The aim of our study was to evaluate the association between insulin resistance and the response to IFN-alpha and ribavirin in pediatric patients with chronic hepatitis C.
MethodsTwenty-six patients with chronic hepatitis C (mean age: 12.5 ± 1.96 years, M/F:3.33) were included in the study. Fasting glucose, insulin, and C-peptide levels, together with HOMA-IR, HOMA-B, and QUICKI values, were assessed. The association between those parameters and treatment response was determined.
ResultsFive (19.2%) of the 26 patients analyzed (2 [21.4%] with treatment response and 3 [16.6%] with no treatment response) had insulin resistance (p = 1.00). There were no significant differences between the patients with and without treatment response with respect to fasting glucose, insulin, and C-peptide levels or HOMA-IR, HOMA-B, and QUICKI values (p > 0.05).
ConclusionsNo significant association was establihed between insulin resistance and response to IFN-alpha and ribavirin, in children with chronic hepatitis C.
El objetivo de nuestro estudio fue evaluar la asociación entre la resistencia a la insulina y la respuesta al IFN-alfa y ribavirina en pacientes pediátricos con hepatitis C crónica.
MétodosEl estudio incluyó a 26 pacientes con hepatitis C crónica (edad promedio: 12.5 ± 1.96 años, M/F: 3.33). Previo ayuno se evaluaron la glucosa, la insulina y los niveles de péptido C, en conjunto con valores de HOMA-IR, HOMA-B, y QUICKI. Se determinó la asociación entre estos parámetros y la respuesta al tratamiento.
ResultadosCinco (19.2%) de los 26 pacientes analizados (2 [21.4%] con respuesta al tratamiento y 3 [16.6%] sin respuesta al tratamiento) presentaron resistencia a la insulina (p = 1.00). No existieron diferencias significativas entre los pacientes con y sin respuesta al tratamiento respecto a la glucosa, la insulina, los niveles de péptido C o los valores de HOMA-IR, HOMA-B, y QUICKI (p > 0.05).
ConclusionesNo se estableció una asociación significativa entre la resistencia a la insulina y la respuesta al IFN-alfa y ribavirina en niños con hepatitis C crónica.
Hepatitis C virus (HCV) infection commonly leads to chronic liver disease, with an estimated worldwide prevalence of about 3%, signifying that over 170 million people may be affected and at risk for chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma.1
Insulin resistance (IR) is defined as an impaired ability to clear glucose from the circulation, at a given level of circulating insulin.2 There are many studies reporting the incidence of IR in patients with HCV infection and its effect on disease progression and treatment response, carried out predominantly on adult patients.2–19
The standard treatment for HCV infection consists of the combination of interferon (INF) plus ribavirin (RBV). Several viral and host factors, such as baseline viral load, steatosis, obesity, IR, type 2 diabetes mellitus (DM2), age, sex, ethnicity, and genotypes, have been associated with nonresponse to treatment. Nonalcoholic fatty liver disease and IR are the major determinants of fibrosis progression and response to antiviral therapy.2
In the present study, IR and its association with the response to treatment with IFN-alpha and ribavirin in children with chronic hepatitis C (CHC) were determined.
Materials and methodsTwenty-six children diagnosed with HCV and followed up between 1998 and 2014 at the Division of Pediatric Gastroenterology were prospectively evaluated. The patients with hepatic decompensation, concurrent hepatitis B infection, autoimmune hepatitis, and metabolic diseases, such as hemochromatosis and α1-antitrypsin deficiency, were excluded from the study. None of the patients had DM or a history of DM in first-degree relatives or were receiving drugs that interact with glucose metabolism.
All patients had a baseline complete blood count, underwent biochemical testing for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphotase (ALP), fasting glucose, insulin, and c-peptide levels, and were tested for anti-HCV and HCV RNA. Anti-HCV was measured using a commercial enzyme immunoassay (Cobas 8000modular analyzer, Roche Diagnostics, Mannheim, Germany). Serum insulin and C-peptide were determined by electroimmunoassay (Cobas8000 modular analyzer, Roche Diagnostics, Mannheim, Germany). The homeostasis model assessment for insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated within 6 months of chronic infection detection, before beginning treatment.
CHC diagnosis was based on elevated levels of ALT and the detection of anti-HCV antibodies and HCV RNA, by ELISA and RT-PCR, respectively. Liver biopsy was performed according to the Menghini technique and liver involvement stage and grade were scored according to the Knodell histology activity index (HAI), in all cases. All of the patients were treated with IFN-alpha and RBV. Treatment response was defined as loss of HCV RNA 6 months after the completion of therapy.
IR was calculated using the HOMA method, with the following equation: insulin resistance (HOMA-IR) = fasting insulin (μU/mL) x fasting glucose (mmol/l)/22.5.20 Patients with HOMA-IR levels >2.5 were considered insulin-resistant.
The HOMA estimating β-cell function as a percentage (HOMA-B%) was calculated as 20 × fasting insulin (μU/mL)/(fasting glucose [mmol]/l − 3.5), assuming that normal young adults have 100% β-cell function.20
The QUICKI was calculated from fasting plasma glucose (FPG) and fasting immunoreactive insulin (FIRI) levels, according to the report by Katz et al.,21 with the formula: QUICKI = 1/(log [FIRI in mU/l] + log [FPG in mg/dl]).
Statistical analysisThe statistical analysis was performed using SPSS 11.0 software (SPSS Inc, Chicago, IL, USA). Results were expressed as means ± SD and percentage. The analysis was conducted using unpaired Student’s t tests, the Fisher’s exact test, chi-square test, and ANOVA. Statistical significance was set at a p < 0.05.
Ethical considerationsWritten statements of informed consent were obtained from all of the patients’ parents, before performing biopsy and the other procedures. The experiments were only carried out in humans. The authors followed the protocols of their work center on the publication of patient data, preserving patient anonymity. The authors declare that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013). The study was approved by the hospital ethics committee (17-09-2019/1349).
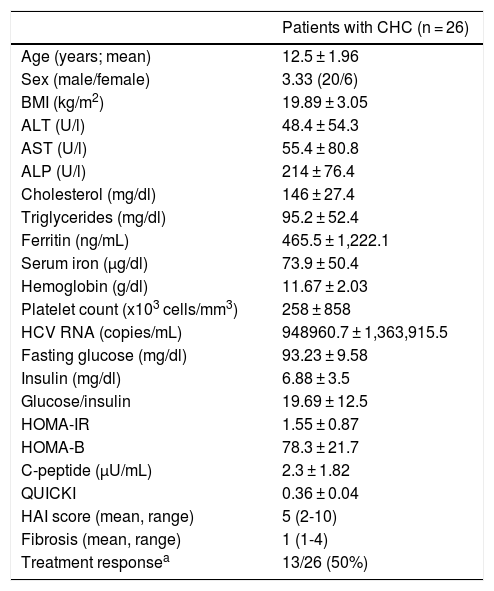
ResultsPatient age ranged from 8.5 years to 16 years (mean 12.5 ± 1.96), and the male:female ratio was 3.33. Five (19.2%) of the 26 patients included in the study had IR: 2 of the 13 patients with treatment response and 3 of the 13 patients with no treatment response (p = 1.00). The demographic and clinical characteristics of the patients are shown in Table 1.
Characteristics and laboratory findings in children with hepatitis C.
| Patients with CHC (n = 26) | |
|---|---|
| Age (years; mean) | 12.5 ± 1.96 |
| Sex (male/female) | 3.33 (20/6) |
| BMI (kg/m2) | 19.89 ± 3.05 |
| ALT (U/l) | 48.4 ± 54.3 |
| AST (U/l) | 55.4 ± 80.8 |
| ALP (U/l) | 214 ± 76.4 |
| Cholesterol (mg/dl) | 146 ± 27.4 |
| Triglycerides (mg/dl) | 95.2 ± 52.4 |
| Ferritin (ng/mL) | 465.5 ± 1,222.1 |
| Serum iron (µg/dl) | 73.9 ± 50.4 |
| Hemoglobin (g/dl) | 11.67 ± 2.03 |
| Platelet count (x103 cells/mm3) | 258 ± 858 |
| HCV RNA (copies/mL) | 948960.7 ± 1,363,915.5 |
| Fasting glucose (mg/dl) | 93.23 ± 9.58 |
| Insulin (mg/dl) | 6.88 ± 3.5 |
| Glucose/insulin | 19.69 ± 12.5 |
| HOMA-IR | 1.55 ± 0.87 |
| HOMA-B | 78.3 ± 21.7 |
| C-peptide (µU/mL) | 2.3 ± 1.82 |
| QUICKI | 0.36 ± 0.04 |
| HAI score (mean, range) | 5 (2-10) |
| Fibrosis (mean, range) | 1 (1-4) |
| Treatment responsea | 13/26 (50%) |
ALP: alkaline phosphatase; ALT: alanine aminotranferase; AST: aspartate aminotransferase; CHC: chronic hepatitis C; HAI: hepatic activity index; HOMA-B: homeostasis model assessment of beta-cell function; HOMA-IR: homeostasis model assessment of insulin resistance; QUICKI: quantitative. insulin sensitivity check index.
No significant differences were observed in the HOMA-IR, HOMA-B, or QUICKI values, or in the fasting glucose, insulin, and c-peptide levels, according to liver disease stage and grade (p > 0.05).
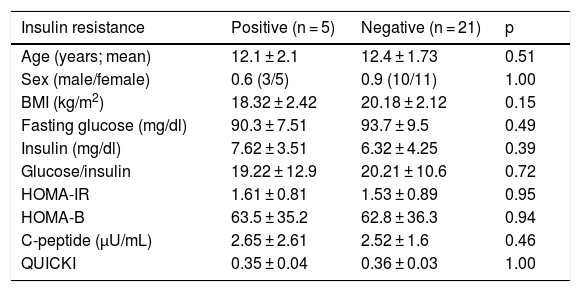
The patients with and without treatment response had no significant differences regarding age, sex, BMI, HCV viral load, ALT levels, or liver disease stage and grade (p > 0.05). There were no significant differences between the groups with and without treatment response, in terms of fasting glucose, insulin, or C-peptide levels, or regarding IR, HOMA-IR, HOMA-B, or QUICKI values, nor were there significant differences in the patients with IR in the two groups in relation to age, sex, BMI, HCV viral load, ALT levels, liver disease stage and grade, fasting glucose, insulin, or c-peptide levels, or with respect to HOMA-IR, HOMA-B, or QUICKI values (p > 0.05). The comparison of patients, according to IR, is shown in Table 2.
Comparison of the patients with hepatitis C, according to insulin resistance.
| Insulin resistance | Positive (n = 5) | Negative (n = 21) | p |
|---|---|---|---|
| Age (years; mean) | 12.1 ± 2.1 | 12.4 ± 1.73 | 0.51 |
| Sex (male/female) | 0.6 (3/5) | 0.9 (10/11) | 1.00 |
| BMI (kg/m2) | 18.32 ± 2.42 | 20.18 ± 2.12 | 0.15 |
| Fasting glucose (mg/dl) | 90.3 ± 7.51 | 93.7 ± 9.5 | 0.49 |
| Insulin (mg/dl) | 7.62 ± 3.51 | 6.32 ± 4.25 | 0.39 |
| Glucose/insulin | 19.22 ± 12.9 | 20.21 ± 10.6 | 0.72 |
| HOMA-IR | 1.61 ± 0.81 | 1.53 ± 0.89 | 0.95 |
| HOMA-B | 63.5 ± 35.2 | 62.8 ± 36.3 | 0.94 |
| C-peptide (µU/mL) | 2.65 ± 2.61 | 2.52 ± 1.6 | 0.46 |
| QUICKI | 0.35 ± 0.04 | 0.36 ± 0.03 | 1.00 |
A p < 0.05 was statistically significant.
Patients with chronic hepatitis have impaired glucose metabolism, with hyperinsulinemia that has been shown to be due to decreased insulin catabolism, rather than pancreatic hypersecretion22,23 or IR.16 Up to 60-80% of patients with cirrhosis have glucose intolerance, and 20% of them develop DM.24 An increase in incidence and prevalence of DM has been reported in HCV infection.10,12
The precise mechanisms of HCV-associated IR are unclear. HCV induces IR through several pathogenetic mechanisms, including both viral and host factors. Not all patients with CHC develop IR, suggesting a complex interaction between those factors, which are also implicated in treatment resistance and an increase in fibrosis progression.
HCV can directly induce IR5,8 because the viral proteins can directly interfere with intracellular insulin signalling.5 The chronic inflammatory response can indirectly induce IR due to increased levels of interleukin (IL)-1, tumor necrosis factor (TNF)-α, and IL-6.2,5,25–27 Oxidative stress due to HCV infection5,25 and β-cell dysfunction have also been reported to possibly contribute to IR in CHC. Increased hepatic SOCS-3 expression is predictive for the outcome of anti-viral therapy in patients with HCV infection.28
IR impairs the sustained response rate to IFN plus RBV in patients with CHC.4,6,7,18 The effect of sustained virologic response (SVR) on IR was first demonstrated in 50 nondiabetic patients treated with IFN and RBV.4 In the sustained responders, HOMA-IR decreased significantly by the end of follow-up, compared with pretreatment values, but nonresponders experienced no significant change in IR.
A reduction in HOMA-IR in patients with HCV and IR that had treatment response and achieved SVR has been described.3,4,11,13,14 Aghemo et al.29 reported that the achievement of SVR with IFN and RBV prevented the development of de novo IR. Treatment failure and a 10% increase in body mass index were significantly associated with the development of de novo IR in nondiabetic patients with CHC. There is also evidence that improved HCV-related IR results in a reduced risk of subsequent DM and a reduction in liver-related complications and mortality.3,13,14 Contrastingly, Fattovich et al.30 reported that HOMA-IR is not a predictor of SVR, regardless of the presence of the HCV genotype in patients with CHC. In our study, no relation was established between IR and treatment response.
Interactions between HCV, IR, steatosis, and hepatic fibrosis are complex and genotype-specific. IR is most strongly associated with HCV genotype 1.25 The presence of IR in CHC predicts nonresponse to antiviral therapy, in addition to a predisposition to DM, in genotype 1,4,6 genotype 2, and genotype 3 infections.5,18,19 In contrast, Bortoletto et al.31 demonstrated that SVR was reduced in patients with an HOMA-IR > 3, but that difference was not sustained when analyzed for HCV genotypes 1, 2, and 3. Eslam et al.15 reported that elevated HOMA-IR was associated with a lower cure rate in HCV patients treated with PEG-INF and RBV, regardless of viral genotype. A limitation of our study was the fact that we could not make a genotype comparison because all of our patients had genotype 1 HCV. Other limitations were a single measurement of the HOMA-IR and QUICKI, instead of multiple measurements, to evaluate changes over time, and the fact that our study was conducted at only one hospital center, on a small number of patients.
There is no consensus regarding the HOMA-IR cutoff value that defines IR, given that different values (e.g., 2, 2.5, 2.7, and 3) are employed in the literature. The patients in our study that had HOMA-IR levels > 2.5 were considered insulin-resistant.
Even though several factors associated with low treatment response rates cannot be modified, such as sex, ethnicity, age, and genotype, there are other preventable risk factors, such as steatosis, IR, and obesity, that can be controlled to achieve SVR.
In conclusion, we found no significant association between treatment response and IR in children with HCV. Further studies with larger study groups and longer follow-up periods are needed to clarify whether IR plays an important role in treatment response in pediatric patients with HCV, as it does in adult patients.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Urganci N, Kalyoncu D, Geylani-Gulec S. Resistencia a la insulina en niños con hepatitis C crónica y su asociación con la respuesta al IFN-alfa y ribavirina. Revista de Gastroenterología de México. 2021;86:140–144.