatigue | 3.8 (4) | 2 (2) | 2.9 (6) | 0.43 |
| Dizziness | 6.7 (7) | 5 (5) | 5.9 (12) | 0.59 |
| Paresthesia | 1 (1) | 0 (0) | 1 (1) | 0.32 |
| Dry mouth | 1 (1) | 4 (4) | 2.5 (5) | 0.16 |
| Somnolence | 4.8 (5) | 9 (9) | 6.9 (14) | 0.23 |
| Upper respiratory infection | 1 (1) | 2 (2) | 1.5 (3) | 0.53 |
| Anxiety | 1.9 (2) | 1 (1) | 1.5 (3) | 0.58 |
| Insomnia | 5.8 (6) | 2 (2) | 3.9 (8) | 0.16 |
| Tendinitis | 1 (1) | 0 (0) | 0.5 (1) | 0.32 |
| Arthralgia | 3.8 (4) | 5 (5) | 4.4 (9) | 0.68 |
| Photosensitivity | 1 (1) | 0 (0) | 0.5 (1) | 0.32 |
| Pruritus | 0 (0) | 1 (1) | 0.5 (1) | 0.30 |
| Other | 1.9 (2) | 2 (2) | 2 (4) | 0.40 |
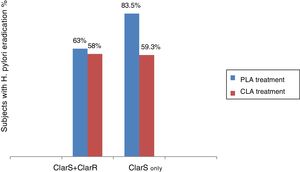
Because H. pylori eradication rates have declined worldwide in the last decade, the issue of empirical therapy effectiveness has been challenged and antibiotic resistance, mainly related to abuse and self-prescription, has become a research priority.18,19 In the present clinical trial, the test treatment (group 2) was noninferior to the gold standard (group 1 therapy), but when the resistance-guided treatment was implemented, it became clear that the combination given to group 2 was superior to the triple treatment (group 1 combination) in clarithromycin-sensitive infection (fig. 4).
Duration of treatment has been proven to be an important issue: meta-analyses have reported that compared with 7-day treatment, a 10-day course improves the eradication rate by 4%. Hence, the 10-day treatment allows adequate exposure time for effective treatment.3 In a recent meta-analysis, first-line levofloxacin therapies were compared with standard triple treatment. No difference was found when comparing levofloxacin+amoxicillin+a PPI versus clarithromycin+amoxicillin+a PPI. However, the levofloxacin+clarithromycin combination resulted in improved eradication.20
In relation to azithromycin, its use in 10-day therapeutic schemes has not been shown to increase the risks associated with this macrolide, particularly cardiovascular ones. The observational studies that evaluated the increased risk of cardiovascular events with azithromycin use linked the increase to serum concentrations obtained after oral administration in multiple daily doses. That risk decreased dramatically when the plasma concentrations fell after the end of treatment, regardless of the concentration of the macrolide in tissue.21
The proposed scheme involved a single dose per day of azithromycin, sufficient to achieve persistently high gastric tissue concentrations. In addition, the maximum serum level of azithromycin after an oral dose of 500mg is 5 times lower than that obtained after an equivalent dose of clarithromycin, which is more highly associated with the risk of severe ventricular arrhythmias. Therefore, it is not likely that azithromycin increased those risks in the proposed 10-day scheme.
Our results confirm that clarithromycin resistance in Mexico is high (28.9% overall). That percentage is surprisingly higher than the figures reported in other case series, in which the frequency of resistance to clarithromycin ranges from 4 to 25%, according to geographic area and to the laboratory method for evaluating antibiotic resistance. The differential frequency found between the 2 cities assessed is also surprising: Toluca revealed almost twice the frequency observed in Mexico City. Although less than one third of the total population of the study was recruited in Toluca, the difference is nevertheless striking. While the indiscriminate use of antibiotics is a common practice in Mexico, favoring the occurrence of random mutations in H. pylori strains and consequently creating resistance to clarithromycin, the regional differences in the resistance patterns could be due to other factors. Therefore, new clinical and epidemiologic studies are necessary to clarify that supposition.
In addition, our findings suggest that the 23S ribosomal ribonucleic acid (rRNA) point mutation is shared by clarithromycin and azithromycin and could perhaps be considered a macrolide resistance marker. Since said resistance marker is possibly common in the phenotype of both macrolides, a cross-resistance effect cannot be ruled out as underpinning the significant differential effect of the levofloxacin-based treatment, when considering the clarithromycin-sensitive subpopulation only or the entire population. This is not a minor occurrence: it would determine the switch in the efficacy of a potentially superior treatment for H. pylori eradication to a noninferior or clinically equivalent one. In that way, the factor of antibiotic resistance to macrolides (direct resistance to clarithromycin and cross-resistance to azithromycin) could explain why the overall eradication rates of H. pylori obtained in the 2 treatments compared herein were not higher than 65%, but that when the subpopulations were analyzed separately, based on sensitivity to clarithromycin (ClarS), the eradication percentage of H. pylori with the PLA treatment was significantly increased (p=0.013) (fig. 4).
Molecular biology methods such as FISH and polymerase chain reaction (PCR) techniques are now widely available for determining H. pylori antibiotic susceptibility. FISH has been shown to be a rapid, accurate, and cost-effective method for H. pylori detection and for determining macrolide resistance in cultured H. pylori colonies, as well as in histopathologic samples.22 Presumably, the resistance rate of H. pylori to levofloxacin is lower than that to macrolides. In addition, the correlation between genotype resistance (measured by the detection of mutations through molecular biology methods, such as FISH) and phenotypic resistance (measured through minimum inhibitory concentrations of antibiotics and clinical outcomes) is high for both antibiotics. Studies show that such agreement between genotypic resistance and clinical outcomes is even better after clarithromycin-based eradication therapy. Thus, the detection of resistance mutations to this antibiotic seems to be an appropriate method to predict potential therapeutic failures.23 However, no testing was done in the present study to support that statement.
In our trial, most AEs were treatment-related (85.1%), but they were mild and self-limited and were not a reason for treatment interruption. Rates were significantly higher in the clarithromycin arm (86%), than in the levofloxacin arm (65.4%), which coincides with specific literature comparing standard triple therapy with levofloxacin-based therapy.20
13C-UBT has been considered the gold standard for verifying treatment eradication results.24 In the present study, 90.33% of the subjects completed such a test. The patients that did not return to the laboratory for testing (9.67%, 22 subjects) represent the same failure percentage described in the literature in relation to the effectiveness of monitoring methods.25,26 Studies in Mexico show that atrophy is seen in 22% of gastric biopsies,27 which could help explain the high percentage of subjects in our study (17.2%) that had negative FISH resistance and positive 13C-UBTs.
One of the drawbacks of the present study was the testing of only one antibiotic resistance, but the results were clearly dependent on clarithromycin resistance. Future research with results of other eradication treatments should include all antibiotic resistance rates to improve the epidemiologic profile. Finally, caution should be exercised regarding the generalization of the antibiotic resistance results of our study, as they should be appreciated as a local variation in the H. pylori eradication challenge.
In conclusion, the azithromycin-levofloxacin combination was noninferior to the triple treatment and was associated with fewer AEs than therapies containing clarithromycin. Levofloxacin may be a suitable and safe antibiotic in H. pylori eradication schemes. However, its use as a first-line or second-line treatment remains to be established, depending on the local pattern of antibiotic resistance. Our findings coincide with those from many other countries in supporting the fact that triple therapy including clarithromycin does not reach the expected eradication rate, and other alternatives must be sought. Knowledge about resistance patterns obtained from local and regional antimicrobial surveillance programs and/or local clinical experience through molecular biology techniques, such as FISH, is an important aid to deciding on the treatment that will have the highest possible predicted success rate. If no available regimen can achieve ≥ 90% eradication, then clinicians should use the most effective regimens locally available.
AuthorshipStudy concept and design: LLG, FC; data collection: LLG, LBQ, BCR, MDS; data analysis and interpretation LLG, LBQ, FC; statistical analysis: LLG, LBQ, FC; first draft of the manuscript: LLG, LBQ, BCR; critical review of the manuscript: LLG, LBQ, SGH, MDS, FC; fundraiser: FC; administrative, technical, and material support and study supervision: FC.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial disclosureThe present study was funded by Asofarma de México, SA de CV.
Conflict of interestThe present study was funded by Asofarma de México S.A. de C. V.
During the study development, Dr. Laura L. de Guevara, Dr. Leticia Bornstein-Quevedo, Dr. Saraí González-Huezo, Dr. Mauricio Di Silvio, and Dr. Beatriz Castañeda received grants from Asofarma de México S.A. de C.V.
Dr. Fernando G. Costa is the Corporate Medical Advisor of Clinical Studies for Asofarma S.A.I. y C. (Argentina).
1 Infinite Clinical Research (ICR) was the Mexican CRO hired to run this study.
2 Ethics Committee of the Centro de Investigación Médico Biológica y Terapia Avanzada SC (CImByTA), Guadalajara, Jalisco (MX). Date of approval: 22 June 2012 (Reference: protocol ASO HP 01, version 2.0)
Please cite this article as: Ladrón-de-Guevara L, Bornstein-Quevedo L, González-Huezo S, Castañeda-Romero B, Costa FG, di Silvio-López M. Erradicación de Helicobacter pylori en México con un esquema basado en levofloxacina versus la triple terapia estándar: resultados de un estudio clínico de fase iiib, abierto, aleatorizado, de no inferioridad. Revista de Gastroenterología de México. 2019;84:274–283.