The manner in which informed consent is obtained varies. The aim of this study is to evaluate the level of knowledge about colonoscopy and comparing 2 methods of obtaining informed consent.
Materials and methodsA comparative, cross-sectional, observational study was conducted on patients that underwent colonoscopy in a public hospital (Group A) and in a private hospital (Group B). Group A received information verbally from a physician, as well as in the form of printed material, and Group B only received printed material. A telephone survey was carried out one or 2 weeks later.
ResultsThe study included a total of 176 subjects (group A [n=55] and group B [n=121]). As regards education level, 69.88% (n=123) of the patients had completed university education, 23.29% (n=41) secondary level, 5.68% (n=10) primary level, and the remaining subjects (n=2) had not completed any level of education. All (100%) of the subjects knew the characteristics of the procedure, and 99.43% were aware of its benefits. A total of 97.7% received information about complications, 93.7% named some of them, and 25% (n=44) remembered major complications. All the subjects received, read, and signed the informed consent statement before the study. There were no differences between the groups with respect to knowledge of the characteristics and benefits of the procedure, or the receipt and reading of the consent form. Group B responded better in relation to complications (P=.0027) and group A had a better recollection of the major complications (P<.0001). Group A had a higher number of affirmative answers (P<.0001).
ConclusionsThe combination of verbal and written information provides the patient with a more comprehensive level of knowledge about the procedure.
La forma de obtener el consentimiento informado es variable, por lo que el objetivo de este trabajo fue evaluar el nivel de conocimientos sobre la colonoscopia comparando 2 modalidades de consentimiento.
Materiales y métodosEstudio observacional, transversal y comparativo realizado en pacientes sometidos a colonoscopia en un hospital público (grupo A) y en un hospital privado (grupo B). El grupo A recibió información verbal por un médico e impresa y el grupo B solo impresa. Una o 2 semanas después se realizó una encuesta telefónica.
ResultadosSe incluyó a 176 sujetos (grupo A n=55 y grupo B n=121). El 69.88% (n=123) de los pacientes tenían nivel educativo universitario, el 23.29% (n=41) nivel educativo secundario, el 5.68% (n=10) nivel educativo primario completo y los restantes (n=2) no habían completado estudios. El 100% conocía las características y el 99.43% los beneficios del procedimiento. El 97.7% recibió información sobre complicaciones, el 93.7% nombró alguna y el 25% (n=44) recordó complicaciones mayores. Todos respondieron, recibieron y leyeron el consentimiento informado antes del estudio. No hubo diferencias entre los grupos en el conocimiento de las características, los beneficios, la recepción y la lectura del consentimiento. El grupo B respondió mejor sobre las complicaciones (p=0.0027) y el grupo A recordaba más las complicaciones mayores (p<0.0001). El grupo A tuvo mayor número de respuestas positivas (p<0.0001).
ConclusionesLa combinación de información verbal y escrita logra mejor nivel de conocimientos por el paciente.
Informed consent (IC) is a key part of the medical act that is related to the principle of autonomy1,2 it is every patient's right to receive the information necessary for making a joint decision about the actions to be taken in regard to his or her health.
Over the last few decades, elements of legal protection (“defensive medicine”)3 have been incorporated into IC and it is sometimes perceived by patients as an instrument for exempting the medical professionals and institutions from responsibilities.4–6
IC is one of the parameters of quality recommended by different scientific societies that are concerned with gastrointestinal endoscopy. It forms part of a process of providing information to the patient before, during, and after the procedure and because of the importance of its role today, should be considered an indicator of quality.7,8
Patient safety is a global health preoccupation and the World Health Organization has led different initiatives to improve the safety of those receiving medical care; among these initiatives, that of “safe surgery” includes informed consent as an indicator of quality.9,10
The manner in which information is given to the patient undergoing endoscopy varies. Some patients receive information directly from the physician in a previous consultation, others are informed by auxiliary personnel (nurses, technicians, or administrators), while others receive information in the form of printed material (or via email) that also serve as IC.
The distinct manners of providing the information to the patients and documenting it, added to their different educational levels, comprehension capacities, and cultural factors, result in a varied understanding of the information that can negatively affect the doctor/patient relationship.11 Every patient should be thoroughly informed about the procedure he or she is to undergo, including its benefits, risks, results, and alternatives.
The aim of our study was to evaluate the level of knowledge about colonoscopy (COL) through the comparison of 2 IC methods.
MethodsA comparative, cross-sectional, observational study was carried out. The Hospital Militar Central (HMC) has a closed access endoscopy system in which the patient must have a consultation with a specialist before the procedure. The Gastroenterología Diagnóstica y Terapeútica (GEDYT) referral center employs an open access endoscopy system, in which the patient requests his or her turn for the procedure and the information is provided by the auxiliary personnel through printed material. Our study simply observed the customary work methods of each service without intervening in any other way.
The study included patients that underwent diagnostic, programmed, and outpatient COL at the Gastroenterology Service of the HMC (group A) and the GEDYT (group B) in Buenos Aires, Argentina, during the month of May 2014.
Inclusion criteria: patients that were 18 years of age or older, that underwent a colonoscopy, were outpatients, had a programmed study, and had a diagnostic study that included simple polypectomy.
Exclusion criteria: patients that had undergone previous gastrointestinal endoscopic studies, patients incapable of understanding due to neurologic and/or cognitive alterations, patients that underwent advanced therapeutic endoscopic procedures, and patients that did not wish to participate in the study.
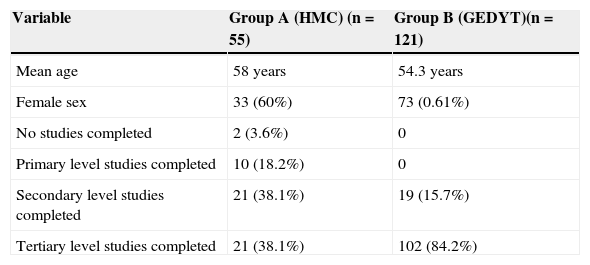
Patients were consecutively incorporated into each group and the data necessary for carrying out a telephone survey (first and last names, age, sex, and highest level of education) were registered (Table 1).
Questionnaire for the patient.
| Age: | ||
| Sex: | ||
| 1. Educational level. | ||
| 2. Were you informed about the characteristics of the endoscopy before undergoing the procedure? | Yes | No |
| 3.- Were you informed as to why the endoscopy was ordered and what benefits you could expect from it? | Yes | No |
| 4.- Were you informed as to the potential complications that could arise from the endoscopic procedure?If your answer was yes, name the complications you recall that could arise as a consequence of a colonoscopy. | Yes | No |
| 5. Were you asked to read and sign a statement of informed consent or authorization before undergoing the endoscopy? | Yes | No |
| 6. Did you read the entire statement of informed consent that you were given before the procedure? | Yes | No |
| 7. Were you informed as to alternative procedures available (other studies you could have undergone) had you not wished to undergo colonoscopy?If your answer was yes, name the alternate studies you were told of as alternatives to colonoscopy: | Yes | No |
The group A patients (HMC) received verbal information related to the colonoscopy in a previous consultation with a physician from the Gastroenterology Service and at that time were given a printed pamphlet with information along with an IC form to sign and bring with them the day of the procedure. The group B patients (GEDYT) received the information pamphlet and IC form when they requested their turn for colonoscopy. It was given to them by the administrative personnel either personally or by email and they were also instructed to present the signed IC at the time of the procedure.
The same pamphlets and forms were used in both groups and they were evaluated by the Flesch-Kincaid Readability Test adapted to Spanish by Fernández-Huerta.12 The scores obtained for the informative pamphlets and the IC form were 62.9 and 67.8, respectively, and were deemed adequate for an adult reader.
The telephone survey was carried out 7 to 15 days after the COL by auxiliary personnel that did not participate in the previous consultation or procedure. The questionnaire consisted of 7 closed, structured questions and 2 open, additional questions (added to questions 4 and 7).
The variables were: age, sex, educational level (no completed studies, primary level completed, secondary level completed, and university level completed), and knowledgeability about COL (the proportion of affirmative answers to all the questions; in the second part of questions 4 and 7, the number of patients that remembered some complication and alternative study were compared).
Ethical aspects: Because of the study design (observational), the signed IC and verbal acceptance to answer the confidential telephone survey were sufficient for study participation.
Statistical analysis: The general data (age, sex, and educational level) were analyzed and expressed as means, range, and SD. The results of each question were evaluated globally through descriptive and comparative statistics (nonparametric test, chi-square test). A value equal to or less than 0.05 was accepted as statistically significant.
ResultsA total of 176 patients were enrolled in the study (group A [n=55], group B [n=121]). The mean age was 55.4 years (range: 23-84 years; SD 10.9) and all the patients agreed to answer the survey. A total of 69.88% (n=123) of the patients had completed university education, 23.29% (n=41) had completed secondary education, 5.68% (n=10) had completed primary education, and the remaining patients (n=2) had not completed any levels of study (Table 2).
Demographic and educational level variables.
| Variable | Group A (HMC) (n=55) | Group B (GEDYT)(n=121) |
|---|---|---|
| Mean age | 58 years | 54.3 years |
| Female sex | 33 (60%) | 73 (0.61%) |
| No studies completed | 2 (3.6%) | 0 |
| Primary level studies completed | 10 (18.2%) | 0 |
| Secondary level studies completed | 21 (38.1%) | 19 (15.7%) |
| Tertiary level studies completed | 21 (38.1%) | 102 (84.2%) |
All the surveyed patients stated that they had been informed about the characteristics of the COL and 99.43% (n=175) stated that they had received information pertaining to the benefits of the procedure. A total of 97.7% received information with respect to potential complications, 93.7% named some of the complications they had been informed of in the IC, but only 25% (n=44) remembered major complications (perforation and/or bleeding). All the patients said they had received the IC form before the study and had read it.
Only 23.8% of the patients recalled having received information about diagnostic alternatives, but just 7.95% (n=14) could name any of them.
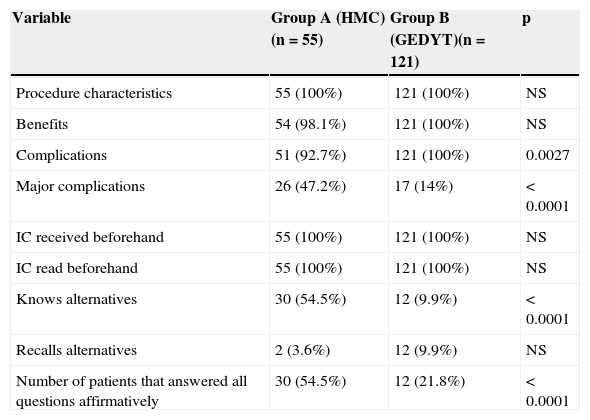
The comparative analysis showed no statistically significant differences in relation to knowledgeability about the characteristics of the study, the benefits, and the prior receipt and reading of the IC (Table 3).
Knowledgeability level comparison (quantity of affirmative answers).
| Variable | Group A (HMC) (n=55) | Group B (GEDYT)(n=121) | p |
|---|---|---|---|
| Procedure characteristics | 55 (100%) | 121 (100%) | NS |
| Benefits | 54 (98.1%) | 121 (100%) | NS |
| Complications | 51 (92.7%) | 121 (100%) | 0.0027 |
| Major complications | 26 (47.2%) | 17 (14%) | < 0.0001 |
| IC received beforehand | 55 (100%) | 121 (100%) | NS |
| IC read beforehand | 55 (100%) | 121 (100%) | NS |
| Knows alternatives | 30 (54.5%) | 12 (9.9%) | < 0.0001 |
| Recalls alternatives | 2 (3.6%) | 12 (9.9%) | NS |
| Number of patients that answered all questions affirmatively | 30 (54.5%) | 12 (21.8%) | < 0.0001 |
NS: not significant.
All the group B patients stated that they had been informed about complications, whereas the percentage in group A was lower (p<0.05). However, a greater number of patients that had a specialized consultation (group A) recalled a higher number of complications considered major (perforation and/or bleeding) than those that did not have a previous consultation (group B) (p<0.05).
The level of information about alternatives was low in group B, with statistically significant differences (p<0.0001). There were no differences between the groups in regard to the ability to remember alternative diagnostic studies.
Thirty patients in group A answered the 7 closed questions in the affirmative, as opposed to 12 patients in group B. The mean of the affirmative answers was 5.5 for group A and 5.01 for group B, and the difference between the two was statistically significant.
DiscussionThere are regional variations in the use of IC and the quantity and quality of the information given to the patients. A Croatian study showed strikingly low levels of IC use and information about alternative procedures and endoscopy-related mortality.13 In China it was observed that a little over two-thirds of the patients that underwent gastrointestinal endoscopy received information prior to the procedure.14 A European study conducted in 2002 revealed a great variation among countries: prior information was provided by an endoscopist in less than one-fourth of the countries, alternatives were not discussed in 15% of them, and information about procedure-related mortality was only provided in 23%.15 Another study reported that providing information to the patients was delegated to hospital or office personnel by 30% of the endoscopists in Great Britain and that there was a tendency to not equally reveal all information in regard to complications and alternatives.16
Our study showed that the patients had adequate levels of information in relation to the majority of the aspects that should be contemplated in the IC process: procedure characteristics, benefits, and complications. At the same time, the knowledgeability of major complications and diagnostic alternatives was low.
The combination of verbal and written information before surgical and endoscopic procedures has resulted in lower levels of anxiety17 and an improved level of understanding in general, as well as about complications. There was a positive increase in these results when audiovisual or multimedia resources were used.18–21
In our population, the comparison of the 2 methods of obtaining the IC showed a higher level of overall knowledge in the group that had a previous interview with a gastroenterologist and the participation of a specialist was associated with a greater capacity to remember major complications and alternative procedures. It is important to take into account the capacity to remember the risks of the procedure as an indicator of IC quality, given that patients undergoing COL want to know about major complications.22
Even though we believe it is necessary to continue studying the quality of the IC process, and despite the methodological limitations of our study (sample size and differences in the number of patients in the groups), we feel that our results are relevant, given the scarcity of articles related to this theme in our country and region.
If the knowledge patients have of the procedure they are undergoing improves with their educational level,23 then we believe a limitation of our study was the fact that the majority of the patients had high school and university educations, which did not enable a correct comparison with lower educational levels.
The satisfaction of the patients that agree to undergo endoscopy through an open access system (such as the group that received printed information with no prior consultation) is high;24 other variables such as not reading the IC and having a higher level of education are related to greater dissatisfaction with respect to the information received.25
The best interests of the patient are identified and respected through IC, giving each patient the opportunity to make an autonomous decision. In its truest form, IC is a process; the document is only a concrete signal of the fact that said process has taken place. This means recognizing the ethical value inherent in self-determination and attempting to recognize the value system of each patient and his or her individual life goals and how these factors influence his or her decisions.
IC is particularly important in the surgical sphere and due to the characteristics of endoscopic procedures it should be obtained in a manner similar to those types of surgeries or procedures in which signed statements of informed consent are a necessity.26
There are a variety of conceivable settings for the time and place in which the IC process could be begun, ranging from the physician's office to the bedside of the hospitalized patient. Depending on the type of practice for which the informed consent is obtained and the level of discussion that the patient needs, the process may require a previous doctor's appointment or include written information, images, or explanatory videos of the procedure or practice. These resources, together with the use of less technical language, can be a way for the patient to have greater understanding, leading to a better joint decision that will be to his or her benefit.21
Current documents for IC should first of all offer a clear description of the planned procedure, its risks, and its benefits. Second, the document should adequately describe the anticipated results, both positive and negative, for the near and distant future, as well as the existing alternatives to the procedure or practice to be performed.27–29
We can conclude that the two methods for providing information were adequate, achieving a high level of knowledge about the different aspects that should be part of IC. The participation of a specialist resulted in higher general levels of knowledge and specific aspects such as major complications and types of alternative procedures. The use of one information system or the other for the patient is determined, among other variables, by the type of institution (basically the number of physicians and patients) and by its access system; these factors should be taken into account when deciding upon the information method.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Prof. Roque Saénz Fuenzalida, MD; Justus Krabshius, MD (Ask a Librarian WGO).
Please cite this article as: Sanguinetti JM, Lotero Polesel JC, Iriarte SM, Ledesma C, Canseco Fuentes SE, Caro LE. Consentimiento informado en colonoscopia: un estudio comparativo de 2 modalidades. Revista de Gastroenterología de México. 2015;80:144–149.