The role of serum albumin level in patients with non-variceal upper gastrointestinal bleeding (NVUGB) has not been extensively studied. Our aim was to evaluate the role of serum albumin on admission in terms of in-hospital mortality in patients with NVUGB.
Materials and methodsPatients admitted with NVUGB during a 4-year period were prospectively included. Demographic, clinical, and laboratory data were collected. ROC curve analysis was used to determine the cutoff value for serum albumin on admission that made a distinction between deceased patients and survivors with respect to serum albumin on admission, as well as its overall performance compared with the Rockall score.
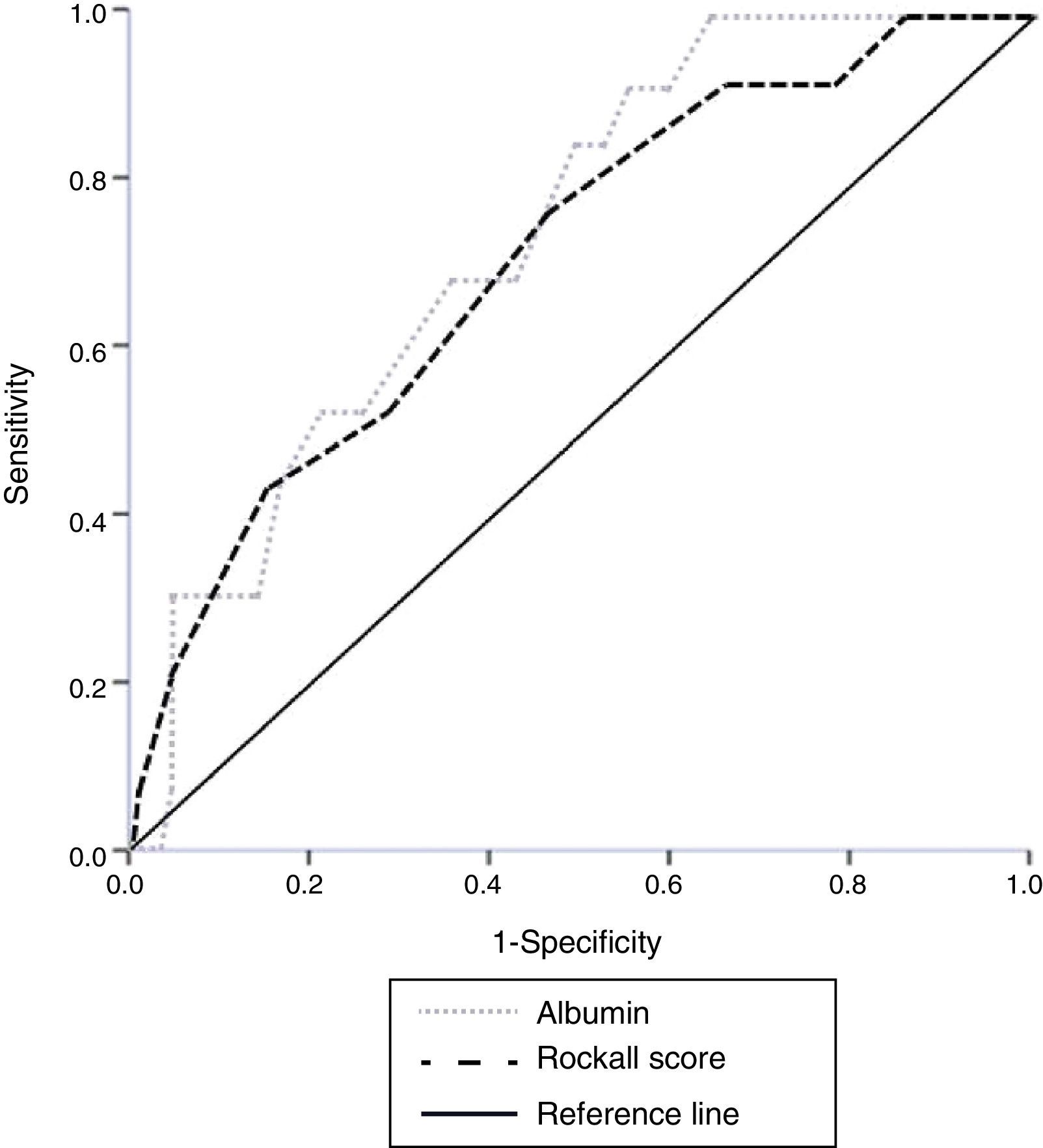
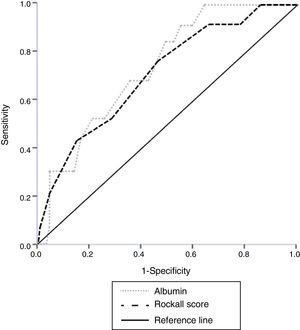
Results185 patients with NVUGB were evaluated. Men predominated (56.7%) and a mean age of 59.1±19.9 years was found. Mean serum albumin on admission was 2.9±0.9g/dl with hypoalbuminemia (< 3.5g/dl) detected on admission in 71.4% of cases. The ROC curve found that the best value for predicting hospital mortality was an albumin level of 3.1g/dl (AUROC 0.738). Mortality in patients with albumin ≥ 3.2g/dl was 1.2% compared with 11.2% in patients with albumin<3.2g/dl (P=.009; OR 9.7, 95%CI 1.2-76.5). There was no difference in overall performance between the albumin level (AUORC 0.738) and the Rockall score (AUROC 0.715) for identifying mortality.
ConclusionsPatients with hypoalbuminemia presenting with NVUGB have a greater in-hospital mortality rate. The serum albumin level and the Rockall score perform equally in regard to identifying the mortality rate.
El papel de los niveles séricos de albúmina en pacientes con sangrado de tubo digestivo alto no variceal (SDA-NV) no ha sido estudiado ampliamente. Nuestro objetivo fue evaluar el papel de los niveles de albúmina en la mortalidad de pacientes con SDA-NV.
Material y métodosSe incluyó a pacientes con SDA-NV de forma prospectiva durante un periodo de 4 años. Se recolectaron datos demográficos, clínicos y de laboratorio. Se usó análisis ROC para determinar el mejor punto de corte para la albúmina sérica al momento de admisión que discrimine entre aquellos que sobrevivieron y aquellos que fallecieron, así como para comparar el desempeño global con la escala de Rockall.
ResultadosCiento ochenta y cinco pacientes con SDA-NV fueron analizados. El sexo masculino predominó (56.7%) y la edad media fue de 59.1±19.9 años. La media de albúmina al momento de admisión fue de 2.9±0.9g/dl, detectando hipoalbuminemia (< 3.5g/dl) en el 71.4% de los casos. La curva ROC encontró un nivel de albúmina de 3.1g/dl (AUROC 0.738) como el mejor punto de corte que predice mortalidad hospitalaria. La mortalidad en pacientes con albúmina ≥ 3.2g/dl fue del 1.2% comparado con el 11.2% en el grupo con un valor<3.2g/dl (p=0.009; RM 9.7, IC del 95%, 1.2-76.5). El desempeño global para identificar mortalidad fue similar entre albúmina (AUORC 0.738) y la escala de Rockall (AUROC 0.715).
ConclusionesLos pacientes con SDA-NV con hipoalbuminemia presentan una mayor mortalidad hospitalaria. La albúmina sérica y la escala de Rockall mostraron un rendimiento similar para identificar mortalidad.
Gastrointestinal bleeding is a common medical emergency with an estimated incidence of 48 to 160 events per 100,000 adults, accounting for approximately 300,000 hospital admissions per year in the United States.1–3 Despite major advances in the management of non-variceal upper gastrointestinal bleeding (NVUGB) in the last decades, its related mortality rate continues to be considerable at 5 to 10%.4,5 Peptic ulcer bleeding is still the most common cause and is responsible for approximately 31 to 67% of all cases, followed by erosive disease, esophagitis, malignancy, and Mallory-Weiss tears.3
Different studies have shown that serum albumin levels have prognostic value for clinical complications in different scenarios including elective surgery, surgical oncology, hospital stay in patients admitted to internal medicine or pediatrics, hospital mortality in stroke patients and patients with major trauma, among other conditions.6–8 Despite the prognostic value of serum albumin in different settings, its clinical value in patients with NVUGB has not been widely evaluated in prospective studies.9 We previously evaluated a cohort of 1,067 patients and found that the serum albumin level upon admission was an independent predictor of in-hospital mortality.10 We also analyzed a group of patients with chronic liver disease (CLD) and NVUGB, and again, hypoalbuminemia was an independent predictor of mortality.4 However, the previously mentioned studies included patients with comorbidities such as CLD, end-stage chronic renal disease (ESRD), and neoplasia, which have been clearly associated with hypoalbuminemia.11–13 These entities are confounding factors associated with final outcomes in these studies.
In this prospective study, we sought to examine the role of serum albumin upon admission in relation to clinical course and in-hospital mortality in patients with NVUGB with no related CLD, ESRD, or neoplasia.
Materials and methodsAll patients admitted to our hospital with NVUGB during the period of August 2010 to August 2014 were evaluated. Inclusion criteria were adult patients with confirmed NVUGB receiving endoscopic therapy within the first 24h of admission. Patients that met the established criteria were prospectively recorded in a database. We excluded patients transferred to another institution before completing follow-up, patients with CLD, ESRD (on dialysis or not), those with neoplasia as a cause of bleeding, or patients with the presence of cancer at another site.
Variables studiedInformation on the following variables was collected: demographics (age and sex), a previous history of gastrointestinal bleeding, and the use of nonsteroidal antiinflammatory drugs. We considered the presence of severe comorbidities, such as diabetes mellitus, cardiovascular disease (including high blood pressure, cerebrovascular disease, or myocardial ischemia), chronic obstructive pulmonary disease, and stable nephropathy excluding patients with ESRD. Data related to the severity of bleeding, laboratory results upon admission (hemoglobin, serum albumin, blood urea nitrogen, and creatinine), need for transfusion and the number of units transfused, and the use of proton pump inhibitors and/or H2 receptor antagonists were included. Albumin determination was performed in a UniCel® DxC 800 Synchron Clinical System (Beckman Coulter, Inc., Pasadena, CA, USA) using the bromocresol purple method. Each sample was analyzed within one hour of being drawn and the equipment was calibrated every day with an established control.
The endoscopic lesions responsible for bleeding were recorded. The endoscopic therapy used was based on the Forrest classification, which stratifies patients with peptic ulcer disease (the most common cause of NVUGB) into high and low-risk categories for mortality. This classification predicts the risk of rebleeding and is the standard evaluation method of the endoscopic intervention modalities.14 The Rockall prognostic score, which has reported usefulness as a predictor of in-hospital mortality, was also used.15,16
The procedures were performed by professors or residents in gastroenterology involved in the project. Patients had daily follow-up visits during hospitalization or until discharge or death. All-cause in-hospital mortality was considered. Approval was obtained from the research and ethics committee of the School of Medicine and the Hospital Universitario “Dr. José E. González” of the Universidad Autónoma de Nuevo León. Informed consent was obtained from all participants for the diagnostic and therapeutic maneuvers required in each case.
Statistical analysisCategorical variables were expressed in percentage frequencies, and continuous variables as means and standard deviations. ROC curve analysis was applied to evaluate the performance of serum albumin and the Rockall score in identifying mortality in patients with NVUGB, as well as to determine the optimal operating point that made a distinction between deceased patients and survivors with respect to serum albumin upon admission. After exploring this optimal value for serum albumin, a comparative analysis was performed to establish the differences between groups. Odds ratios were determined for all the evaluated variables. Categorical and continuous variables were analyzed with the X2 and Mann-Whitney U tests. Statistical analyses were performed using the SPSS v17.0 (Chicago, IL, USA) and MedCalc® for Windows, version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium) programs.
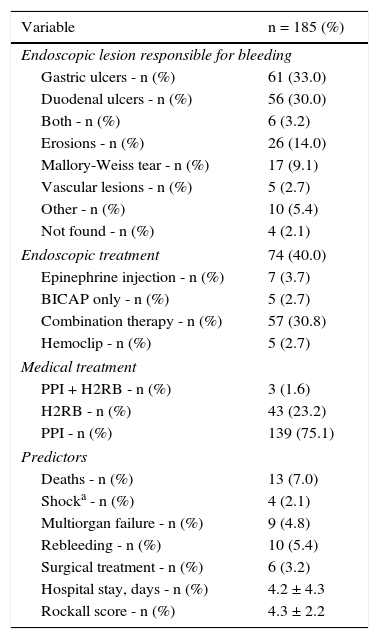
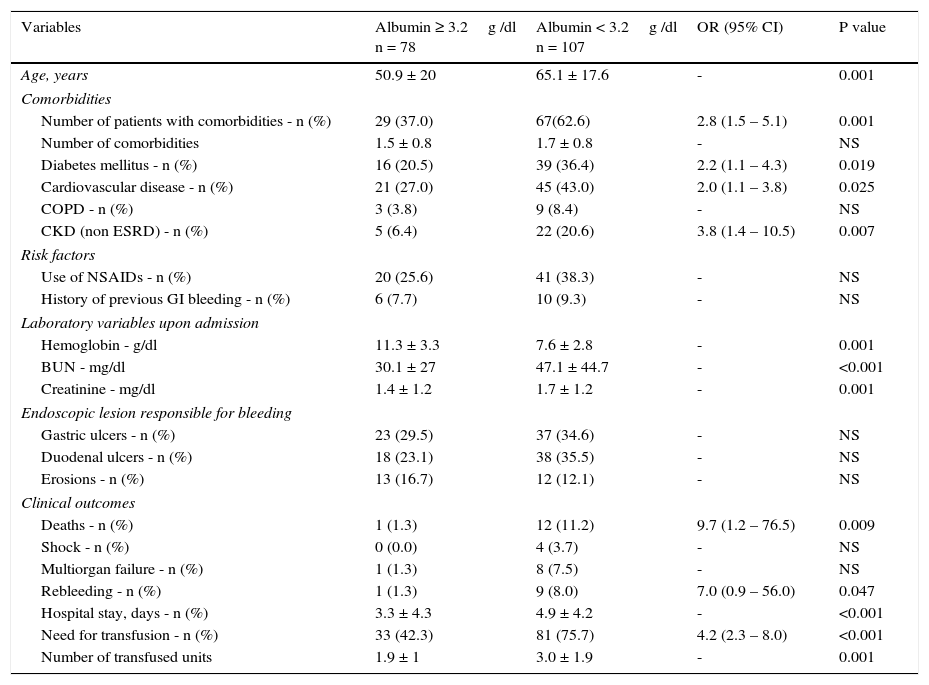
ResultsDuring the study period, 444 patients with upper gastrointestinal bleeding were evaluated. Bleeding was deemed to be of non-variceal origin in 235 of the patients. Of this population, 33 had CLD, 7 were diagnosed with some form of cancer, and 10 presented with ESRD. They were excluded from the study. A final total of 185 patients, 105 men and 80 women with a mean age of 59.1 ± 19.9 years, were included. Mean serum albumin upon admission was 2.9 ± 0.9g/dl, and 132 patients (71.4%) had hypoalbuminemia (< 3.5g/dl). Overall in-hospital mortality was 7.0%. The global medical and endoscopic findings, as well as outcomes, are shown in Table 1. ROC curve analysis was performed with the serum albumin value upon admission, resulting in an AUROC of 0.738. A value of 3.1g/dl was found to be the discriminating figure between deceased patients and survivors with a sensitivity of 92.3%, specificity of 44.2%, PPV of 10.6%, and NPV of 98.8%. Based on this serum albumin cutoff value, there were differences in the clinical endoscopic variables and outcomes between those with an albumin level ≥ 3.2g/dl and those with a level < 3.2g/dl (Table 2).
Results of patient treatment and management.
| Variable | n = 185 (%) |
|---|---|
| Endoscopic lesion responsible for bleeding | |
| Gastric ulcers - n (%) | 61 (33.0) |
| Duodenal ulcers - n (%) | 56 (30.0) |
| Both - n (%) | 6 (3.2) |
| Erosions - n (%) | 26 (14.0) |
| Mallory-Weiss tear - n (%) | 17 (9.1) |
| Vascular lesions - n (%) | 5 (2.7) |
| Other - n (%) | 10 (5.4) |
| Not found - n (%) | 4 (2.1) |
| Endoscopic treatment | 74 (40.0) |
| Epinephrine injection - n (%) | 7 (3.7) |
| BICAP only - n (%) | 5 (2.7) |
| Combination therapy - n (%) | 57 (30.8) |
| Hemoclip - n (%) | 5 (2.7) |
| Medical treatment | |
| PPI + H2RB - n (%) | 3 (1.6) |
| H2RB - n (%) | 43 (23.2) |
| PPI - n (%) | 139 (75.1) |
| Predictors | |
| Deaths - n (%) | 13 (7.0) |
| Shocka - n (%) | 4 (2.1) |
| Multiorgan failure - n (%) | 9 (4.8) |
| Rebleeding - n (%) | 10 (5.4) |
| Surgical treatment - n (%) | 6 (3.2) |
| Hospital stay, days - n (%) | 4.2 ± 4.3 |
| Rockall score - n (%) | 4.3 ± 2.2 |
Values are presented as mean ± standard deviation.
BICAP: bipolar coagulation; H2RB: histamine 2 receptor blockers; PPI: proton pump inhibitor.
Comparison of clinical variables and outcomes of patients with distinct serum albumin level upon admission.
| Variables | Albumin ≥ 3.2g /dl n = 78 | Albumin < 3.2g /dl n = 107 | OR (95% CI) | P value |
|---|---|---|---|---|
| Age, years | 50.9 ± 20 | 65.1 ± 17.6 | - | 0.001 |
| Comorbidities | ||||
| Number of patients with comorbidities - n (%) | 29 (37.0) | 67(62.6) | 2.8 (1.5 – 5.1) | 0.001 |
| Number of comorbidities | 1.5 ± 0.8 | 1.7 ± 0.8 | - | NS |
| Diabetes mellitus - n (%) | 16 (20.5) | 39 (36.4) | 2.2 (1.1 – 4.3) | 0.019 |
| Cardiovascular disease - n (%) | 21 (27.0) | 45 (43.0) | 2.0 (1.1 – 3.8) | 0.025 |
| COPD - n (%) | 3 (3.8) | 9 (8.4) | - | NS |
| CKD (non ESRD) - n (%) | 5 (6.4) | 22 (20.6) | 3.8 (1.4 – 10.5) | 0.007 |
| Risk factors | ||||
| Use of NSAIDs - n (%) | 20 (25.6) | 41 (38.3) | - | NS |
| History of previous GI bleeding - n (%) | 6 (7.7) | 10 (9.3) | - | NS |
| Laboratory variables upon admission | ||||
| Hemoglobin - g/dl | 11.3 ± 3.3 | 7.6 ± 2.8 | - | 0.001 |
| BUN - mg/dl | 30.1 ± 27 | 47.1 ± 44.7 | - | <0.001 |
| Creatinine - mg/dl | 1.4 ± 1.2 | 1.7 ± 1.2 | - | 0.001 |
| Endoscopic lesion responsible for bleeding | ||||
| Gastric ulcers - n (%) | 23 (29.5) | 37 (34.6) | - | NS |
| Duodenal ulcers - n (%) | 18 (23.1) | 38 (35.5) | - | NS |
| Erosions - n (%) | 13 (16.7) | 12 (12.1) | - | NS |
| Clinical outcomes | ||||
| Deaths - n (%) | 1 (1.3) | 12 (11.2) | 9.7 (1.2 – 76.5) | 0.009 |
| Shock - n (%) | 0 (0.0) | 4 (3.7) | - | NS |
| Multiorgan failure - n (%) | 1 (1.3) | 8 (7.5) | - | NS |
| Rebleeding - n (%) | 1 (1.3) | 9 (8.0) | 7.0 (0.9 – 56.0) | 0.047 |
| Hospital stay, days - n (%) | 3.3 ± 4.3 | 4.9 ± 4.2 | - | <0.001 |
| Need for transfusion - n (%) | 33 (42.3) | 81 (75.7) | 4.2 (2.3 – 8.0) | <0.001 |
| Number of transfused units | 1.9 ± 1 | 3.0 ± 1.9 | - | 0.001 |
Values are presented as mean ± standard deviation.
BUN: blood urea nitrogen; CI: Confidence Interval; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; GI: gastrointestinal; NS: not statistically significant; NSAIDs: Nonsteroidal anti-inflammatory drugs; OR: Odds ratio.
We performed a secondary data analysis, comparing the overall performance of serum albumin upon admission and the Rockall score for identifying the mortality rate. No statistical difference for predicting mortality was found between the serum albumin AUROC (0.738) and the total Rockall score (0.715) (fig. 1).
Discussion and conclusionsThe serum albumin level in the setting of NVUGB is an in-hospital mortality marker. To the best of our knowledge, there is only one previous retrospective analysis that specifically addresses the role of serum albumin levels in NVUGB9, although several other studies have reported the association of hypoalbuminemia and mortality.4,10 This is the first prospective report addressing the value of serum albumin upon admission as a risk factor for in-hospital mortality in patients with NVUGB.
Several studies have shown that an alteration in albumin plasma levels has prognostic value in relation to different clinical conditions and scenarios.6–8 Our research team recently published a cohort of 1,067 patients with NVUGB in whom a serum albumin level upon admission < 2.6g/dl, the Rockall score, the presence of rebleeding, and hospital stay duration were independent predictors of in-hospital mortality.10 However, we included patients with CLD, ESRD, and neoplasia. These three comorbidities have been previously identified as predictors of a low albumin level and poor outcome.11–13 In addition, it has been stated that serum albumin presents oxidative damage in cirrhosis and an alteration in its structure and turnover.12,17,18 ESRD has been shown to have a higher risk for gastrointestinal bleeding, with the likely contribution of several factors (glomerular filtration rate, age, Helicobacter pylori infection, and albumin level).19 Finally, hypoalbuminemia in the setting of malignancy without gastrointestinal bleeding has been extensively associated with poor survival.20
When the demographic and clinical variables, the etiology of bleeding, and the mortality rate in our population were compared with other studies in Italy (PNED),5 Canada (RUGBE),21 the United States (AIMS65),22 and Spain,23 we noted that our patients tended to be younger (59.1 ± 19.9 years) than those in the Italian PNED (68 ± 16 years), Canadian RUGBE (66 ± 17 years), American (AIMS65) (75 years, IQR1-3, 60-83), and Spanish (68 ± 15 years) studies. In addition, our study had similar results in regard to the etiology of bleeding, the rebleeding rate, and hospital stay. However, overall mortality in our patients was higher (7.0% vs PNED 4.5%, RUGBE 5.4%, AMIS65 3.2-2.7%, and the Spanish study 2.5-1%). This may be related to the major presence of hypoalbuminemia (71.4%) due to subclinical comorbidities such as CLD caused by nonalcoholic steatohepatitis,24,25 the presence of subclinical malnutrition, and the high prevalence of comorbidity, cardiovascular disease, and diabetes in our group (Table 2).
When comparing our cohort with a previous report from Taiwan,9 we also found that our patients were younger, and the number of men was smaller. However, in our study, similar to the Taiwanese study, patients with normal serum albumin compared with patients in the hypoalbuminemia group were older, they presented with a major prevalence of comorbidities, lower levels of hemoglobin, and elevated blood urea nitrogen and creatinine upon admission. In addition, these patients had longer hospital stay, greater blood transfusion requirements, and higher rebleeding and mortality rates. We did not find any difference between groups in the type of endoscopic lesion responsible for bleeding.
There was no statistically significant difference when albumin values were analyzed and compared with the total Rockall score. This could suggest that serum albumin upon admission has a clinical value as a marker similar to that of the Rockall score for classifying the risk for in-hospital mortality in NVUGB patients without CLD, ESRD, or cancer (fig. 1). The high sensitivity and negative predictive value enabling those patients (with a serum albumin ≥ 3.2g/dl) with a very low likelihood of poor outcome to be distinguished, is remarkable.
Even though albumin plays an important role in maintaining normal physiologic processes and it has been described as a marker of worse prognosis, treatment with its replacement in patients with hypoalbuminemia has not led to better survival in other settings, such as in critically ill patients and those with sepsis.26,27 In the setting of peptic ulcer bleeding, one prospective study demonstrated that albumin administration in patients with hypoalbuminemia, compared with a control group (also with hypoalbuminemia), shortens the duration of hospital stay (9 vs 15 days, p = 0.02). However, no significant difference was found in the rate of rebleeding and mortality.28 Therefore, based on these pieces of evidence and given the higher presence of comorbidity in the hypoalbuminemia group in our study, it appears that albumin itself is not a determinant of mortality, but a surrogate marker of a worse baseline condition.
One of the strengths of our study is the fact that it is a prospective cohort study with predefined inclusion criteria in patients with NVUGB receiving early endoscopic therapy that do not have evident CLD, ESRD, or cancer. We also found that the serum albumin level (< 3.2g/dl) upon admission was a marker of increased in-hospital mortality. The main limitations of our study were that we did not evaluate the nutritional status of the patients, which may be an important factor causing hypoalbuminemia,29 and we were not able to assess 30-day mortality, which may have resulted in an underestimated mortality, since the overall hospital mortality rate was not significantly higher than that reported in other studies.
In conclusion, hypoalbuminemia appears to be an important surrogate marker of poor clinical condition, which subsequently suggests a poor outcome in NVUGB, with an overall performance for identifying mortality similar to that of the Rockall score.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed conformed to the ethical standards of the responsible committee on human experimentation and were in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Dr. Sergio Lozano-Rodriguez, M.D for his assistance in editing this article.
Please cite this article as: González-González JA, Vázquez-Elizondo G, Monreal-Robles R, et al. Hipoalbuminemia en el desenlace clínico de pacientes con sangrado de tubo digestivo alto no variceal. Revista de Gastroenterología de México. 2016;81:183–189.
See related content at DOI: http://dx.doi.org/10.1016/j.rgmx.2016.08.001, Montaño Loza A. Impacto clínico de la albúmina sérica en la hemorragia de tubo digestivo alto no variceal. Revista de Gastroenterología de México. 2016;81:181–182.