Anastomosis leak occurs in 1–19% of colorrectal surgeries. Our objective was to present the first Mexican case series on colorrectal surgery using indocyanine green fluorescence angiography to evaluate perfusion prior to carrying out the anastomosis.
Materials and methodsA retrospective, analytic, descriptive study was conducted. We studied the case records of consecutive patients that underwent colorrectal surgery with indocyanine green angiography performed by the same group of colorrectal surgeons.
ResultsTwenty-one case records were reviewed. Eleven (52.3%) of the patients were women, mean patient age was 57 years (38–82), and mean body mass index was 25 kg/m2 (17–34). Fifteen (71.4%) patients were diagnosed with malignant disease. Indocyanine green angiography changed our therapeutic decision in three (14.2%) patients. Two colorrectal anastomoses (14.2%) were performed at fewer than 5 cm from the anal verge and 13 (61.9%) were performed at more than 5 cm from the anal verge. Three of the anastomoses were ileocolic (14.2%), two were coloanal (9.5%), and one was ileoanal (4.7%). There were six (28.5%) complications, no cases of anastomotic leak, and no complications associated with the use of indocyanine green. The mortality rate was 0%.
ConclusionThe present case series is the first on colorrectal surgery conducted in Mexico using indocyanine green fluorescence angiography, with excellent results.
La dehiscencia de anastomosis ocurre en 1 al 19% de los pacientes con cirugía colorrectal. El objetivo es presentar la primera serie de cirugía colorrectal en México en la que se utilizó la angiografía por fluorescencia con verde de indocianina para valorar la perfusión, previo a realizar la anastomosis.
Material y métodosEstudio retrospectivo, analítico y descriptivo. Se analizaron los expedientes de pacientes consecutivos con cirugía colorrectal de un mismo grupo de cirujanos colorrectales en los que se utilizó la angiografía con verde de indocianina.
ResultadosSe revisaron 21 expedientes de los cuales 11 (52.3%) eran mujeres, media de edad 57 años (38 a 82) y media de índice de masa corporal (IMC) 25 kg/m2 (17 a 34). El diagnóstico fue enfermedad maligna en 15 (71.4%) pacientes. La angiografía cambió nuestra decisión terapéutica en tres (14.2%) casos. Dos anastomosis se hicieron colorrectales a menos de 5 cm del margen anal (14.2%), a menos de 5 cm del margen anal, 13 (61.9%) se hicieron a más de 5 cm, tres (14.2%) fueron ileo-colónicas, dos (9.5%) se hicieron colo-anales y una (4.7%) se hizo ileo-anal. Tuvimos seis (28.5%) complicaciones, no existió dehiscencia de anastomosis ni complicación asociada con el uso de verde de indocianina. Mortalidad de 0%.
ConclusiónPresentamos la primera serie de cirugía colorrectal en México en la que se utilizó la angiografía por fluorescencia con verde de indocianina con excelentes resultados.
Colorectal surgery has a 20–30% complication rate and hospital stay varies from 6 to 12 days1–8. Anastomosis leak (AL) is one of the complications most dreaded by patients and surgeons alike because it results in high rates of morbidity and mortality. It is also associated with longer hospital stay and higher hospitalization costs. AL, in malignant colonic or rectal disease, has also been associated with a higher recurrence rate and a lower survival rate9–13. AL occurs in 1–19% of patients that undergo colorectal surgery and incidence is higher, the more distal the anastomosis1,10,12,14. Incidence varies, according to location, and is reported at 1–8% for ileocolic anastomosis, 2–3% for colocolic anastomosis, 3–7% for ileorectal anastomosis, and 5–19% for colorectal or coloanal anastomosis10. The AL-associated mortality rate ranges from 6 to 22%14.
Multiple risk factors for AL have been described and include the surgical technique employed, location of the anastomosis, and patient characteristics. However, the risk factor most widely associated with AL is ischemia10. Anastomotic blood flow is traditionally evaluated subjectively, assessing macroscopic characteristics, such as the color of the serosa, palpable pulse, peristaltic movements, or active bleeding of the terminal arteries10,12,14. Reports in the literature have shown that the risk for AL is not adequately assessed through macroscopic analysis of colonic blood flow9,10,14.
Indocyanine green fluorescence angiography (ICGFA) has been used to observe perfusion in real time, providing a superior evaluation of the segments to anastomose and reducing AL in colorectal surgery9,10.
AimOur aim was to present the first Mexican case series on colorectal surgery using ICGFA to evaluate perfusion prior to performing the anastomosis.
Materials and methodsIndocyanine green is a dye approved by the US Food and Drug Administration (FDA) for diagnostic studies in cardiology, ophthalmology, and gastroenterology, among others9,14,15. It is injected intravenously, binding to the plasma proteins with low permeability into the interstitium, and is eliminated almost exclusively by the liver. Indocyanine green is fluorescent when exposed to infrared light9,14.
We conducted a retrospective, analytic, descriptive study that included patients that underwent colorectal surgery with anastomosis and the prior performance of ICGFA. All the patients signed written statements of informed consent and were operated on by the same group of colorectal surgeons (Coloncare Hospital Ángeles Valle Oriente, Monterrey, Nuevo León, Mexico).
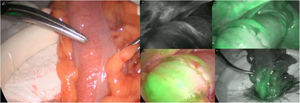
Patients seen from November 2019 to March 2020 were selected. We used the Stryker 1688 platform and included patients with benign and malignant disease. In the first surgical stage, all the patients underwent colonic or rectal resection and the site of adequate blood flow was identified according to its macroscopic characteristics. The anastomosis site was chosen based on the aspect of the mucosa, the seromuscular layer of the colon, and bleeding at the incision site, according to the experience and criteria of the surgeon. Twenty-five milligrams of indocyanine green were then diluted in 10 ml of injectable water and administered at 0.2 mg/kg. The previously chosen site was reassessed by means of ICGFA. The time interval from intravenous injection to the appearance of fluorescence in the colon varied from 35 to 90 s. Therapeutic decision modification was defined as those cases in which ICGFA made us change our previously established anastomosis site (Fig. 1). A second dose (same dose) was then administered to evaluate the proximal and distal segment of the intestine to be anastomosed. The anastomosis was performed at the adequate site indicated by the ICGFA. A pneumatic and visual test was routinely carried out through rectosigmoidoscopy in all the patients to assess the anastomosis. In addition, the anastomotic doughnuts were thoroughly checked in all the patients that had a mechanical anastomosis. The minimum follow-up in all patients was two weeks.
Statistical analysisA descriptive analysis was carried out, obtaining means with standard deviation. No comparisons between groups were performed.
Ethical considerationsProtection of human and animal subjects. No experiments were performed on humans or animals. The present study was retrospective. All the patients signed written statements of informed consent regarding the procedure.
Confidentiality of data. We have followed the protocols of our work center on the publication of patient data, preserving the anonymity of the patient data.
All the patients signed written statements of informed consent in relation to the surgical procedure, in which all its risks and benefits were explained.
ResultsA total of 21 patients were studied, 11 (52.3%) of whom were women. Mean patient age was 57 years (38–82) and mean body mass index (BMI) was 25 kg/m2 (17–34). Fifteen (71.4%) of the patients had malignant disease, 5 (23.8%) benign disease, and there was one (4.7%) case of ulcerative colitis and cancer of the sigmoid colon (Table 1). The most frequent comorbidities were arterial hypertension, presenting in 7 (33.3%) patients, smoking in 4 (19%), diabetes mellitus in 3 (14.2%), and one (4.7%) patient had a history of pelvic radiotherapy due to a gynecologic malignancy. Five (23.8%) of the patients received neoadjuvant chemotherapy and 2 (9.5%) received neoadjuvant radiotherapy. Three (14.2%) patients presented with fistulizing disease and 2 (9.5%) presented with symptomatic partial colonic obstruction (Table 2).
Demographic characteristics.
| n = 21 | |
|---|---|
| Sex (%) | 11 (52.3%) |
| Women | |
| Men | 10 (47.7%) |
| Age (mean) | 57 (38−82) years |
| BMI (mean) | 25 (17−34) kg/m2 |
| Malignant disease (%) | 15 (71.4%) |
| Adenocarcinoma of the rectum (%) | 6 (28.5%) |
| Adenocarcinoma of the sigmoid colon (%) | 6 (28.5%) |
| Adenocarcinoma of the right colon (%) | 3 (18%) |
| Benign disease (%) | 5 (23.8%) |
| Diverticular disease (%) | 3 (14.2%) |
| Colocutaneous fistula | 1 (4.7%) |
| Pericolic abscess | 1 (4.7%) |
| Uncomplicated diverticular disease | 1 (4.7%) |
| Actinic rectovaginal fistula | 1 (4.7%) |
| Volvulus of the sigmoid colon | 1 (4.7%) |
| Benign and malignant disease (%) | 1 (4.7%) |
| IBD (ulcerative colitis + cancer of the sigmoid colon) | 1 (4.7%) |
BMI: body mass index; IBD: inflammatory bowel disease.
Comorbidities and clinical presentation.
| n = 21 | |
|---|---|
| Comorbidities | |
| Arterial hypertension (%) | 7 (33.3%) |
| Smoking (%) | 4 (19%) |
| Diabetes mellitus (%) | 3 (14.2%) |
| Previous radiotherapy (%) | 1 (4.7%) |
| Cardiovascular diseases (%) | 0 (0%) |
| Clinical presentation | |
| NA CT (%) | 5 (23.8%) |
| Fistulizing disease (%) | 3 (14.2%) |
| NA RT (%) | 2 (9.5%) |
| Symptomatic partial colonic obstruction (%) | 2 (9.5%) |
NA CT: neoadjuvant chemotherapy; NA RT: neoadjuvant radiotherapy.
Nineteen (90.4%) of the surgeries performed were completely laparoscopic, one (4.7%) with the laparoscopic and transanal approach, and one (4.7%) was a hand-assisted laparoscopy. Three (14.2%) patients with fistulizing disease presented with focal abdominal sepsis and no patient presented with disseminated peritonitis. Central pedicle ligation (root of the artery) was performed in the patients diagnosed with colon cancer. The anal anastomoses were endoanal and the rest of the anastomoses were intracorporeal. The splenic angle was mobilized in all patients that had total excision of the mesorectum. No patient received intraoperative transfusions. ICGFA changed the therapeutic decision in 3 (14.2%) of the patients. Two (9.5%) of the anastomoses were colorectal at fewer than 5 cm from the anal verge and 13 (61.9%) were constructed at more than 5 cm from the anal verge. Three (14.2%) of the anastomoses were ileocolic, 2 (9.5%) were coloanal, and one (4.7%) was ileoanal (Table 3). We performed a protective ileostomy on all of our patients that had received neoadjuvant chemotherapy or neoadjuvant radiotherapy, as well as on those that underwent ileoanal anastomosis. All protective ileostomies were closed 6 weeks after the initial procedure.
Intraoperative approach, findings, and therapeutic decision modification due to ICGFA.
| n = 21 | |
|---|---|
| Approach | |
| Laparoscopic (%) | 19 (90.4%) |
| Laparoscopic and transanal (%) | 1 (4.7%) |
| Hand-assisted laparoscopic (%) | 1 (4.7%) |
| Focal abdominal sepsis (%) | 3 (14.2%) |
| Therapeutic decision modification due to ICGFA (%) | 3 (14.2%) |
| Type of anastomosis | |
| Colorectal at less than 5 cm (%) | 2 (9.5%) |
| Colorectal at more than 5 cm (%) | 13 (61.9%) |
| Ileoanal (%) | 1 (4.7%) |
| Coloanal (%) | 2 (9.5%) |
| Ileocolonic (%) | 3 (14.2%) |
ICGFA: indocyanine green fluorescence angiography.
There were 6 (28.5%) complications in our group, 3 (14.2%) of which were classified as Clavien-Dindo I (postoperative ileus) and 3 (14.2%) as Clavien-Dindo II (pneumonia, central venous catheter infection, and surgical wound infection). We had no cases of AL and no complication associated with ICGFA. The mortality rate in our patients was 0% (Table 4).
Discussion and conclusionWe present herein the first Mexican case series of patients that underwent colorectal surgery due to benign or malignant disease, using ICGFA to evaluate perfusion, before performing the anastomosis.
ICGFA has been shown to be a safe procedure that does not increase patient morbidity or mortality. In our case series there were no complications associated with the use of indocyanine green. Boni et al. reported on 107 patients with benign or malignant disease and also found no complications associated with ICGFA14. No ICGFA-associated complications were reported by the Santi group9 either. In addition to being very safe, ICGFA is easily reproducible and does not require a learning curve for its successful application.
Numerous studies in the literature have demonstrated that ICGFA reduces AL in colorectal surgery in benign disease, and particularly, in malignant disease. Total mesorectal excision in surgery for malignant disease is associated with worse perfusion in newly constructed anastomoses, and that is the scenario in which ICGFA has been shown to offer greater benefit. Boni et al. reported therapeutic decision modification with the use of ICGFA in 3.7% of their patients and described only one case of AL, which was associated with poor surgical technique14.
Santi et al. reported therapeutic decision modification upon using ICGFA in 38 patients. That same group described three complications and one case of AL associated with a problem with the mechanical stapler9. Wada et al. conducted a study on 149 patients with lower anterior resection, employing propensity score matching, and reported AL in 8.8% of the patients that underwent ICGFA evaluation, compared with 14.7% that did not. They also found that a higher BMI, neoadjuvant chemotherapy, and lateral lymph node dissection were associated with AL16.
Kudszuz et al. reported therapeutic decision modification in 13.9% of cases and a 4% reduction in AL with the use of ICGFA17, and the PILLAR-II trial reported therapeutic decision modification in 7.9% of the cases using ICGFA18.
In the meta-analysis by Blanco et al. that included patients that underwent colorectal surgery for benign or malignant disease, 555 patients underwent ICGFA evaluation and 747 patients (control group) did not. AL was described in 5.4% of the cases, with no statistically significant differences between groups. Most importantly, they did find a statistically significant decrease in AL in patients with malignant disease that underwent ICGFA evaluation, compared with those that did not. There was therapeutic decision modification in 7.4% of all cases (benign and malignant)10. Shen et al. conducted a meta-analysis on patients with colorectal cancer, comparing a group with ICGFA and a control group without it and also found a statistically significant lower rate of AL, when using ICGFA in patients with colorectal cancer19.
ICGFA has also been employed in robotic-assisted colorectal surgery, with similar findings. Hellan et al. analyzed a group of 40 patients with rectal or left colonic resections performed robotically, using ICGFA. There was therapeutic decision modification in 40% of the patients and AL in 5%13. Jafari et al. presented a case series of patients with rectal resections carried out robotically, comparing a group with ICGFA and a control group without it. There was therapeutic decision modification in 19% of the patients and an AL rate of 6% with the use of ICGFA, compared with 18% without it20. Kim et al. conducted a case series on patients that underwent robotic-assisted sphincter-saving operations and found a decrease in AL cases with the use of ICGFA (0.6%), compared with the control group (5.2%)21.
ICGFA has been very attractive for the enhanced recovery after surgery (ERAS) protocol. Brescia et al. studied 182 patients that underwent colorectal surgery with the ERAS protocol, using ICGFA in 75 patients. There was therapeutic decision modification in 6.7%. There was no AL in the ICGFA group, and the AL rate was 5.6% in the control group without ICGFA. There was no statistically significant difference regarding hospital stay between the two groups1.
The cost of ICGFA has been a matter of discussion in the literature. Compared with the fact that patients with AL require reoperation, admission to the intensive care unit, and longer hospital stay, treatment with ICGFA is much less expensive. Costs are 5-times higher in patients with AL than in patients without said complication22.
ICGFA has also been employed to evaluate tumor perfusion prior to resection of the colon or rectum. In one case, Santi et al. utilized the technique to differentiate a colonic tumor from the right ureter and prevent ureteral injury. Likewise, ICGFA has been used in colonic resections to identify the vascular pedicle feeding the tumor9.
AL continues to occur, even with ICGFA. Perhaps a more objective manner of analyzing fluorescence should be sought23. Fluorescence intensity depends on the distance from which the beam is placed and whether it is carried out intra-abdominally or extra-abdominally. The evaluation of fluorescence is still a subjective one. When the concentration of indocyanine green is low, the reflected intensity has a positive correlation with the concentration, but if the concentration is increased to a certain point, said correlation is lost. Thus, maintaining low concentrations of the dye is recommended13. Even though ICGFA gives us a more objective evaluation, it continues to be a technique whose interpretation by the surgeon is subjective.
Wada et al. quantitatively measured fluorescence, using analysis software that measured maximum fluorescence time, and found lower curves in the patients that presented with AL, compared with the group with no AL16. The future of ICGFA lies in its being able to quantitatively measure fluorescence, thus completely eliminating subjectivity from the perfusion analysis of the segments involved in colorectal surgery.
In conclusion, ours is the first Mexican case series on colorectal surgery using ICGFA, with excellent results. We had no AL or complications associated with the use of ICGFA in the patients analyzed.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestDr. Luis Enrique Salgado Cruz has been a consultant for Stryker.
Please cite this article as: Ortiz de Elguea-Lizárraga JI, Riojas-Garza A, Chapa-Lobo AF, Rangel-Ríos HA, García-García AL, Quevedo-Fernández E, et al. Angiografía por fluorescencia con verde de indocianina para cirugía de colon y recto. Primera serie reportada en México. Revista de Gastroenterología de México. 2022;87:29–34.