The novel SARS-CoV-2 coronavirus is responsible for the infectious disease caused by coronavirus 19 (COVID-19). The current pandemic is growing worldwide and could affect 50-60% of the world population in the months to come. The most severe disease manifestations are atypical pneumonia and sepsis, but the gastrointestinal tract, particularly the liver, has recently been reported to be affected by SARS-CoV-2. Therefore, the aim of the present work was to review the literature available on the topic and provide information about COVID-19, in both healthy and diseased livers, and issue recommendations. The incidence of liver injury specifically associated with COVID-19 varies from 14.8-53%. The majority of case series have reported altered ALT and AST, elevated total bilirubin, and low serum albumin and liver compromise has been associated with the most severe cases of COVID-19. Cirrhosis of the liver has a recognized immune dysfunction status that includes immunodeficiency and systemic inflammation, making it reasonable for those patients to be more susceptible to SARS-CoV-2 infection. The recommendations for those patients, in addition to the general measures of physical distancing and handwashing for all persons, include social, medical, and psychologic support during the period of home quarantine to prevent lapses in treatment. Patients should be made aware that they need to keep abreast of changes in recommendations and social policies.
El coronavirus SARS-CoV-2, un nuevo coronavirus, es responsable de la enfermedad infecciosa por coronavirus 19 (COVID-19). A nivel global, esta pandemia va en crecimiento y pudiera afectar al 50-60% de la población mundial en los siguientes meses. Si bien es una enfermedad cuya manifestación más severa es la neumonía atípica y sepsis, recientemente se ha descrito que el tracto digestivo y en particular el hígado, pueden verse afectados por el SARS-CoV-2. Así pues, el objetivo del presente trabajo es revisar la literatura disponible al respecto y emitir algunas recomendaciones del papel que el COVID-19 ejerce sobre el hígado en la salud y la enfermedad. La incidencia de lesión hepática asociada específicamente a COVID-19 varía de 14.8-53%. La mayoría de las series de casos han reportado alteración en ALT y AST, elevación de bilirrubinas totales y albúmina sérica baja. La afectación hepática se ha asociado a casos más graves de COVID-19. Por otra parte, se reconoce que la cirrosis hepática es un estado de disfunción inmune que comprende inmunodeficiencia e inflamación sistémica, lo cual hace razonable que estos pacientes sean más susceptibles a la infección por SARS-CoV-2. Las recomendaciones para estos pacientes, además de las medidas generales para la población (aislamiento social, lavado de manos) incluyen el apoyo social, médico y psicológico durante el período de estancia en el domicilio para evitar transgresiones a la terapia. Es recomendable orientar a los pacientes a mantenerse informados de los cambios en recomendaciones y políticas sociales.
Coronaviruses are enveloped, positive-sense RNA viruses that vary from 60nm to 140nm in diameter, with projections in the form of spikes that give the appearance of a crown, hence the name. Up to November 2019 there were four known coronaviruses that presented in humans, causing mild respiratory infections: HKU1, NL63, 229E, and OC43.1,2 The appearance of a new human coronavirus has recently been described, first reported in the city of Wuhan, China. The novel coronavirus, SARS-CoV-2, is a beta coronavirus, of the subgenus Sarbecovirus and the subfamily Orthocoronavirinae. The organization of the viral genome of the Wuhan Human-1 coronavirus was determined through the sequencing alignment of two beta coronaviruses: one associated with humans (SARS-CoV Tor2) and one associated with bats (bat SL-CoVZC45).3,4
The infectious disease due to coronavirus 19 (COVID-19), so-named by the World Health Organization (WHO) on February 11, 2020, is caused by the SARS-CoV-2 virus and is the causal agent of a potentially fatal disease that has become a huge public health problem across the globe.5 By February 24, 2020, more than 80,000 confirmed cases were registered worldwide, in at least 37 countries, and more than 2,700 deaths, resulting in a worldwide health emergency declared by the WHO.6
In Mexico, the first case was reported on February 27 and two days later four cases were confirmed by the Mexican Health Department. All cases were traced back to an individual from Bergamo, Italy.7 The progression of this pandemic is rising worldwide, and 3,525,116 confirmed cases were reported by May 5,8 with a mortality rate of 3.4%. In Mexico, by that same date, there were 24,905 confirmed cases and 2,271 deaths due to COVID-19.9 Even though it is a disease whose most severe manifestations are atypical pneumonia and sepsis, the digestive tract, particularly the liver, has recently been reported to be affected by SARS-CoV-2.10,11
Therefore, the aim of the present work was to review the available literature on the topic and provide recommendations related to the role of COVID-19 in both healthy and diseased livers.
Materials and methodsA narrative review of the literature was conducted, based on a thorough search of the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search was carried out within the time frame of January 1, 2020 to April 10, 2020, utilizing the search term “coronavirus” combined with the following terms: “SARS-CoV-2”, “liver”, “hepatic”, “enzymes”, “acute” “chronic”, “cirrhosis”, “liver failure”, “hepatitis”, “COVID-19” and their Spanish equivalents. We found 98 works and included 47 in the present review. The excluded articles were case reports, duplicate publications, or those in a language other than Spanish or English.
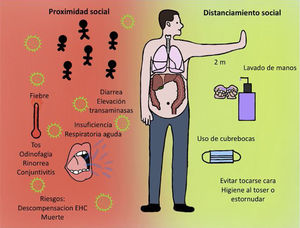
COVID-19 and hepatic manifestationsThe clinical manifestations of COVID-19 are varied and range from an asymptomatic state to the more severe acute respiratory distress syndrome (ARDS) and multiorgan dysfunction. The common signs and symptoms are fever, cough, sore throat, headache, fatigue, myalgia, dyspnea, and conjunctivitis, but can progress to pneumonia, respiratory failure, and death.1 In addition, patients with COVID-19 infection have also presented with gastrointestinal symptoms, such as diarrhea, and a low percentage of patients with MERS-CoV or SARS-CoV present with similar gastrointestinal complaints. Symptoms appear after an incubation period of approximately 5.2 days.5
A metalloproteinase called angiotensin-converting enzyme 2 (ACE2) has been identified as the functional receptor for coronavirus cell entry. ACE2 microRNA expression has been found in multiple organs and human tissue, including the oral and nasal mucosa, nasopharynx, lung, stomach, small bowel, colon, skin, lymph nodes, thymus, bone marrow, spleen, kidney, brain, biliary epithelium, and liver, making its expression a potential target for infection.1,12–15
Thus, the liver is an organ that is affected by COVID-19. There is information that the liver, either healthy or presenting with a pre-existing hepatic disease, is a pathophysiologic target for that family of coronaviruses. Liver injury (hepatitis) in patients with SARS is manifested through mild-to-moderate elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) during the early disease stage, as well as a decrease in serum albumin and an increase in serum bilirubin levels. Autopsy findings in patients with SARS show a large number of viral particles, not only in the lungs, but also in parenchymal cells and the vascular endothelium of other organs, including the liver.12
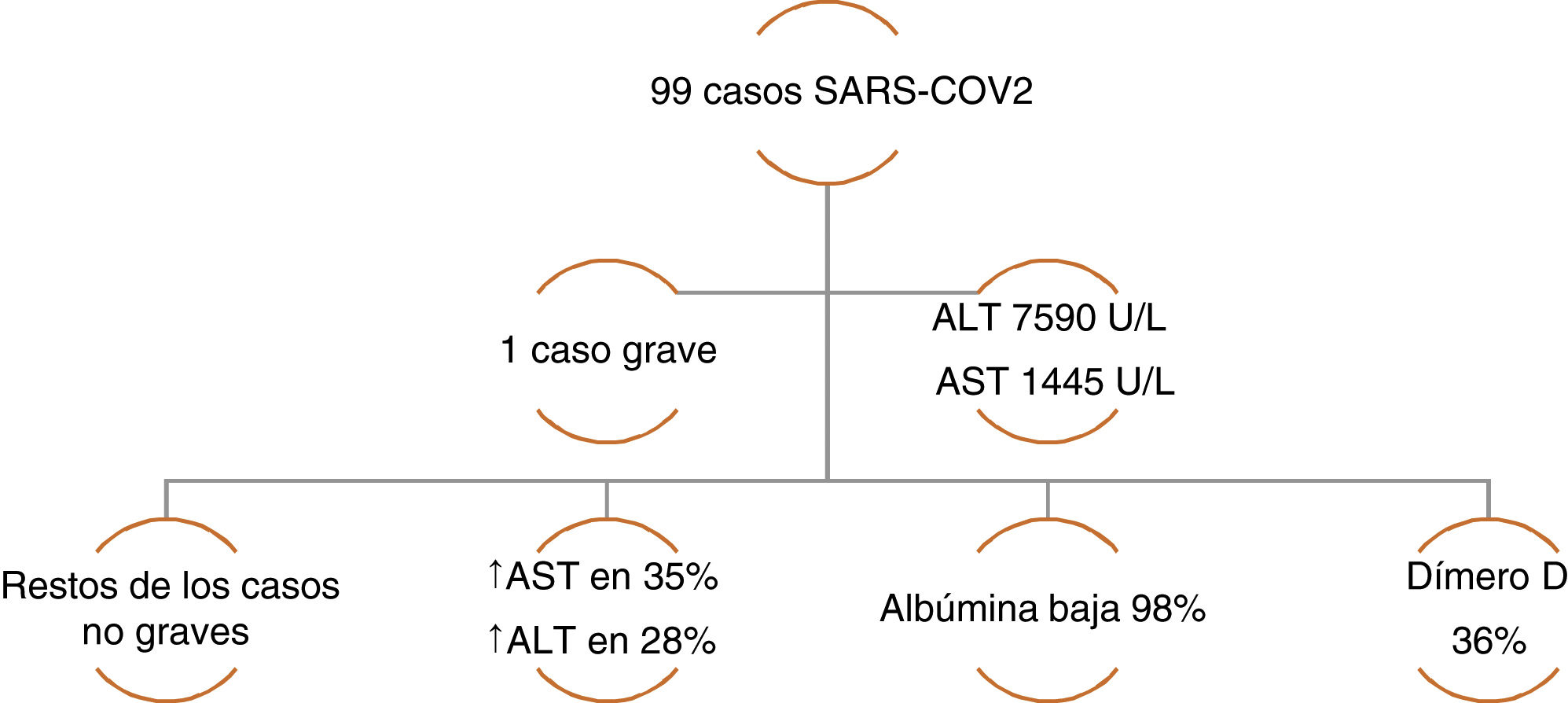
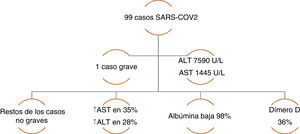
The incidence of liver injury specifically associated with COVID-19 varies from 14.8-53%. The majority of case series have reported alterations in ALT and AST, elevated total bilirubin, and low serum albumin.12 Chen et al. reported a case series of 99 cases of pneumonia due to COVID-19, in which only one patient had a lesion with a severe hepatocellular pattern and elevated ALT and AST, whereas the rest of the patients had moderately elevated aminotransferase levels. A decrease in albumin was present in the majority of the cases. With respect to the coagulation profile, the prothrombin time increased in only 5% of the cases, highlighting an increase in dimer levels in one-third of the patients (Figure 1).16 Likewise, Huang et al. found similar liver biochemistry alterations, describing elevated AST levels in 37% of the patients, with a higher number of those cases in the patients that were admitted to the intensive care unit (ICU) (62% vs. 25%).17 In a case series of 138 COVID-19 cases, Wang et al. also found statistically higher AST levels in patients that required ICU admission (p<0.001).18
Case series of COVID-19 patients with altered liver chemistry. Data taken from: Chen, et al.16
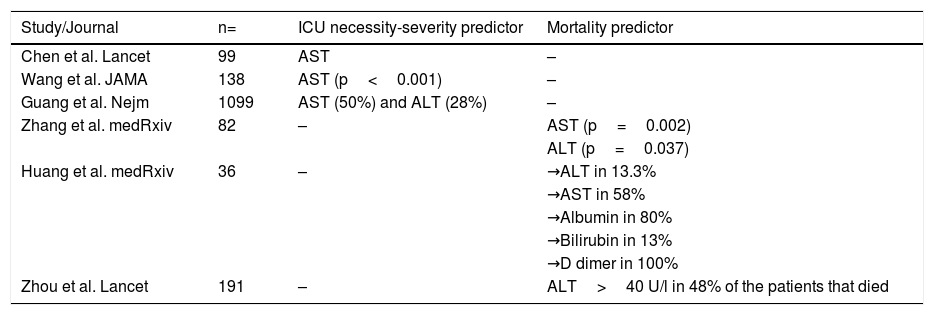
In addition, there are studies in which the presence of liver alterations has been related to death. In their case series on mortality from SARS-CoV-2 in 82 cases, Zhang et al. reported that the median of time from symptom onset until death was 15 days. With respect to transaminase elevation, there was a significant association between AST (p=0.002), ALT (p=0.037), and the time from symptom onset until death.19 In another report, Huang et al. described the clinical characteristics of 36 patients that did not survive COVID-19. The liver chemistry alterations behaved similarly to those in previous reports, with elevated ALT in 13.3%, elevated AST in 58%, a decrease in albumin in 80%, an increase in total bilirubin in 13%, and an increase in D dimer in 100% of the cases.20 Finally, Zhou et al. reported that in 191 patients with COVID-19, 31% presented with an elevation of ALT>40 U/L. In the subgroup of patients that died, ALT was elevated in 48% of the cases, vs. 24% of the patients in the group that survived (p=0.0050).21 In that context, the report on 1,099 patients with COVID-19, conducted by Guang et al., stated that AST elevation was > 40 U/L in 39% of the patients and that ALT elevation was > 40 U/L in 28% of the patients with severe disease. Of the patients that required ICU admission, mechanical ventilation, or that died, 50% had elevated AST and 28% had elevated ALT.22Table 1 summarizes the characteristics of liver chemistry alterations and their outcomes.
Studies describing the main characteristics of liver chemistry alterations and associated clinical outcomes.
| Study/Journal | n= | ICU necessity-severity predictor | Mortality predictor |
|---|---|---|---|
| Chen et al. Lancet | 99 | AST | – |
| Wang et al. JAMA | 138 | AST (p<0.001) | – |
| Guang et al. Nejm | 1099 | AST (50%) and ALT (28%) | – |
| Zhang et al. medRxiv | 82 | – | AST (p=0.002) |
| ALT (p=0.037) | |||
| Huang et al. medRxiv | 36 | – | →ALT in 13.3% |
| →AST in 58% | |||
| →Albumin in 80% | |||
| →Bilirubin in 13% | |||
| →D dimer in 100% | |||
| Zhou et al. Lancet | 191 | – | ALT>40 U/l in 48% of the patients that died |
Due to the problem of access to biopsy or autopsy reports, the histopathologic findings of this new disease are not yet completely defined. A case report by Xu et al.23 presented the post-mortem biopsy results of a 50-year-old man who died from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). With respect to the liver biopsies, there was microvesicular steatosis and mild lobular and portal inflammatory activity. Nevertheless, it was not completely certain if the liver injury was due to SARS-CoV-2 or to drug-induced liver injury (DILI). The latter diagnostic probability is sustained by the fact that numerous drugs with great hepatotoxic potential, such as remdesivir, tocilizumab, chloroquine, and statins, are empirically used in some cases to treat severe SARS-CoV-2, all of which can make the coronavirus patient prone to developing DILI.12 There was also no information in the case report about any comorbidities, to know if the patient could have been at risk due to a pre-existing liver disease. All of this leads to the hypothesis that elevated aminotransferases may be the result of a direct effect on the hepatocyte mediated by the virus, or of a liver injury mediated by immune and inflammatory activation associated with the infection.
Coagulation disorders have been reported as a pathophysiologic component of COVID-19. Thrombotic complications appear to emerge as an important problem in patients with COVID-19. Preliminary reports on the results of the present pandemic showed that infected patients commonly developed thrombocytopenia (36.2%) and D dimer elevation (46.4%) but those figures were higher in patients with severe disease (57.7% and 59.6%, respectively), suggesting that they are at risk for developing disseminated intravascular coagulation (DIC). The increase in D dimer levels and fibrin degradation products, together with prolonged prothrombin time, have been associated with poor outcome in patients affected by the new coronavirus.24 However, whether those hemostatic changes are a specific effect of the SARS-CoV-2 or a consequence of the cytokine storm that precipitates the appearance of the systemic inflammatory response syndrome (SIRS), as has been observed in other viral diseases, is not yet known. Another consideration still to be investigated is that the hemostatic changes seen in COVID-19 infection are related to liver dysfunction. A recent study described three cases of severe COVID-19 and cerebral infarction and one associated with bilateral limb ischemia, in the context of elevated antiphospholipid antibodies. Nutritional deficiencies and liver dysfunction can also interfere with the production of coagulation factors.25
COVID-19 in the context of the patient with chronic liver diseaseCirrhosis of the liver as an immunocompromised statePatients with cirrhosis of the liver (CL) are immunocompromised and more susceptible to developing spontaneous bacterial infections, some of which are due to uncommon pathogens, reflected in a higher mortality rate close to 30%.26 Patients with CL, especially in the decompensated stage, are susceptible to the development of those infections, whose prevalence can increase to 44%.27
Thus, CL is a state of immune dysfunction that encompasses immunodeficiency and systemic inflammation, exhibiting at least two known phenotypes of immune dysfunction in cirrhosis: the proinflammatory and the immunodeficient. They are characterized by the differences in cytokine activity, phagocyte activity, and HLA expression, among others, that present throughout the pathophysiologic stages from compensated stages to decompensated stages, and up to acute-on-chronic liver failure (ACLF).28 Said immunodeficiency also results in a reduced synthesis of pattern recognition receptors (PRRs).29 Different classes of germline-encoded PRRs recognize invasive pathogens and monitor the extracellular and intracellular compartments of host cells to detect signs of invasion by microorganisms.30 The liver is the primary source of PRRs (e.g., C-reactive protein, lipopolysaccharide [LPS]-binding protein, peptidoglycan-recognition protein, soluble CD14) that activate the complement system, induce opsonization, and regulate immune cell function. Hepatocytes synthetize and secrete the majority of those proteins, in response to different proinflammatory cytokines (TNF-α, IL-6) produced in the course of a systemic inflammatory response. Other PRRs are expressed to recognize different viral and bacterial molecules, including the endosomal toll-like receptors (TLRs). The TLR2, TLR4, TLR5, and TLR6 subtypes are expressed in all types of hepatic cells and participate in the uptake and elimination of endotoxins, as well as in the production of cytokines.28,31,32
Cirrhosis of the liver and respiratory infectionsThroughout history, there have been outbreaks and epidemics of viral diseases, especially respiratory diseases. The most recent is the COVID-19 pandemic. Patients with chronic liver disease (CLD) present with multifactorial immune dysfunction.33,34 Due to the fact that the infection site affects outcome in patients with CLD, acute respiratory diseases acquire great importance because they produce an almost 40-fold increase in the hospitalization rate, compared with the general population.34 They also generate a 2.95-fold increase in 30-day mortality in the community setting and up to an 11-fold increase in the hospital setting, the highest among isolated infectious complications.33 The prevalence of pneumonia and influenza reported in 2009 in the United States was 0.38% and 0.027%, respectively.34 As expected, patients with a higher grade of decompensation have a higher mortality rate in those types of events and associated factors are inadequate antibiotic use, bacteremia, leukocyte count, total bilirubin, more advanced age, and alcoholic disease etiology, as well as the development of organ failure and ACLF.33 Models developed during community outbreaks of influenza-like disease indicate an increase in morbidity and mortality in patients with CLD, mainly related to organ failure and complications associated with liver disease, such as gastrointestinal bleeding.35
Patients with CLD are prone to developing respiratory failure due to numerous causes. They can also present with disease modifiers that worsen its natural history, such as smoking, chronic obstructive pulmonary disease, malnutrition, muscle deconditioning and wasting, hypoalbuminemia, coagulopathy, multiple transfusions, etc. In addition, ascites directly affects pulmonary and respiratory mechanics by increasing the intra-abdominal pressure, and hepatic encephalopathy increases the risk for micro-aspirations and ineffective cough.36 Thus, up to 70% of patients with CLD can present with arterial hypoxemia. An assisted ventilation event in patients with cirrhosis, especially in the context of ARDS, has a mortality rate of 59% to 100% in nontransplant hospitals.36 The mortality rate in patients with CLD in ICUs is 46-90%. In the context of ARDS, that outcome appears to be associated more with the development of extrapulmonary organ failure than with the hypoxemia itself.37
Some of the factors that modify the outcome of hospitalized patients with acute respiratory diseases are those inherent to CLD, such as portal pressure alterations that lead to the portal hypertension-related complications of variceal gastrointestinal bleeding, ascites, and hepatic encephalopathy, etc. There is also an increased rate of viral liver disease superinfection or reactivation. The translocation of bacteria from the gut to the mesenteric and/or extraintestinal lymph nodes is the key factor in the development of spontaneous bacterial infections in cirrhosis of the liver, even in the absence of clinically evident bacterial infections. Those spontaneous infections present in up to 30% of patients with decompensated cirrhosis, worsening outcome and elevating the mortality rate.38 The accumulated mortality rate after any infection in patients with cirrhosis is 43.5%, whereas the mortality rate with no infection is only 13.6%.39 Furthermore, short-term and long-term outcomes that occur after an acute respiratory failure event must be added, especially if mechanical ventilation is required, and they are generally worse than outcomes in cirrhotic patients without those conditions.40
COVID-19 in patients with CLDThe first epidemiologic reports characterizing patients with COVID-19 came out of China. One of those reports provided data from 1,099 patients, 23 of whom had chronic hepatitis B virus (HBV) infection.22 The probability of having presented with HBV was greater in the patients with severe COVID-19 disease than in the non-severe cases.41 In another report from Wuhan, China, on a total of 138 patients, four of them presented with CLD but did not require ICU admission. The most current experience at the Renmin Hospital of the University of Wuhan in patients with decompensated liver cirrhosis provided data on 111 patients. Their mean age was 58.7 years, 82 were outpatients, and 29 were inpatients.42 In the most recent report from Lombardy, Italy, on 1,591 critically ill patients, 3% of them had CLD.43
Up to May 5, 2020, the SECURE-Cirrhosis Registry (an open platform with the voluntary reporting of cases from China, Japan, Korea, and the Americas) and the COVID-HEP Registry (which captures cases from the rest of the world), in combination, reported 426 cases of COVID-19 in CLD patients (64.2% males, mean age 59 years) from 25 countries. Of those patients, 185 had cirrhosis, with 24% requiring ICU admission. Fifteen percent needed mechanical ventilation and 37% (n=68) died. The causes of death were pulmonary problems in 82%, cardiogenic shock in 7%, and decompensated hepatopathy in 11%. From the same sources, 176 noncirrhotic patients with CLD plus COVID-19 infection and 58 transplanted patients with COVID-19 were reported, with mortality rates of 6% and 22%, respectively.44,45 In the Morbidity and Mortality Weekly Report (up to March 28, 2020) issued by the Centers for Disease Control and Prevention (CDC), out of 7,162 COVID-19 patients with risk an underlying health condition, 41 (0.6%) had CLD, 16 of whom were hospitalized, with 7 requiring admission to the ICU. It should be emphasized that those are preliminary results, given that the data of all infected persons in the United States is still pending.46 Likewise, the Intensive Care National Audit and Research Centre (ICNARC) report from the United Kingdom on March 27, 2020, stated that of the 775 patients in their database, three presented with severe liver comorbidity.47 At present, neither the epidemiology nor the experience with patients with CLD has been published in Mexico.
Care recommendations in patients with chronic liver diseasePatients with CLD should follow the general recommendations issued for the population at large. To protect themselves and others, the recommendation is to stay home, with the exception of the following scenarios:
- 1
The purchase of basic essential products (food and medications), spacing the intervals between outings for the longest period of time possible. If there is another person in the home that can carry out said task, it should be done by the individual that is least susceptible to infection.
- 2
Outdoor physical activity, once a day, preferably alone.
- 3
The need to receive or give care. When possible, medical care should be received or given remotely (by telephone, video call, etc.).
- 4
Going from home to the workplace, if it is not possible to work from home.
In addition, the recommendations of standard hygiene should be followed, such as:
- 1
Frequent handwashing with soap and water for at least 40 seconds. Utilizing hand sanitizers with alcohol for 20 seconds is an option, if soap and water are not available.
- 2
Avoid contact with persons outside the immediate family, especially if they present with respiratory symptoms or have been in contact with someone with those symptoms.
- 3
Utilize hygienic measures when coughing or sneezing, by doing so into the bend of your arm or into a disposable tissue.
- 4
Avoid contact with unsanitized surfaces. Clean work surfaces and objects frequently, as well as those in the home.
- 5
Wear a simple disposable or homemade facemask (Figure 2).
Recommendations as to disease care are maintained, emphasizing not hesitating to seek attention at a non-respiratory emergency medical center if necessary. Due to the impact physical distancing and home quarantining can have, social, medical, and psychologic support are of vast importance to prevent both treatment interruption and the relapse into alcohol consumption by patients with alcohol-related CLD. Patients must be made aware that they have to keep abreast of changes in the recommendations and social policies and be instructed not to take substances or medications that have not been prescribed by their physician, including supplements or alternative products, as measures of “prevention” or to “strengthen the immune system” etc. How long the pandemic will last is not known, therefore an essential recommendation is that the influenza vaccination regimen in patients with CLD be up to date.
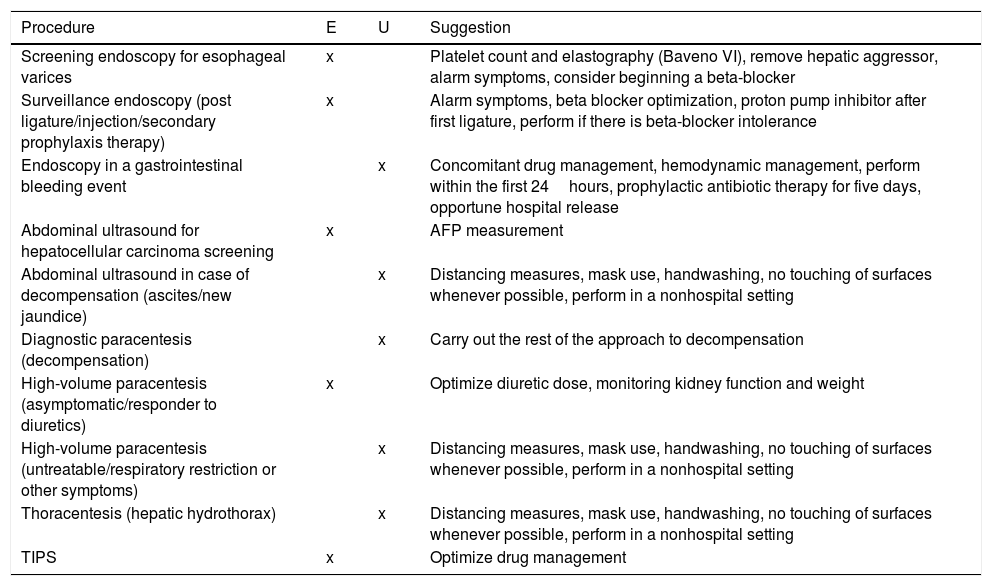
The standard of medical care for patients that are hospitalized due to causes other than COVID-19 remains the same, with certain recommendations as to the selection of emergency or elective procedures, outlined in Table 2. Likewise, despite the fact that there is no standardized treatment for COVID-19 management in patients with severe disease, the indications for liver health summarized in Table 3 should be taken into account.
COVID-19 and CLD: urgent (U) or elective (E) procedures. AFP: alpha fetoprotein; TIPS: transjugular intrahepatic portosystemic stent.
| Procedure | E | U | Suggestion |
|---|---|---|---|
| Screening endoscopy for esophageal varices | x | Platelet count and elastography (Baveno VI), remove hepatic aggressor, alarm symptoms, consider beginning a beta-blocker | |
| Surveillance endoscopy (post ligature/injection/secondary prophylaxis therapy) | x | Alarm symptoms, beta blocker optimization, proton pump inhibitor after first ligature, perform if there is beta-blocker intolerance | |
| Endoscopy in a gastrointestinal bleeding event | x | Concomitant drug management, hemodynamic management, perform within the first 24hours, prophylactic antibiotic therapy for five days, opportune hospital release | |
| Abdominal ultrasound for hepatocellular carcinoma screening | x | AFP measurement | |
| Abdominal ultrasound in case of decompensation (ascites/new jaundice) | x | Distancing measures, mask use, handwashing, no touching of surfaces whenever possible, perform in a nonhospital setting | |
| Diagnostic paracentesis (decompensation) | x | Carry out the rest of the approach to decompensation | |
| High-volume paracentesis (asymptomatic/responder to diuretics) | x | Optimize diuretic dose, monitoring kidney function and weight | |
| High-volume paracentesis (untreatable/respiratory restriction or other symptoms) | x | Distancing measures, mask use, handwashing, no touching of surfaces whenever possible, perform in a nonhospital setting | |
| Thoracentesis (hepatic hydrothorax) | x | Distancing measures, mask use, handwashing, no touching of surfaces whenever possible, perform in a nonhospital setting | |
| TIPS | x | Optimize drug management |
Medications in COVID-19 and liver disease.
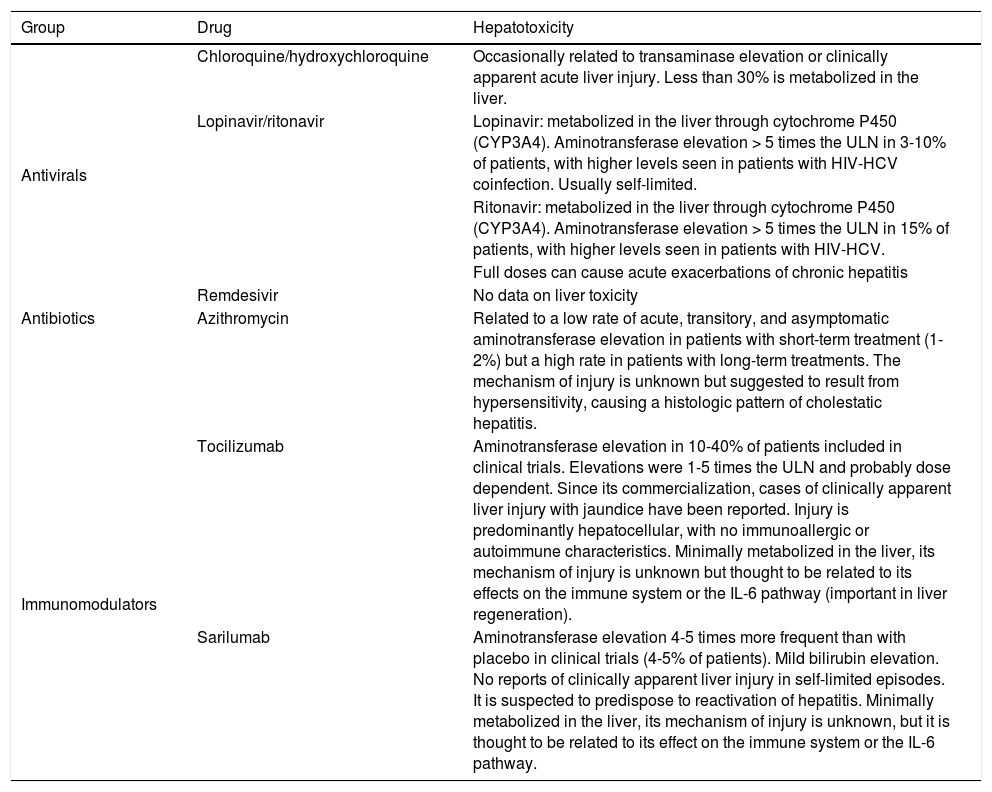
| Group | Drug | Hepatotoxicity |
|---|---|---|
| Antivirals | Chloroquine/hydroxychloroquine | Occasionally related to transaminase elevation or clinically apparent acute liver injury. Less than 30% is metabolized in the liver. |
| Lopinavir/ritonavir | Lopinavir: metabolized in the liver through cytochrome P450 (CYP3A4). Aminotransferase elevation > 5 times the ULN in 3-10% of patients, with higher levels seen in patients with HIV-HCV coinfection. Usually self-limited. | |
| Ritonavir: metabolized in the liver through cytochrome P450 (CYP3A4). Aminotransferase elevation > 5 times the ULN in 15% of patients, with higher levels seen in patients with HIV-HCV. | ||
| Full doses can cause acute exacerbations of chronic hepatitis | ||
| Remdesivir | No data on liver toxicity | |
| Antibiotics | Azithromycin | Related to a low rate of acute, transitory, and asymptomatic aminotransferase elevation in patients with short-term treatment (1-2%) but a high rate in patients with long-term treatments. The mechanism of injury is unknown but suggested to result from hypersensitivity, causing a histologic pattern of cholestatic hepatitis. |
| Immunomodulators | Tocilizumab | Aminotransferase elevation in 10-40% of patients included in clinical trials. Elevations were 1-5 times the ULN and probably dose dependent. Since its commercialization, cases of clinically apparent liver injury with jaundice have been reported. Injury is predominantly hepatocellular, with no immunoallergic or autoimmune characteristics. Minimally metabolized in the liver, its mechanism of injury is unknown but thought to be related to its effects on the immune system or the IL-6 pathway (important in liver regeneration). |
| Sarilumab | Aminotransferase elevation 4-5 times more frequent than with placebo in clinical trials (4-5% of patients). Mild bilirubin elevation. No reports of clinically apparent liver injury in self-limited episodes. It is suspected to predispose to reactivation of hepatitis. Minimally metabolized in the liver, its mechanism of injury is unknown, but it is thought to be related to its effect on the immune system or the IL-6 pathway. |
According to the information reported in the international medical literature, hepatic manifestations of SARS-CoV-2 are common and the more severe alterations are associated with poor outcome. Severe cases are beginning to present in Mexico, underlining the importance of being aware of hepatic manifestations in the general population, so that cases potentially requiring opportune hospitalization can be recognized. On the other hand, COVID-19 infection in patients with pre-existing CLD implies a greater risk and probability of liver dysfunction, and so healthcare professionals in charge of those patients are recommended to be alert regarding the development of potential complications. There may be questions as to the usefulness of the strategies proposed herein, but when dealing with an emerging pathology of the current magnitude it is impossible to provide recommendations that have been proven 100%. The disease, its behavior, and the data reported over the past few months have been dynamic, and thus should not be considered definitive or unalterable. For the benefit of our patients with cirrhosis of the liver, we must continuously be updating our information. It is also important to recognize that more studies on different populations are needed to have a complete understanding of the morbidity and mortality associated with SARS-CoV-2 in the cirrhotic patient.
Financial disclosureNo financial support was received in relation to this document.
Conflict of interestDr. José Antonio Velarde Ruiz Velasco has been a speaker for Takeda and Gilead.
Dr. José María Remes-Troche is a member of the advisory board of Takeda and Asofarma. He received research funding from Sanfer and Asofarma. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, and Astra-Zeneca.
The rest of the authors declare they have no conflict of interest related to the present document.
Dr. José María Remes-Troche is a member of the advisory board of Takeda and Asofarma. He received research funding from Sanfer and Asofarma. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, and Astra-Zeneca.
The rest of the authors declare they have no conflict of interest related to the present document.
Please cite this article as: Velarde-Ruiz Velasco JA, García-Jiménez ES, Remes-Troche JM. Manifestaciones hepáticas y repercusión en el paciente cirrótico de COVID-19. Revista de Gastroenterología de México. 2020;85:303–311.