Levo-pantoprazole, the S-enantiomer of pantoprazole, is a proton pump inhibitor that has been shown in animal studies to be faster and stronger than its racemic formulation. There are no studies on humans and therefore our aim was to evaluate the effects of levo-pantoprazole versus racemic pantoprazole on intragastric pH.
Materials and methodsA randomized controlled study was conducted on patients with erosive gastroesophageal reflux disease that were given 20mg of levo-pantoprazole (n = 15) versus 40mg of racemic pantoprazole (n = 15) for 7 days. Baseline and end-of-treatment symptom evaluation and intragastric pH measurement were carried out.
ResultsThere were no differences between the groups in the baseline evaluations. From 40 to 115min after the first dose of levo-pantoprazole, the mean intragastric pH was higher, compared with that of racemic pantoprazole (p < 0.05). After one week, levo-pantoprazole and racemic pantoprazole significantly reduced intragastric acid production and its esophageal exposure (p < 0.05). Even though there was no statistically significant difference, a larger number of patients that received levo-pantoprazole stated that their heartburn improved within the first 3 days.
ConclusionsThe S-enantiomer of pantoprazole (levo-pantoprazole) had a faster and stronger effect with respect to acid suppression, compared with its racemic formulation. Although the effect on symptoms was faster with levo-pantoprazole, occurring within the first days of treatment, it was equivalent to that of the racemate at one week of treatment.
El S-enantiómero del pantoprazol, el levopantoprazol, es un inhibidor de la bomba de protones que en estudios animales ha mostrado ser más rápido y potente que su formulación racémica. Sin embargo, no existen estudios en humanos por lo que nuestro objetivo fue evaluar los efectos sobre el pH intragástrico de levopantoprazol versus de pantoprazol racémico.
Material y métodosEstudio aleatorizado controlado en pacientes con enfermedad por reflujo gastroesofágico erosivo a quienes se les administró 20mg de levopantoprazol (n=15) versus 40mg de pantoprazol racémico (n=15) durante 7 días. De forma basal y al final del tratamiento se realizó evaluación sintomática y medición del pH intragástrico.
ResultadosNo hubo diferencias entre los grupos en las evaluaciones realizadas de forma basal. A partir de los 40 minutos y hasta los 115 minutos posterior a la primera dosis de levopantoprazol el pH intragástrico promedio fue mayor en comparación que el pantoprazol racémico (p<0.05). Después de una semana, levopantoprazol y pantoprazol racémico redujeron de forma significativa la exposición esofágica y la producción intragástrica de ácido (p<0.05). Aunque no hubo una diferencia significativa, una mayor proporción de pacientes que recibieron levopantoprazol reportaron mejoría de la pirosis en los primeros 3 días.
ConclusionesEl enantiómero S del pantoprazol (levopantoprazol) tiene un efecto más rápido y potente sobre la supresión de ácido en comparación con su formulación racémica. El efecto sobre los síntomas, aunque es más rápido en los primeros días con levopantoprazol, es equivalente a el racemato después de una semana de tratamiento.
Proton pump inhibitors (PPIs) produce more long-lasting and efficacious acid suppression than other classes of drugs utilized for the treatment of acid-related diseases.1 Thus, PPIs are considered the treatment of choice for peptic ulcer disease, gastroesophageal reflux disease, and other states of gastric acid hypersecretion, such as Zollinger-Ellison syndrome. 2–4
In 1989, omeprazole, the first drug of that group, appeared, followed by lansoprazole (1995), rabeprazole (1999), pantoprazole (2000), and more recently, ilaprazole (2003).5–7 To have faster pharmacokinetic effects, the structure of those molecules were then modified, giving rise to the intravenous formulations. The subsequent goal in the pharmacologic development of PPIs was to have longer-lasting effects, which was achieved through formulations with magnesium (omeprazole, esomeprazole, and pantoprazole), the use of isomers, and delayed release presentations (esomeprazole and dexlansoprazole).7–9 Even though all PPIs are efficacious in the management of acid suppression, studies show varying rates in relation to intragastric pH control and clinical response. It should be stated that the percentage time for which intragastric pH is > 4 is the most widely used of all the parameters for correlating PPI efficacy with their capacity to suppress acid.10 For example, in patients with erosive GERD, healing rates are higher, the longer the intervals at which intragastric pH > 4 is maintained.
Despite the fact that, in general terms, the effects of all PPIs could be considered equivalent (as long as comparable doses are utilized), there are some differences that confer certain advantages to some molecules, in particular.6 For example, pantoprazole undergoes less hepatic metabolism, with the consequent lower risk for medication interaction.6,11–12 Rabeprazole and esomeprazole have shown faster action in controlling symptoms and higher esophagitis cure rates have been reported with esomeprazole.6,11–13
Chirality is a practically ubiquitous property in the molecules of basic amino acids, carbohydrates, and fats that are components of the human organism and other life forms.14 The two forms of a chiral molecule are called enantiomers, isomers, or stereoisomers. Each of the molecules of a chiral or enantiomeric pair has an identical chemical composition and can be represented similarly on a two-dimensional plane. However, their chirality produces significant differences in the way in which each enantiomer interacts with other molecules at the receptor level. Consequently, the effects of one enantiomer are different from those observed when a mixture of both enantiomers of a chiral molecule (a racemate or racemic formulation) is used.14
The currently available PPIs are racemic benzimidazoles that contain the “R” (dextrorotary) and “S” (levorotary) enantiomers at a proportion of 1:1.15 Each of those enantiomers has distinct pharmacokinetic and pharmacodynamic properties, and based on those different properties, S-pantoprazole (or levo-pantoprazole), considered a chirally pure PPI, has recently been developed.16–18
Studies on animals have shown that levo-pantoprazole is absorbed more quickly and can be stronger (1.5 to 1.9 times) and more efficacious (3 to 4 times) than its racemic formulation.17–18 A controlled clinical trial on 369 patients showed that 20mg de levo-pantoprazole was more efficacious than 40mg of racemic pantoprazole in the remission of heartburn and regurgitation at 28 days.19 However, there are no studies on humans evaluating intragastric pH behavior after the administration of 20mg of levo-pantoprazole, compared with 40mg of its racemic formulation.
The primary aim of the present study was to evaluate whether the administration of 20mg of levo-pantoprazole was equivalent to or better than 40mg of racemic pantoprazole in suppressing intragastric acid, initially and at 7 days of treatment in patients with erosive GERD. The secondary aim was to evaluate the effect of the two drugs on GERD symptoms.
Materials and methodsStudy populationA randomized controlled study was conducted on consecutive patients recently diagnosed with erosive GERD that came to our hospital center. Patients that had esophageal erosions found at endoscopy (Los Angeles classification grades A-B),20 had heartburn as a primary symptom in the clinical evaluation, and that were not under treatment with a PPI were included.
Study protocol and interventionsFor the baseline data, the demographic characteristics of all the subjects were evaluated and they answered a validated Spanish version of the GERD-Q® questionnaire.21 That instrument consists of 6 questions related to the symptoms or situations associated with GERD and has a maximum score of 18. The result is considered positive if the patient has a score > 8. Heartburn (considered the most typical symptom of GERD) intensity was also evaluated, utilizing a Likert scale from 0 to 3 (0=nothing, 1=mild, 2=moderate, and 3= severe). Then (day 0), after an 8h fast, all the patients underwent high-resolution esophageal manometry (Given, Yoqneam, Israel) to accurately locate the esophagogastric junction (EGJ). To perform the 24h esophageal impedance-pH monitoring (Sandhill, Denver, Colorado, USA) on the patients, a two-sensor catheter (a 10cm intragastric sensor under the EGJ and a 5cm sensor above the EGJ) was introduced transnasally. On the following morning (day 1), before the pH monitoring system was removed, the subjects were randomized to receive 20mg of levo-pantoprazole or 40mg of racemic sodium pantoprazole. The randomization was performed by an independent researcher via a computer program that created a 1:1 intervention allocation ratio. The treatment allocations were kept in sealed envelopes and the researcher did not know beforehand which drug he was going to prescribe to the patient. Once the interventions were allocated, the patients took the medication. They remained fasting for 2h, after which they had a standardized breakfast (150ml of orange juice, 2 pieces of toast, and 2 scrambled eggs with ham), continuing the pH monitoring for one more hour. The pH monitoring system was then removed, and the patient was instructed to take the assigned medication 30min before breakfast for the next 6 days. During that period, the patients recorded the presence of heartburn at the end of the day, utilizing the Likert scale (0 to 3). On the last treatment day (day 7), the patients returned for a second esophageal pH monitoring study, following the protocol described above.
Parameters evaluatedAt the baseline and throughout the study, the presence and intensity of heartburn was evaluated as previously described. Improvement was considered when there was a decrease of at least one point on the Likert scale, in relation to the baseline score. In accordance with the data from the 24h monitoring studies, the following baseline and final parameters in the two groups were evaluated and compared: the %time for which intragastric pH was > 4, the %time for which intragastric pH was < 4 (normal < 4.2%), and the DeMeester score (normal < 14.7). On day 1, specifically after the administration of the first dose of the assigned drug, intragastric pH was evaluated at 5min intervals for the first 2hours (the hours of fasting) and at 15min intervals during the hour after breakfast was eaten.
Statistical analysisDescriptive statistics were employed, utilizing the chi-square test, the Mann-Whitney U test, and the Wilcoxon signed rank test, as appropriate, for the comparison between groups. All the differences were considered significant with a p < 0.05. The analysis was carried out with SPSS version 21.0 (SPSS Inc, Chicago IL, USA) software. A sample size of 15 patients per group was calculated (80% power, alpha error 0.05), assuming a 50% difference in intragastric pH at some point within the first 2h of medication administration.
Ethical disclosuresThe patients signed statements of informed consent to participate as volunteers in the present study. We, the authors, declare we have followed the protocols of our work center regarding the publication of patient data, absolutely maintaining patient confidentiality and anonymity.
The study was approved by the Research and Ethics Committee of the Instituto de Investigaciones Médico-Biológicas of the Universidad Veracruzana (013-2016) and was carried out within the time frame of January 1, 2016 and May 31, 2016.
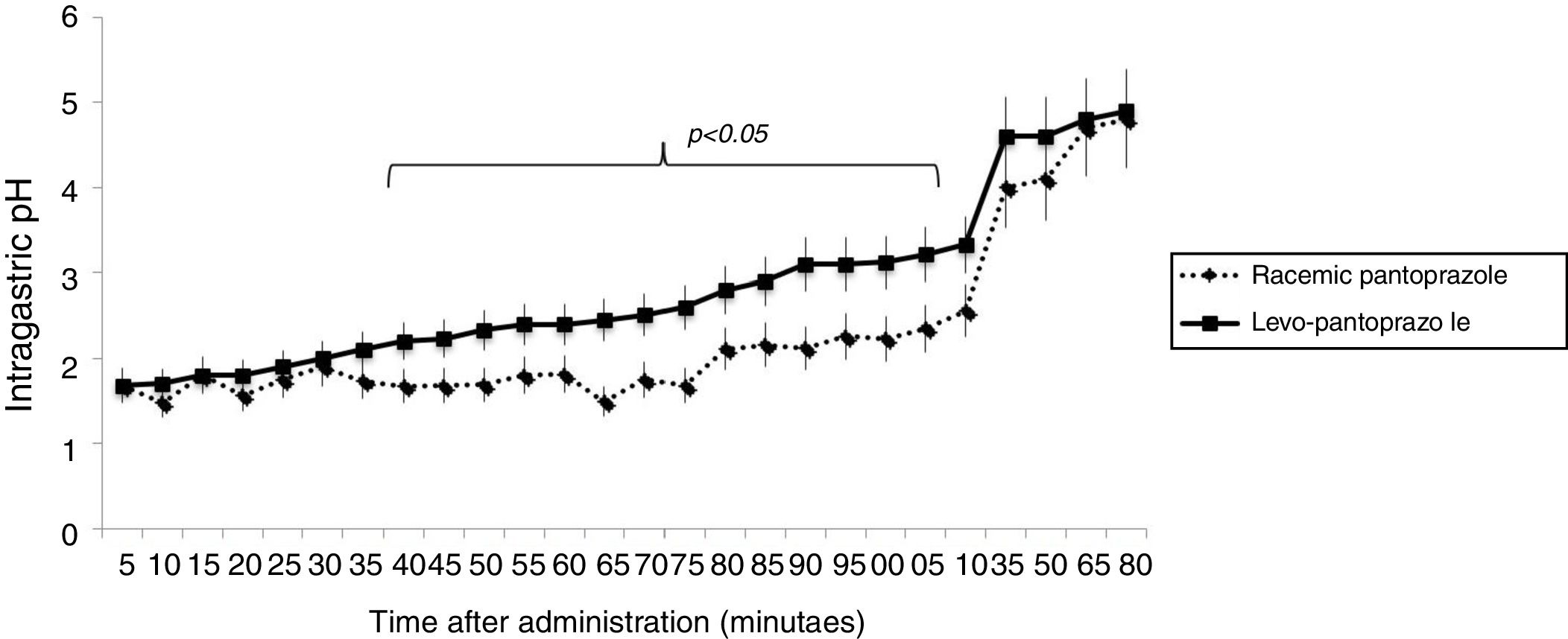
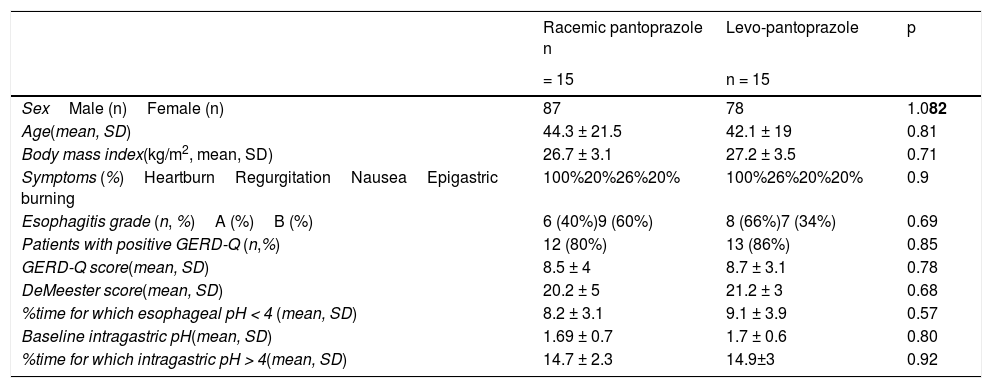
ResultsThe demographic characteristics, the GERD-Q scores, and the pH monitoring study parameters of the two groups are shown in Table 1. There were no statistically significant differences between groups. Figure 1 shows the mean intragastric pH at 5min intervals for 3hours, from the administration of the first dose of 20mg levo-pantoprazole or 40mg of racemic pantoprazole. Curve behavior was similar for the first 35min (p > 0.05), but from 40min to 115min, the mean intragastric pH was significantly higher in the patients that received levo-pantoprazole (p < 0.05). From 120min, when breakfast was administered, and up to one hour afterwards, the mean intragastric pH in the patients that received levo-pantoprazole and racemic pantoprazole was similar (p > 0.05).
Sociodemographic characteristics and 24h pH study findings in the baseline evaluation of the study groups.
| Racemic pantoprazole n | Levo-pantoprazole | p | |
|---|---|---|---|
| = 15 | n = 15 | ||
| SexMale (n)Female (n) | 87 | 78 | 1.082 |
| Age(mean, SD) | 44.3 ± 21.5 | 42.1 ± 19 | 0.81 |
| Body mass index(kg/m2, mean, SD) | 26.7 ± 3.1 | 27.2 ± 3.5 | 0.71 |
| Symptoms (%)HeartburnRegurgitationNauseaEpigastric burning | 100%20%26%20% | 100%26%20%20% | 0.9 |
| Esophagitis grade (n, %)A (%)B (%) | 6 (40%)9 (60%) | 8 (66%)7 (34%) | 0.69 |
| Patients with positive GERD-Q (n,%) | 12 (80%) | 13 (86%) | 0.85 |
| GERD-Q score(mean, SD) | 8.5 ± 4 | 8.7 ± 3.1 | 0.78 |
| DeMeester score(mean, SD) | 20.2 ± 5 | 21.2 ± 3 | 0.68 |
| %time for which esophageal pH < 4 (mean, SD) | 8.2 ± 3.1 | 9.1 ± 3.9 | 0.57 |
| Baseline intragastric pH(mean, SD) | 1.69 ± 0.7 | 1.7 ± 0.6 | 0.80 |
| %time for which intragastric pH > 4(mean, SD) | 14.7 ± 2.3 | 14.9±3 | 0.92 |
SD: standard deviation
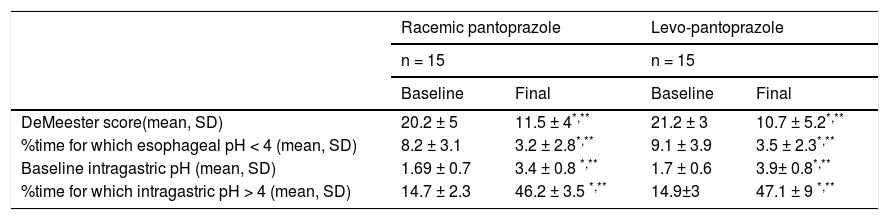
Both levo-pantoprazole and racemic pantoprazole significantly reduced esophageal exposure to acid and intragastric acid production (parameters evaluated in the pH study) after 7 days of treatment (Table 2). The percentage time for which intragastric pH was > 4 was also significantly higher after one week (p < 0.001), compared with the baseline values in the 2 groups, with 47.1% and 46.2% for levo-pantoprazole and racemic pantoprazole, respectively. Upon comparing the effects of the two medications with each other, there were no differences (Table 2). Likewise, the GERD-Q score decreased after 7 days of treatment in the patients that received levo-pantoprazole (8.7 ± 3.1 vs. 3.9 ± 2.9, p = 0.001), as well as in those that received racemic pantoprazole (8.5 ± 4 vs. 4.2 ± 1.8, p = 0.001). There was no difference between levo-pantoprazole and racemic pantoprazole after 7 days of treatment in relation to the GERD-Q score comparison (p = 0.65), thus the efficacy of the two medications was considered equivalent.
24h pH study parameters before and after the intervention in each group.
| Racemic pantoprazole | Levo-pantoprazole | |||
|---|---|---|---|---|
| n = 15 | n = 15 | |||
| Baseline | Final | Baseline | Final | |
| DeMeester score(mean, SD) | 20.2 ± 5 | 11.5 ± 4*,** | 21.2 ± 3 | 10.7 ± 5.2*,** |
| %time for which esophageal pH < 4 (mean, SD) | 8.2 ± 3.1 | 3.2 ± 2.8*,** | 9.1 ± 3.9 | 3.5 ± 2.3*,** |
| Baseline intragastric pH (mean, SD) | 1.69 ± 0.7 | 3.4 ± 0.8 *,** | 1.7 ± 0.6 | 3.9± 0.8*,** |
| %time for which intragastric pH > 4 (mean, SD) | 14.7 ± 2.3 | 46.2 ± 3.5 *,** | 14.9±3 | 47.1 ± 9 *,** |
SD: standard deviation.
0.001 baseline vs final (Wilcoxon test).
**p>0.05 final racemic pantoprazole vs. final levo-pantoprazole (Mann-Whitney U test).
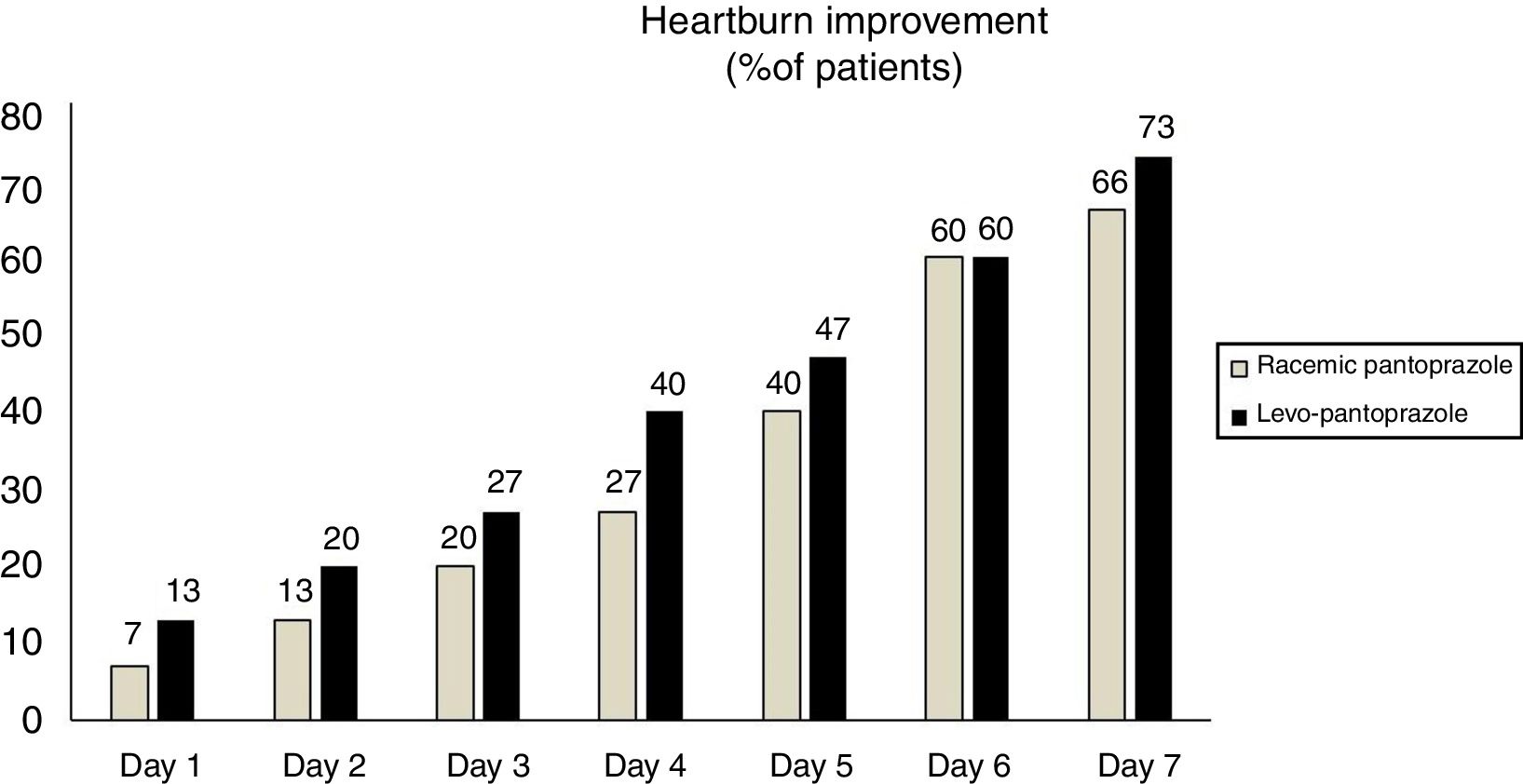
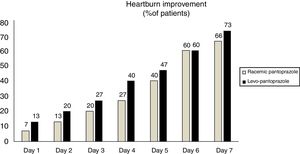
With respect to the primary symptom (heartburn), a larger number of patients that received levo-pantoprazole stated that their heartburn improved within the first 4 days, albeit with no statistically significant difference (fig. 2). There was also no statistically significant difference regarding the symptoms of nausea (p = 0.87), epigastric burning sensation (p = 0.56), and regurgitation (p = 0.9), during the 7 days.
All the patients completed the treatment and 2 of the patients that received levo-pantoprazole stated they experienced effects related to the medication (one reported headache and the other diarrhea that resolved the first day), whereas 2 of the patients that received racemic pantoprazole had a side effect (one reported nausea and the other headache).
Discussion and conclusionThe present study evaluated the acute and 7-day effects that the administration of the S-isomer of pantoprazole (levo-pantoprazole) or its racemic formulation had on intragastric pH. Behavior was different during the first hours, but it was equivalent at the end of the evaluation period. The increase in intragastric pH with levo-pantoprazole use was significantly higher than its racemic formulation at 40min from the first dose and the difference was maintained for 75 more minutes, showing that levo-pantoprazole was the molecule that acted more quickly and strongly. It should be mentioned that the effect of the increase above 4 in intragastric pH that was reached in both groups at 120min after drug administration, was the result of the administration of breakfast.
Even though there is evidence in animal models that levo-pantoprazole is faster and stronger than its racemic formulation, our study is the first to demonstrate said effect in humans. For example, in an animal model, Cao et al.17–18 showed that the racemic mixture of pantoprazole was absorbed in 0.5h, whereas the levo-pantoprazole enantiomer was absorbed in only 5min. They also showed that the area under the curve analysis produced by levo-pantoprazole was 1.5 times higher than that of the racemate.17–18
The use of an isomer of a PPI has been described to have pharmacokinetic and pharmacodynamic advantages that increase the strength of its effect.22 Esomeprazole (the S-enantiomer of omeprazole), dexlansoprazole, and R (+) rabeprazole are good examples that have been shown to have a clinical benefit and the same safety in patients that did not have a clinical response to their conventional formulations (racemic mixtures).7–9,22 Nevertheless, it is important to state that in the case of esomeprazole, its clinical efficacy became evident at a dose corresponding to the double of the dose of its racemic formulation. In other words, 40mg de esomeprazole was the equivalent of 20mg of omeprazole.5 In the case of levo-pantoprazole, it should be pointed out that clinical efficacy and equivalence became evident at half the dose of its racemic formulation, signifying that 20mg of levo-pantoprazole was the equivalent of 40mg of racemic pantoprazole. The decrease in the therapeutic dose of a PPI due to chiral purification reduces the metabolic burden on the body, potentially making it safer.14,15
Pantoprazole is completely metabolized in the liver, utilizing the cytochrome P (CYP) 450 pathway, specifically through CYP 2C19 and CYP 3A4, and 80% of its inactive metabolites are excreted by the kidneys. Studies utilizing the expression of human isoenzymes of CYP450 have revealed that the metabolism of one enantiomer of a molecule is significantly affected by the presence of its other enantiomer.23-25 In the case of pantoprazole, the difference between the plasma concentrations of its two enantiomers was minimal in the fast metabolizers (FMs), but substantial in the slow metabolizers (SMs).25-26 The R-enantiomers showed greater variability in the substrates metabolized by CYPC 2C19 than the S isomers, especially in the SMs, as opposed to the FMs.27-28 That difference resulted in greater concentrations of the R-enantiomer in the SMs, which could increase the probability of adverse effects or medication interactions. In addition, the S-enantiomer can be metabolized through alternate metabolic pathways, such as CYP 3A4 and other sulfonyl transferases. Thus, it appears that the pharmacokinetics of levo-pantoprazole is less dependent on the CYP 2C19 polymorphisms, resulting in plasma levels that can be safer and more stable, compared with its racemic formulation. Even though the prevalence of SMs and FMs is not known in Mexico, the use of levo-pantoprazole could be considered one of the safer and more efficacious options in the prescription of a PPI. However, further studies are needed that are conducted specifically on Mexican populations, evaluating the effect and safety of levo-pantoprazole and its relation to the CYP 450 isoenzymes.
The increase that PPIs produce on intragastric pH, especially if it is > 4, is known to be correlated with cicatrization of the esophagus, but its correlation with symptom improvement is less evident. Since 1992, and thanks to a meta-analysis carried out by Bell et al.,3] the importance of the increased intragastric pH and esophageal healing has been emphasized, but the total time of intragastric pH required for optimum cicatrization to be achieved is not precisely known. In our study, at the end of a week of treatment, the percentage time for which intragastric pH was > 4 was 47.1% and 46.2%, for levo-pantoprazole and racemic pantoprazole, respectively. Those percentages are comparable to the ones described by Miner et al.9 after the fifth administration of standard doses of esomeprazole (58.43%), rabeprazole (50.53%), omeprazole (49.16%), lansoprazole (47.98%), and sodium pantoprazole (41.94%). In a prospective study conducted by Cho et al.30 on 149 patients that were randomized to receive levo-pantoprazole or racemic pantoprazole, they showed that the wound-healing percentage for esophagitis was 85% and 84% at 4 weeks, and 94% and 97% at 8 weeks, respectively. Thus, the expected cicatrization percentage would be above 80% with 4 weeks of treatment.
In our study, there was no statistically significant difference between the two formulations in the number of patients that reached clinical improvement (heartburn) and we could conclude that their efficacy was equivalent. However, it is important to note that during the first 4 days of treatment, heartburn improved in a higher number of patients receiving levo-pantoprazole. That could be explained by the pharmacologic findings reported in our study, specifically the fact that levo-pantoprazole has a faster effect than its racemic formulation. With respect to the other symptoms of nausea, epigastric burning sensation, and regurgitation, there were no differences between the two treatments, which was probably due to the short period of time of the study.
Clinical improvement with levo-pantoprazole, compared with its racemic formulation, has been previously demonstrated. In a phase IV study conducted on 280 patients in India, there was a significant decrease in the frequency and severity of heartburn, regurgitation, nausea, epigastric pain, and abdominal pain after 14 days of the administration of 20mg of levo-pantoprazole (p < 0.0001).31 Pai et al.19 reported that 64% of the patients that took 20mg of pantoprazole had symptom improvement after 14 days of treatment, compared with 57% of the patients that took racemic pantoprazole, whereas after 28 days, improvement was 86% and 74%, respectively. Importantly, longer-term studies on Mexican populations are needed to evaluate whether there are differences in efficacy between 20mg of levo-pantoprazole and 40mg of its racemic formulation beyond 7 days.
The two formulations of pantoprazole utilized in our study had similar results, with respect to safety and side effects, and none of the patients had to suspend either drug. In the study by Jain et al.,31 levo-pantoprazole was well-tolerated, and no patient had to suspend treatment due to adverse events, such as headache, abdominal pain, flatulence, diarrhea, nausea/vomiting, or erythema/pruritus. The incidence of adverse events was 6.43% on day 14 and 1.79% on day 28.
Among the limitations of our study, one was the fact that, as stated above, longer-term studies are needed to evaluate the clinical efficacy of pantoprazole in the Mexican population. In addition, even though it was not the primary aim of the study, we decided to carry out the clinical evaluation based on heartburn, the typical symptom most associated with GERD. Nevertheless, it should be emphasized that the effect of levo-pantoprazole on symptomatology that includes regurgitation, dyspeptic symptoms, and other extraesophageal manifestations, needs to be evaluated. On the other hand, even though we utilized a probe that enabled the measurement of intraluminal esophageal impedance, it is known that the diagnostic gain of that technique is for those patients that present with refractory GERD, in whom it is necessary to document whether symptoms are associated with episodes of non-acid reflux or not. Given that our patients were treatment-naïve, and the majority presented with typical symptoms, said evaluation was not a primary aim of the present analysis, but that type of evaluation would be interesting in future studies.
In conclusion, our study showed that the S-enantiomer of pantoprazole (levo-pantoprazole) had a faster and stronger effect, in relation to acid suppression, compared with its racemic formulation. Although the effect on symptoms was faster with levo-pantoprazole during the first days of treatment, it was equivalent to that of the racemate after one week of treatment.
Financial disclosureThe present study was financed by a grant for researcher initiative support from Laboratorios SANFER de México.
Conflict of interestDr. José María Remes-Troche is a member of the Advisory Board of Takeda Pharmaceuticals and Asofarma. He received development and research grants from Sanfer, Asofarma, CONACYT, and the Universidad Veracruzana. He is a speaker for Takeda, Asofarma, Sanfer, Carnot, Alfasigma, and Dr. Schär. Dr. Mercedes Amieva-Balmori is a speaker for Takeda, Sanfer, and Chinoin. Dr. Fausto Daniel García-García, Dr. Gabriela Rojas-Loureiro, Dr. Xaira Rivera-Gutiérrez, and the medicinal chemist/chemical biologist (QFB) declare that they have no conflict of interest.
Please cite this article as: Remes-Troche JM, García García FD, Rojas-Loureiro G, Rivera-Gutiérrez X, Reyes-Huerta J, Amieva-Balmori M. Efecto sobre el pH intragástrico de 20 mg de levopantoprazol versus 40 mg de pantoprazol racémico durante los primeros 7 días de tratamiento en pacientes con enfermedad por reflujo gastroesofágico. Revista de Gastroenterología de México. 2020;85:48–55.