Thiopurine-related leukopenia is associated with polymorphisms in the thiopurine methyltransferase (TPMT) and nucleoside diphosphate-linked moiety X type motif 15 (NUDT15) genes. However, those polymorphisms explain only a fraction of thiopurine-related leukopenia. Our aim was to study the role of an inosine triphosphate pyrophosphatase (ITPA) polymorphism in patients with inflammatory bowel disease (IBD) and thiopurine-related leukopenia that was unexplained by the TPMT and NUDT15 polymorphisms.
Material and methodsWe enrolled consecutive IBD patients on thiopurines (azathioprine or 6-mercaptopurine) from January 2019–March 2020, at a tertiary care center in North India. The presence of the ITPA (C.94C > A) polymorphism was evaluated in all patients, along with its association with thiopurine-related leukopenia.
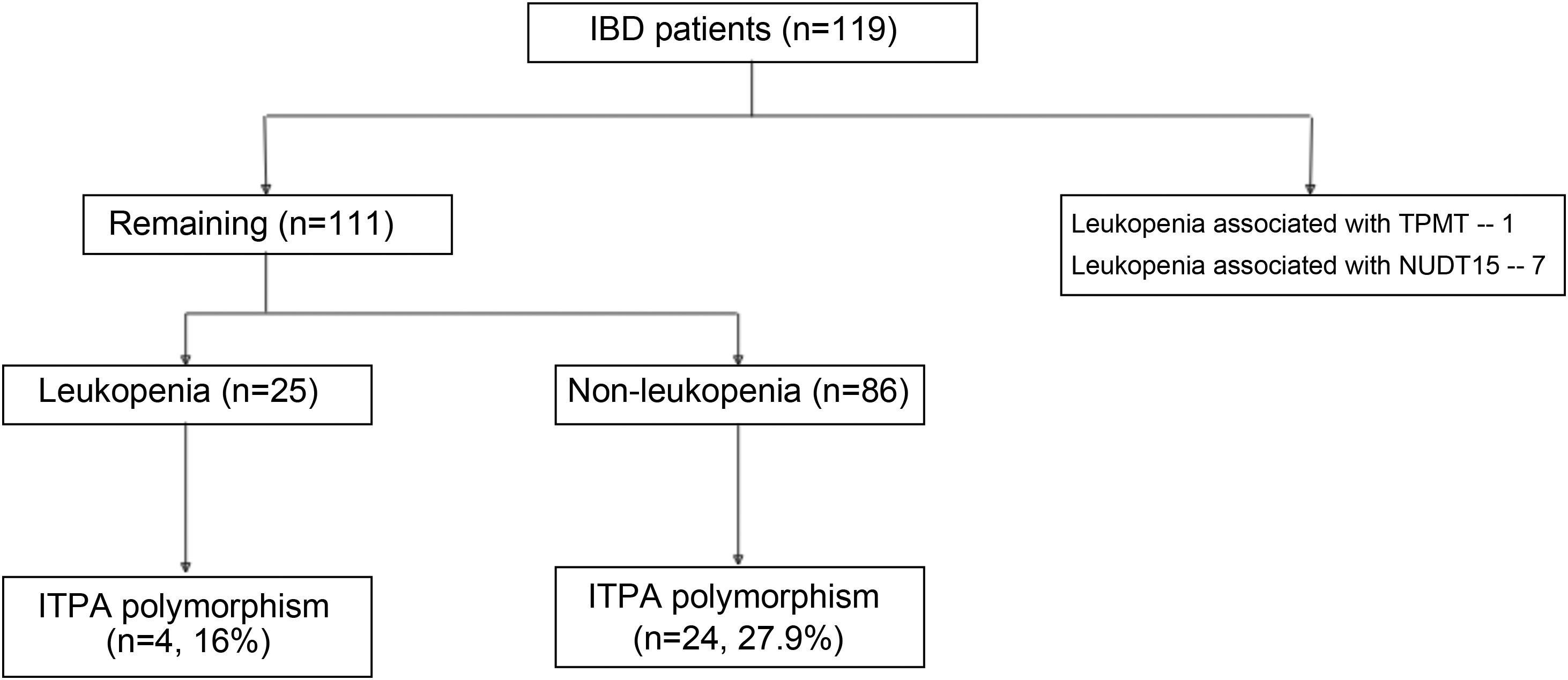
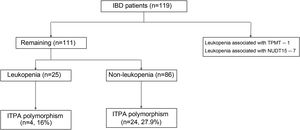
ResultsOf the 33 patients (from a total of 119 patients) that developed leukopenia, 8 had the TPMT (n = 1) or NUDT15 (n = 7) polymorphism. Of the remaining 111 patients, their mean age was 36.36 ± 13.54 years and 57 (51.3%) were males. Twenty-five (21.01%) had unexplained leukopenia. The ITPA polymorphism was detected in 4 (16%) patients in the unexplained leukopenia group and 24 (27.9%) patients in the non-leukopenia group (p = 0.228). The odds ratio for predicting leukopenia with the ITPA polymorphism was 0.4921 (95% CI 0.1520–1.5830, p = 0.234).
ConclusionThe ITPA (C.94C > A) polymorphism was frequently detected in the study population but was not predictive for leukopenia in patients with IBD on thiopurine therapy.
La leucopenia relacionada con tiopurina está asociada con polimorfismos en la tiopurina metiltransferasa (TPMT, por sus siglas en inglés) y con el gen NUDT15. Sin embargo, estos polimorfismos explican solo una fracción de la leucopenia relacionada con tiopurina. Nuestro objetivo fue estudiar el papel de un polimorfismo de pirofosfatasa inosina trifosfato (ITPA, por sus siglas en inglés) en pacientes con enfermedad inflamatoria intestinal (EII) y la leucopenia relacionada con tiopurina que no fue explicada por los polimorfismos de TPMT o NUDT15.
Material y métodosReclutamos a pacientes consecutivos con EII tratados con tiopurinas (azatioprina o 6-mercaptopurina) entre enero del 2019 y marzo del 2020, en un centro de tercer nivel en el norte de la India. La presencia de polimorfismo en ITPA (C.94C > A) fue evaluada en todos los pacientes, junto con su asociación a la leucopenia relacionada con tiopurina.
ResultadosDe los 33 pacientes (de un total de 119 pacientes) que desarrollaron leucopenia, 8 presentaron polimorfismo en TPMT (n = 1) o NUDT15 (n = 7). De los 111 pacientes restantes, la edad promedio fue de 36.36 ± 13.54 años y 57 (51.3%) fueron hombres. Veinticinco (21.01%) presentaron leucopenia no explicada. El polimorfismo en ITPA fue detectado en 4 (16%) pacientes del grupo de leucopenia no explicada y en 24 (27.9%) pacientes del grupo sin leucopenia (p = 0.228). La razón de momios para predecir la leucopenia con polimorfismo en ITPA fue 0.4921 (95% IC 0.1520–1.5830, p = 0.234).
ConclusiónEl polimorfismo en ITPA (C.94C > A) fue frecuentemente detectado en la población de estudio pero no fue predictivo para leucopenia en pacientes con EII en terapia con tiopurina.
Thiopurines are an important therapy in the management of many autoimmune conditions (autoimmune hepatitis, inflammatory bowel disease [IBD], sarcoidosis, etc.), malignancies (lymphomas), and post-transplantation immunosuppression. The metabolism of thiopurines is complex, with numerous basic intermediates, such as nucleoside triphosphates, 6-thio-GTP, and 6-thio-dGTP. Polymorphisms that impact the activity of thiopurine methyltransferase (TPMT) could lead to leukopenia. NUDT15 gene polymorphisms have also been reported to be more important in East Asian and South Asian populations1,2. However, not all events of cytopenia are explained by the polymorphisms of those 2 genes, and many other genes have been proposed as possible reasons for thiopurine-related cytopenia.
The inosine triphosphate pyrophosphatase (ITPA) enzyme aids in the metabolism of azathioprine and 6-mercaptopurine. The information in the literature on the role of ITPA polymorphisms in causing thiopurine-related leukopenia is conflicting3–5. Initial reports suggested that ITPA mutations were associated with leukopenia, but those studies were primarily conducted in the Western world, including the Netherlands, Germany, the United Kingdom, and Venezuela3–5. However, a meta-analysis that included studies from Sweden, the Netherlands, and New Zealand showed no association between ITPA polymorphisms and side effects of thiopurines, such as myelosuppression5,6.
There is no available data regarding the relevance of ITPA polymorphisms in South Asian patients, including India. To address this knowledge gap, our aim was to study the impact of ITPA mutations on patients with IBD at a center in North India. We included the IBD patients that did not have the TPMT and NUDT15 polymorphisms, evaluating whether the presence of the ITPA (C.94C > A) polymorphism had any association with thiopurine-related leukopenia.
Material and methodsStudy group and designWe conducted a prospective observational study to evaluate the impact of ITPA polymorphisms in predicting thiopurine-related leukopenia. The study was structured, in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study included cases (individuals with IBD that were on thiopurine therapy and developed leukopenia) and controls (patients with IBD on thiopurine therapy that did not develop leukopenia) from the cohort of a previous study on TPMT and NUDT15 polymorphisms2. Only patients that had unexplained leukopenia (given that they did not have the TPMT or NUDT15 polymorphisms) were included as cases.
Enrollment and follow-upWe enrolled consecutive IBD patients from January 2019-March 2020 that were on thiopurines (azathioprine or 6-mercaptopurine). The study was conducted at a tertiary care center in North India. Only the patients that had received thiopurines for at least 3 months or more were included. All patients underwent a clinical history interview, clinical examination, and studies, after their informed consent. The diagnosis of ulcerative colitis or Crohn’s disease was based on the ECCO guidelines, along with the use of a combination of clinical, endoscopic, radiologic, and histologic assessment7,8. Leukopenia was defined as a total white blood cell count below 3000 per microliter. The patients were monitored for adverse events and blood tests were carried out at regular intervals at follow-up. Azathioprine was started at 1 mg/kg and 6-MP was started at 0.5 mg/kg. If there was no leukopenia, the dose was increased to 2 mg/kg for azathioprine and 1 mg/kg for 6-MP. Complete blood counts were performed every week for one month and then monthly, if normal.
Sample size was calculated to maintain the possibility that 20% of the patients on thiopurines would develop cytopenia and half of them would have a detectable mutation, compared with 10% of patients that would not develop cytopenia. With a power of 80% and a 2-sided confidence level of 95%, the calculated sample size was 78.
Inosine triphosphate pyrophosphatase polymorphism analysisThe blood samples taken in EDTA tubes were utilized for DNA extraction, using the QIAamp DNA blood kit (Qiagen Inc.), and tested for thiopurine analogue metabolism-related polymorphisms (TPMT, NUDT15, and ITPA). For ITPA genotyping (C.94C > A), exon 2 and exon 3 primers were used to amplify the genomic DNA. The 328-base pair product was digested with endonuclease NspI at 37 degrees Celsius. Agarose gel at 2% was used to resolve the fragments.
Statistical analysisAll data were collected on Excel® sheets. The statistical analysis was performed, using SPSS® 23.0 (SPSS Inc, USA) software. A p value of less than 0.05 was considered statistically significant. We used the chi-square test to compare the categorical variables. Because the data were not normally distributed, we used the Mann-Whitney U test to compare the continuous data.
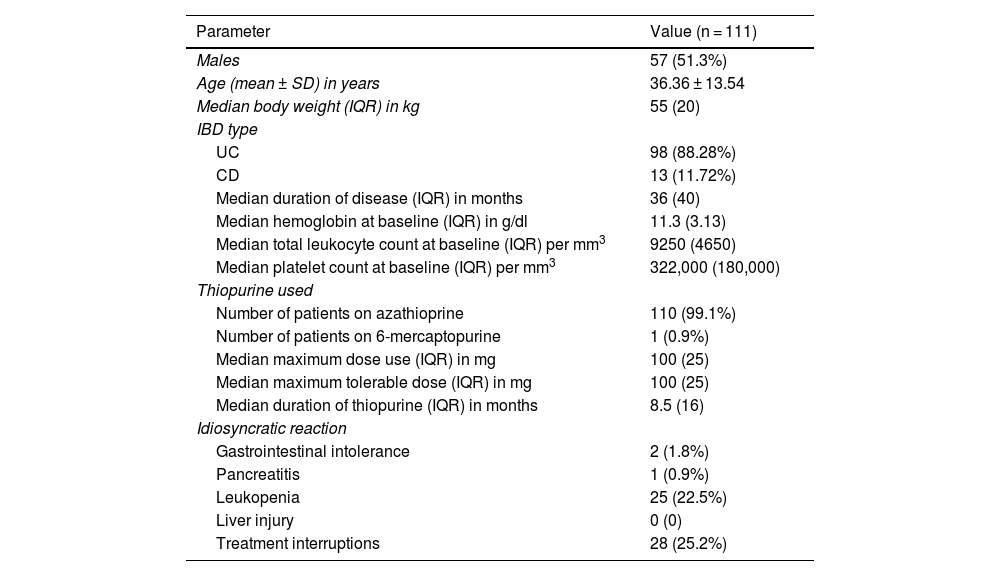
ResultsA total of 119 patients on thiopurines at our IBD clinic were initially selected. Leukopenia (WBC count < 3000/mm3) was seen in 33 (27.73%) patients. Of the 33 patients with leukopenia, 8 patients had the NUDT15 or TPMT polymorphisms, explaining the leukopenia. The TPMT mutation was seen in one patient with leukopenia, whereas the NUDT15 polymorphism was seen in 7. Therefore, 111 patients were included in the present study to assess the ITPA polymorphism (Fig. 1). The mean age of the patients was 36.36 ± 13.54 years, and 51.3% of the cases were males (Table 1). Regarding disease characteristics, 88.28% of the cases presented with ulcerative colitis and the remaining patients had Crohn’s disease. The median disease duration was 36 months, the median duration of thiopurine therapy was 8.5 months, and the median maximum tolerated dose was 100 mg.
Baseline characteristics of the study population.
| Parameter | Value (n = 111) |
|---|---|
| Males | 57 (51.3%) |
| Age (mean ± SD) in years | 36.36 ± 13.54 |
| Median body weight (IQR) in kg | 55 (20) |
| IBD type | |
| UC | 98 (88.28%) |
| CD | 13 (11.72%) |
| Median duration of disease (IQR) in months | 36 (40) |
| Median hemoglobin at baseline (IQR) in g/dl | 11.3 (3.13) |
| Median total leukocyte count at baseline (IQR) per mm3 | 9250 (4650) |
| Median platelet count at baseline (IQR) per mm3 | 322,000 (180,000) |
| Thiopurine used | |
| Number of patients on azathioprine | 110 (99.1%) |
| Number of patients on 6-mercaptopurine | 1 (0.9%) |
| Median maximum dose use (IQR) in mg | 100 (25) |
| Median maximum tolerable dose (IQR) in mg | 100 (25) |
| Median duration of thiopurine (IQR) in months | 8.5 (16) |
| Idiosyncratic reaction | |
| Gastrointestinal intolerance | 2 (1.8%) |
| Pancreatitis | 1 (0.9%) |
| Leukopenia | 25 (22.5%) |
| Liver injury | 0 (0) |
| Treatment interruptions | 28 (25.2%) |
CD: Crohn’s disease; IBD: inflammatory bowel disease; IQR: interquartile range; SD: standard deviation; UC: ulcerative colitis.
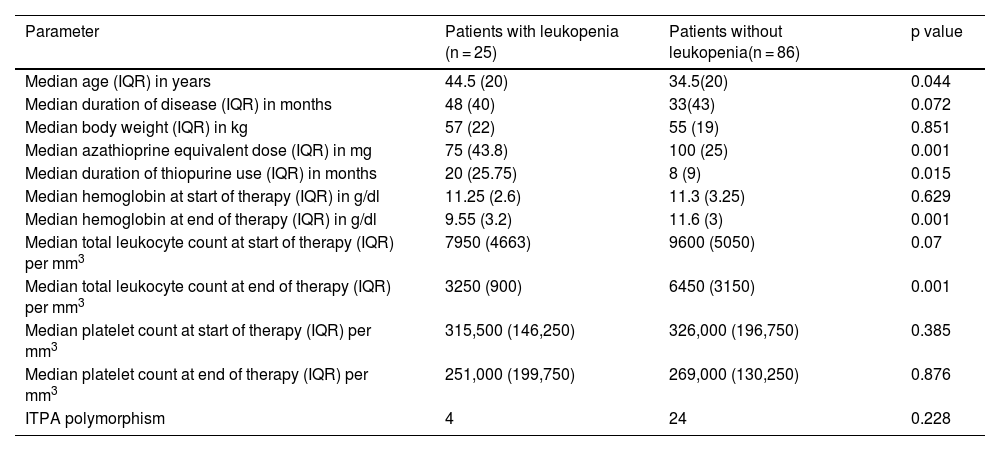
Of the 111 patients, 25 (21.01%) presented with unexplained leukopenia (Table 2). Four patients (16%) that developed leukopenia had the ITPA polymorphism (C.94C > A). Of the patients that did not develop leukopenia while taking thiopurines, 24 (27.9%) presented with the ITPA polymorphism. There was no significant association between the presence of leukopenia and the ITPA polymorphism (p = 0.228). Patients with IBD that had leukopenia were on lower tolerated azathioprine equivalent doses (p = 0.001) and also had a lower duration of thiopurine therapy (p = 0.015).
Comparison of patients with inflammatory bowel disease, with and without leukopenia.
| Parameter | Patients with leukopenia (n = 25) | Patients without leukopenia(n = 86) | p value |
|---|---|---|---|
| Median age (IQR) in years | 44.5 (20) | 34.5(20) | 0.044 |
| Median duration of disease (IQR) in months | 48 (40) | 33(43) | 0.072 |
| Median body weight (IQR) in kg | 57 (22) | 55 (19) | 0.851 |
| Median azathioprine equivalent dose (IQR) in mg | 75 (43.8) | 100 (25) | 0.001 |
| Median duration of thiopurine use (IQR) in months | 20 (25.75) | 8 (9) | 0.015 |
| Median hemoglobin at start of therapy (IQR) in g/dl | 11.25 (2.6) | 11.3 (3.25) | 0.629 |
| Median hemoglobin at end of therapy (IQR) in g/dl | 9.55 (3.2) | 11.6 (3) | 0.001 |
| Median total leukocyte count at start of therapy (IQR) per mm3 | 7950 (4663) | 9600 (5050) | 0.07 |
| Median total leukocyte count at end of therapy (IQR) per mm3 | 3250 (900) | 6450 (3150) | 0.001 |
| Median platelet count at start of therapy (IQR) per mm3 | 315,500 (146,250) | 326,000 (196,750) | 0.385 |
| Median platelet count at end of therapy (IQR) per mm3 | 251,000 (199,750) | 269,000 (130,250) | 0.876 |
| ITPA polymorphism | 4 | 24 | 0.228 |
IQR: interquartile range; ITPA: inosine triphosphate pyrophosphatase.
With respect to the other parameters, there was no significant association between sex (p = 0.326), IBD type (p = 0.143) or risk of idiosyncratic reaction (p = 0.18), and the adverse event of thiopurine-related leukopenia. The odds ratio for predicting leukopenia with the ITPA polymorphism was 0.4921 (95% CI 0.1520–1.5830, p = 0.234).
Discussion and conclusionThiopurines are used for the treatment of IBD because of their role as steroid-sparing agents in long-term maintenance therapy. Thiopurines are known to cause side effects, such as leukopenia, pancreatitis, hepatotoxicity, and gastrointestinal intolerance9. Those adverse events can lead to thiopurine discontinuation or disruption. The role of TPMT testing for thiopurine maintenance is well recognized. NUDT15 mutations are more frequently reported in the South Asian population than TPMT mutations and they are significantly associated with adverse events1. The FDA presently recommends testing for TPMT and NUDT15 in patients that develop severe myelotoxicity or repeated myelosuppression during thiopurine therapy. The role of ITPA in predicting myelosuppression in the South Asian population is largely unknown. In our prospective study on a South Asian population, the ITPA (C.94C > A) polymorphism was detected in 28 patients (25.2%) with IBD, making it a more common polymorphism than NUDT15 or TPMT. The ITPA polymorphism was detected in 16% of the patients that developed leukopenia, compared with 27.9% of the patients that did not develop leukopenia. We demonstrated that the ITPA polymorphism was not a predictor for leukopenia in patients on thiopurine therapy.
Inosine triphosphate pyrophosphatase helps convert inosine triphosphate (ITP) to inosine monophosphate (IMP). Patients on thiopurine with ITPA polymorphisms may have an increased accumulation of ITP. However, the clinical significance of those changes is unclear. The gene location for ITPA is chromosome 20. There are at least 5 single nucleotide polymorphisms reported for ITPA10. G138A, G561A, and G708A are usually silent, whereas the two remaining polymorphisms (C94A and IVS2 + A21C) are associated with reduced ITPA activity. The most commonly reported ITPA polymorphism is C94A. The mutations can be homozygous or heterozygous. ITPA deficiency leads to the accumulation of ITP nucleotides and toxic 6-thio-ITP. Theoretically, ITPA polymorphisms can cause cytopenia.
Asians (14-19%) harbor ITPA mutations more commonly than Whites and Africans (6-7%)11. A study of 63 patients with acute lymphoblastic leukemia from Northern India found an ITPA mutation in 17.5%12. Another report on 82 Lithuanian patients with IBD found no statistically significant impact of ITPA polymorphisms on leukopenia13. A study on 232 patients with IBD from Venezuela revealed a positive correlation between an ITPA polymorphism and azathioprine-related adverse events, such as myelosuppression and joint pain14. A Japanese study revealed that seven patients with IBD (from a total of 19 cases with an ITPA polymorphism) developed leukopenia15. A recent large Chinese study on 1419 patients with various diseases found no statistically significant association of the ITPA (94C > A) polymorphism with leukopenia16, but those authors recommended ITPA and NUDT15 genotype testing, before starting thiopurine therapy. They attributed various epigenetic factors associated with NUDT15 to the occurrence of adverse events. In our study, ITPA mutations were found in 25.2% of patients. We found no association between ITPA mutations and leukopenia. Therefore, testing for ITPA polymorphisms prior to the start of thiopurine therapy in Asian populations, such as ours, might not be clinically helpful in predicting leukopenia.
The strength of our study lies in the fact that it reports on the ITPA (C.94C > A) polymorphism in patients with IBD in South Asia. An important limitation of the study is its short follow-up of the patients on thiopurines. A long-term follow-up period is necessary for monitoring the side effects of thiopurines, given that leukopenia could also occur later in the disease course. Because azathioprine biologically converts to 6-MP and the dose relationship between the two is linear, we have included the single patient in whom 6-MP was used.
In addition, the polymorphism analyses are relevant to both drugs. To ensure that the inclusion of that patient did not impact the dose-related analysis, we used the equivalent azathioprine dose (Table 2) for such an analysis.
In conclusion, ITPA mutations were seen in 25.2% of the Indian population studied and there was no significant association between adverse events and the ITPA polymorphism. The presence of an ITPA polymorphism was not a predictor for leukopenia. We suggest there is no need for routine ITPA testing in Indian patients prior to starting thiopurine therapy.
Ethical considerationsAll patients gave their informed consent to participate in this research. The work complies with the current regulations on bioethical research. This study was conducted after approval from the institutional ethics committee. The authors declare that this article does not contain personal information that can identify the patients.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.