Since the publication of the 2007 dyspepsia guidelines of the Asociación Mexicana de Gastroenterología, there have been significant advances in the knowledge of this disease. A systematic search of the literature in PubMed (01/2007 to 06/2016) was carried out to review and update the 2007 guidelines and to provide new evidence-based recommendations. All high-quality articles in Spanish and English were included. Statements were formulated and voted upon using the Delphi method. The level of evidence and strength of recommendation of each statement were established according to the GRADE system. Thirty-one statements were formulated, voted upon, and graded. New definition, classification, epidemiology, and pathophysiology data were provided and include the following information: Endoscopy should be carried out in cases of uninvestigated dyspepsia when there are alarm symptoms or no response to treatment. Gastric and duodenal biopsies can confirm Helicobacter pylori infection and rule out celiac disease, respectively. Establishing a strong doctor-patient relationship, as well as dietary and lifestyle changes, are useful initial measures. H2-blockers, proton-pump inhibitors, prokinetics, and antidepressants are effective pharmacologic therapies. H.pylori eradication may be effective in a subgroup of patients. There is no evidence that complementary and alternative therapies are beneficial, with the exception of Iberogast and rikkunshito, nor is there evidence on the usefulness of prebiotics, probiotics, or psychologic therapies. The new consensus statements on dyspepsia provide guidelines based on up-to-date evidence. A discussion, level of evidence, and strength of recommendation are presented for each statement.
Desde la publicación de las guías de dispepsia 2007 de la Asociación Mexicana de Gastroenterología ha habido avances significativos en el conocimiento de esta enfermedad. Se realizó una revisión sistemática de la literatura en PubMed (01/2007 a 06/2016) con el fin de revisar y actualizar las guías 2007 y proporcionar nuevas recomendaciones basadas en evidencia. Se incluyeron todas las publicaciones en español e inglés, de alta calidad. Se redactaron enunciados que fueron votados utilizando el método Delphi. Se estableció la calidad de la evidencia y la fuerza de las recomendaciones según el sistema GRADE para cada enunciado. Treinta y un enunciados fueron redactados, votados y calificados. Se informan nuevos datos sobre definición, clasificación, epidemiología y fisiopatología. La endoscopia debe realizarse en dispepsia no investigada cuando hay datos de alarma o falla al tratamiento. Las biopsias gástricas y duodenales permiten confirmar infección por Helicobacter pylori y excluir enfermedad celiaca, respectivamente. Establecer una fuerte relación médico-paciente, cambios en la dieta y en el estilo de vida son útiles como medidas iniciales. Los bloqueadores H2, inhibidores de la bomba de protones, procinéticos y fármacos antidepresivos son efectivos. La erradicación de H.pylori puede ser eficaz en algunos pacientes. Con excepción de Iberogast y rikkunshito, las terapias complementarias y alternativas carecen de beneficio. No existe evidencia con respecto a la utilidad de prebióticos, probióticos o terapias psicológicas. Los nuevos enunciados proporcionan directrices basadas en la evidencia actualizada. Se presenta la discusión, el grado y la fuerza de la recomendación de cada uno de ellos.
Dyspepsia is a symptomatic complex that has a high frequency in the general population.1 It is defined as the presence of chronic and recurrent discomfort in the epigastrium that includes a wide variety of symptoms such as pain, a burning sensation, bloating, early satiety, fullness, burping, nausea, and vomiting that can be continuous or intermittent.2 This syndrome can be the manifestation of different organic, systemic, or metabolic diseases (organic or secondary dyspepsia) or not have an apparent cause (functional dyspepsia). Thus, dyspepsia encompasses a heterogeneous group of diseases whose clinical manifestations are common, but caused by different pathophysiologic mechanisms, and so may require specialized treatment. This condition is a challenge for the physician, due to the frequent overlapping with other digestive syndromes, the erratic therapeutic response, and the constant search for a diagnostic strategy and efficient treatment for each subject.
In 2007, the Asociación Mexicana de Gastroenterología brought together a group of gastroenterologists that formulated the diagnostic and treatment guidelines for dyspepsia.2–5 Since then, new concepts about this disease have emerged in areas such as epidemiology (especially in Mexico), physiopathogenesis, new diagnostic criteria, the correct identification of dyspepsia subgroups, the recognition of overlap with other diseases, the differential diagnosis, and quality studies on the effectiveness of drugs and other treatment alternatives. All these advances justify the creation of a document that complements the 2007 diagnostic and treatment guidelines. In March 2016, the Asociación Mexicana de Gastroenterología summoned the coordinators of this consensus to carry out a review of the advances in the different aspects of dyspepsia, evaluate the evidence, and formulate statements on the current status of this illness.
The aim of this document is to present a consensus review of the most recent information on dyspepsia to bring the diagnostic and treatment guidelines published in 2007 up to date, incorporating the new internationally published scientific evidence, with special emphasis on studies conducted in Mexico.
MethodsThis consensus was formulated utilizing the Delphi method.6 The consensus coordinators carried out a bibliographic review, performing a search with the words “dyspepsia”, “functional dyspepsia”, and “non-ulcer dyspepsia”, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “inflammation”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “therapy”, “management”, “review”, “guidelines” and “meta-analysis”, as well as the equivalent terms in Spanish. The search was conducted in PubMed and included all articles published in English and Spanish within the time frame of January 2007 and June 2016. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses, but was not limited to these types of articles. Complementary manual and electronic searches were carried out in the archives of the Revista de Gastroenterología de México and all the publications up to June 2016 that the coordinators considered relevant.
After each theme was reviewed, a series of statements was formulated that addressed the main aspects of the disease. They were sent to all the members of the consensus group for the first round of anonymous electronic voting. Each statement was voted upon as “in agreement” or “in disagreement”. When agreement was equal to or greater than 75%, the statement was left unchanged for the next voting round. When disagreement was 75% or higher, the statement was eliminated from the document. When statements had less than 75% agreement or disagreement, the coordinator of each theme restructured them, taking the comments of the participants into account. Three rounds of distance electronic voting were carried out and one round of face-to-face voting took place in Culiacán (Sinaloa, Mexico) in June 2016. The final vote was conducted utilizing a 4-point scale: a) complete agreement b) partial agreement, c) partial disagreement, and d) complete disagreement.
Once the final consensus statements were obtained, the coordinators’ task was to establish the level of evidence that sustained each statement and give a recommendation grade, when applicable, employing the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system.7 This system was developed in an effort to overcome the limitations of previous systems, optimizing the evaluation of the quality of evidence and the grading of the strength of the recommendations, and was used in a recently published consensus endorsed by the Asociación Mexicana de Gastroenterología.8 In the GRADE system, the quality of evidence is not established by the methodology, alone, of the study being analyzed, but is also classified based on the design utilized for answering a previously posed specific question.7,9 In this manner, the quality of evidence is defined as “high”, when the publication of new research study results will not modify our confidence in the estimate of effect, as “moderate”, when the publication of new research study results can modify our confidence in the estimate of effect, as “low” when the publication of new research study results will most likely importantly impact our confidence in the estimate of effect, and as “very low” when any estimate of effect is uncertain. The GRADE system also establishes the strength of the recommendations as strong or weak in favor of or against the intervention or statement. The results of this system are expressed through a defined code that uses capital letters for the quality of evidence, followed by a number indicating the strength of the recommendation in favor of or against the intervention or statement.7,9Table 1 shows the GRADE system code.
Classification of the quality of evidence and strength of recommendations according to the GRADE system.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | Code |
|---|---|
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
The final consensus statements are presented below.
Definition, pathophysiology, and epidemiology of dyspepsia in adults1. Dyspepsia is a syndrome that is defined as the presence of chronic and recurrent discomfort in the epigastrium that involves different symptoms, such as pain, burning sensation, bloating, early satiety, fullness, burping, or nauseaDyspepsia is perhaps one of the more confusing terms in gastroenterology, given that it is frequently used indistinctly to refer to functional dyspepsia. It should be remembered that, strictly speaking, dyspepsia refers to a symptomatic complex and not to a diagnosis. Although this concept has been clarified for some time, it frequently continues to be used inappropriately.10,11 In an effort to promote its better use, the consensus group established the use of the term dyspepsia in reference only to a group of symptoms with a possibly common origin. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Dyspeptic symptoms do not enable a reliable distinction between their organic or functional origin to be made. This group of discomforts usually indicates the presence of pathology at the gastroduodenal level, but the symptoms themselves are not sufficient for determining the cause of the underlying disorders.12 The challenge for the physician is to study the patient appropriately and systematically to determine whether there is a structural disease causing the dyspeptic symptoms or whether they are the result of the sole presence of functional dyspepsia. The same as the previous statement, the consensus group considered that the correct usage of these terms is necessary for standardizing the nomenclature used in this disorder GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Functional dyspepsia is defined by the presence of fullness, plenitude, or epigastric pain or burning sensation, with no evidence of organic, metabolic, or systemic diseases that explain those symptoms.13 In general, organic or structural diseases are classified in terms of organ morphology, whereas functional diseases are classified in relation to symptoms and how the patients interpret them.14 Nevertheless, in the last two decades there has been an important change in the knowledge of the underlying pathophysiologic mechanisms that affect the gastroduodenal region.15,16 Numerous molecular, neuronal, and cellular mechanisms have been described that interact with the microenvironment and macroenvironment to modify gastric and intestinal function. These mechanisms are observed in many of the patients affected by dyspepsia, but in themselves, do not explain all the cases. The most recent definition states that gastrointestinal functional disorders are brain-gut interaction disturbances that generate symptoms caused by the combination of any of the following factors: altered motility, visceral hypersensitivity, and alterations of the mucosa, of the immune function, and of the gut microbiota, as well as of central nervous system processing.17 GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
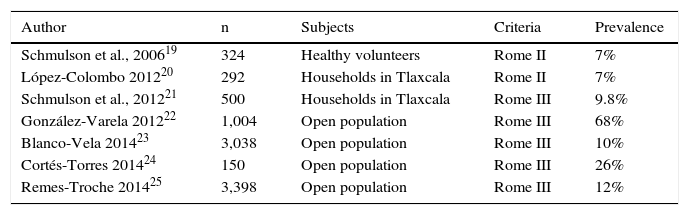
Dyspepsia affects 25-40% of the population at some time of life and is the reason for 3-5% of first-contact care consultations in the United States.1 However, 50-60% of the subjects with dyspepsia have no structural lesions that explain their complaints. The prevalence of uninvestigated dyspepsia widely varies according to the population studied and the definition used to establish its presence. A meta-analysis that included more than 300 international studies estimated the prevalence of uninvestigated dyspepsia to be 21%.18 In Mexico, the prevalence of this disorder has been reported at 7 to 68%19–25 (Table 2). The largest population study conducted in Mexico at present that included more than 3,000 subjects in an open population from 8 different regions of the country reported a 12% prevalence of uninvestigated dyspepsia.26 Because the diagnostic criteria currently require the performance of endoscopy to diagnosis functional dyspepsia, there are no studies that allow its prevalence to be known. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Dyspepsia prevalence studies conducted on the Mexican population.
| Author | n | Subjects | Criteria | Prevalence |
|---|---|---|---|---|
| Schmulson et al., 200619 | 324 | Healthy volunteers | Rome II | 7% |
| López-Colombo 201220 | 292 | Households in Tlaxcala | Rome II | 7% |
| Schmulson et al., 201221 | 500 | Households in Tlaxcala | Rome III | 9.8% |
| González-Varela 201222 | 1,004 | Open population | Rome III | 68% |
| Blanco-Vela 201423 | 3,038 | Open population | Rome III | 10% |
| Cortés-Torres 201424 | 150 | Open population | Rome III | 26% |
| Remes-Troche 201425 | 3,398 | Open population | Rome III | 12% |
As with irritable bowel syndrome, postinfectious dyspepsia has been described as a different entity, based on retrospective studies that demonstrated the existence of a group of patients that developed dyspeptic symptoms after having presented with symptoms of acute gastroenteritis. Cohort studies have confirmed that the risk for presenting with dyspepsia increases 5-fold after one year of having had acute bacterial gastroenteritis.27 Different microbes have been associated with the development of postinfectious dyspepsia, but Salmonella spp., Escherichia coli O157, Campylobacter jejuni, Giardia lamblia, and norovirus stand out among them. A recent meta-analysis that included 19 good-quality studies reported a 9.55% prevalence of functional dyspepsia in adults after infectious gastroenteritis (OR: 2.54, 95% CI: 1.76-3.65).28 Some factors that increase the risk for postinfectious dyspepsia are female sex, younger age, smoking, and associated psycho-morbidity. This entity has been related to the presence of aggregated T cell foci, diminished CD4+ lymphocytes, and increased macrophage counts in the duodenum that persist several months after the acute infection. Despite being conceived as a different entity, there is no specific treatment with confirmed usefulness for this disorder. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 90%. Partial agreement, 10%.
For more than two decades, positive diagnoses of the different functional disorders have been attempted to be made through symptom-based diagnostic criteria, and not by exclusion. However, in uninvestigated dyspepsia, the accepted diagnostic strategy is a thorough clinical history and identification of the alarm signs and symptoms, whose presence indicates the immediate performance of endoscopy.29 Even though this tactic is widely accepted, the value of these alarm signs and symptoms are far from optimal. None of these clinical data has been shown to have high individual diagnostic accuracy.30 A meta-analysis that evaluated the usefulness of immediate endoscopy as a diagnostic strategy in uninvestigated dyspepsia in Asia found that the alarm signs and symptoms had very limited usefulness for diagnosing cancer.31 On the other hand, it is possible to detect organic lesions in one of every 4 patients that meet the Rome III criteria for functional dyspepsia, which is why the sensitivity and specificity of these criteria are considered insufficient.32 A careful evaluation of the patients is clearly necessary to detect other possible associated factors and potential indicators of the presence of organicity. Therefore, the diagnosis of functional dyspepsia can be clinically suspected, applying the new version of the Rome criteria, but it continues to be an exclusion diagnosis requiring the performance of endoscopy.29 GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
The Rome III consensus proposed two substantial changes in the definition of functional dyspepsia. On the one hand, it described this disorder based on 4 main symptoms (pain, burning sensation, fullness, and satiety) for the purpose of increasing the specificity of this symptomatic complex. On the other hand, it established the subdivision of this disorder into two subgroups: epigastric pain syndrome characterized by pain or burning sensation, and the postprandial distress syndrome in the presence of early satiety and fullness. Different studies have suggested that the risk factors and pathophysiology can be different in the two subgroups. For example, Helicobacter pylori (H. pylori) infection, not being married, sleep disturbances, and depression are associated with postprandial distress syndrome.33 Other studies found a higher prevalence of altered gastric accommodation and increased duodenal eosinophils in epigastric pain syndrome.34 Epidemiologic studies confirmed the defined presence of both groups, but the separation was not always clear, due to the great overlapping between them, in the Mexican population, as well.26,35 More recent studies have shown that the subgroups can be reclassified and the overlap between them reduced, when symptom induction through food consumption is recognized.36 The Rome IV criteria have been slightly modified, recognizing that not only postprandial distress syndrome, but also epigastric pain and burning sensation, can be induced or worsened through food consumption. Functional dyspepsia subtype identification has promoted the recognition of distinct underlying pathophysiologic factors and therefore the need for specialized treatment.37 There is increasing evidence supporting this concept and for the time being it is accepted that initial treatment can be selected based on the subgroup. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
The overlap of dyspepsia with these conditions is obvious, not only due to the frequent coexistence of symptoms in the same patient, but also because of the shared pathophysiologic phenomena (e.g. postinfectious states, low-grade inflammation, or motor disorders). In clinical practice, it may not only be artificial, but also impossible, to exclude symptoms such as heartburn, acid regurgitation, vomiting, or the complaints that disappear upon evaluating the clinical criteria of functional dyspepsia.34 One out of every 4 patients with functional dyspepsia shows gastric emptying delay and 86% of the patients with gastroparesis meet the clinical criteria for functional dyspepsia.12 The line that separates functional dyspepsia from gastroparesis is not clearly defined, due to the poor correlation between gastric emptying delay and the pattern and severity of symptoms, as well as to the lack of emptying delay stability over time.38,39 The Rome IV criteria recognize the frequent coexistence of these symptoms in the same patient, but they clearly exclude them from the clinical criteria of functional dyspepsia for the purpose of increasing their specificity.29 GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 85%. Partial agreement, 15%.
H. pylori eradication has consistently shown that it improves dyspeptic symptoms in some patients with functional dyspepsia.40–42 Numerous studies conducted on Asian, European, and American populations have evaluated the potential benefit of eliminating H. pylori in patients with functional dyspepsia and the results have been positive in favor of treatment. A meta-analysis that included 21 trials with more than 3,500 patients showed a consistent therapeutic gain in favor of eradication when compared with placebo, with a number necessary to treat (NNT) of 14.40 A meta-analysis that included 14 randomized and controlled clinical trials containing information on the effect of eradication over a 12-month period showed significant improvement in patients with treated functional dyspepsia, compared with the control group (OR: 1.38; 95% CI: 1.18-1.62, p < 0.0001).41 One study demonstrated that 82% of the patients that achieved eradication of the bacterium had a complete or satisfactory response in relation to their symptom resolution, compared with 62.5% of those with persistent infection.42 Nevertheless, antimicrobial treatment benefits should be considered for each particular case, taking into account the regional resistance rates, costs, and risks. Importantly, symptom improvement from eradication treatment can occur after 6 months, and those cases with good response should not continue to be regarded as part of functional dyspepsia. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
Endoscopy of the proximal digestive tract enables the direct recognition of lesions causing dyspepsia, such as ulcers, erosions, or neoplasias. However, only a minority of subjects with uninvestigated dyspepsia present with significant lesions at endoscopy. A systematic review and meta-analysis that included 9 studies and more than 5,300 patients showed that erosive esophagitis was the most frequently detected abnormality, followed by peptic ulcer (prevalence 13.4 and 8.0%, respectively).43 Because endoscopy is an invasive and costly procedure that has certain risks, applying the selection criteria to identify those subjects that will benefit the most from it is a widely-accepted line of conduct. As mentioned above, the detection of alarm symptoms has low sensitivity, but high specificity, for the detection of malignancy in subjects with dyspepsia.44 Some studies have shown that early endoscopy can be a recommendable strategy in Asian populations, in whom factors such as age and alarm symptoms are not very reliable for detecting malignancy.30 Even though the diagnostic performance of endoscopy in patients with persistent or refractory symptoms has not been established in the Mexican environment, it is currently recommendable to perform the diagnostic study in this context, mainly in patients with no H. pylori infection.45 GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Biopsies can establish the definitive diagnosis in some patients with dyspeptic symptoms and provide complementary information in others. Given that diagnostic endoscopy is frequently performed on patients with treatment-refractory dyspepsia, obtaining the greatest amount of information possible during the procedure should be one of the physician's priorities. The American Gastroenterological Association (AGA) has formulated specific recommendations for the correct use of esophageal, gastric, and duodenal biopsies in patients with no identifiable lesions in the mucosa46,47 and the consensus group considered that these can also be a useful guide in the Mexican environment. The currently available evidence indicates that biopsies taken from the esophagus and the normal-appearing gastroesophageal junction will most probably not identify significant abnormalities or impact the clinical management of those patients.46,47 Given the evidence that at least one group of patients with dyspepsia can benefit from H. pylori eradication, taking biopsies from the gastric body and antrum, even when they appear normal, can be useful if possible H. pylori infection is unknown. This becomes relevant in the patient whose other treatments have failed.46,47 The AGA does not recommend taking duodenal biopsies in the absence of lesions, if there are no other symptoms or signs that increase the risk for detecting celiac disease.46,47 A meta-analysis of 15 studies that reported the prevalence of celiac disease in patients with dyspepsia showed that the prevalence of positive serology and biopsy-confirmed enteropathy were more frequent in dyspeptic subjects, compared with controls, but the results did not reach an important statistical difference.48 A recent review has reproduced those findings and detected at least one group of dyspeptic patients at greater risk for presenting with celiac disease: young women between 20 and 37 years of age.49 More recently, some specific populations have shown an increased prevalence of celiac disease in patients with dyspepsia.50 In addition, even though there is little evidence in the Mexican environment, at least 3 Mexican studies have reported a high prevalence of villous atrophy in patients with dyspepsia studied through systematic endoscopic biopsy,51 increased frequency of lymphocytic infiltration in patients with refractory dyspepsia,52 and a two-fold greater detection of specific diagnoses through duodenal biopsies in subjects with dyspepsia, compared with controls.53 Some experts have suggested that the routine taking of duodenal biopsies can be useful as part of the diagnostic algorithm in those patients.50 Considering the current evidence, the consensus group does not recommend the routine use of duodenal biopsies in patients with dyspepsia, but suggests contemplating the use of this diagnostic tool in subjects that have a failed initial treatment, individualizing each case. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 75%. Partial agreement, 20%. Complete disagreement, 5%.
There is no general agreement about what to consider normal in the endoscopic examination in an adult with dyspeptic symptoms. Some experts consider that gastritis (a histologic term that should not be employed synonymously with dyspepsia), with no erosions, ulcers, or associated neoplasia, forms part of the clinical spectrum of functional dyspepsia.54 What is very clear, is that the detection of structural lesions (erosions, ulcers, or neoplasias) in any segment of the proximal gastrointestinal tract at endoscopy has an impact on the diagnosis, prognosis, and treatment of the disease.45 The value and impact of recognizing minimal lesions in the integrated management of the patient with dyspepsia is unknown. Interobserver agreement is low, regardless of the experience of the endoscopist, for detecting lesions based solely on the aspect and coloration of the mucosa.55–57 Until there are clearly defined and standardized classification systems, findings such as redness, pallor, nodularity, increased vascular pattern, and erythematous stippling have no clear value in the integrated management of the patient with dyspeptic symptoms and do not rule out the diagnosis of functional dyspepsia. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Determining whether the disorder has an organic cause or is a functional disorder, cannot be discerned through the dyspeptic symptoms, alone. The challenge for the physician is to identify the clinical characteristics and risk factors that lead to the suspicion of underlying structural or organic disorders. Even though abdominal ultrasound is an accessible method, there is very little information on its utility in the diagnostic process of the patient with dyspepsia. One study evaluated the diagnostic usefulness of endoscopy and abdominal ultrasound in 709 patients seen consecutively for different functional disorders (Rome III).58 The diagnosis of functional disease was confirmed in 61% of the patients and 39% had some type of organic disease. There are consensus criteria for establishing the presence of gastric emptying delay and gastroparesis that can be useful in the differential diagnosis of subjects with dyspepsia.59 The main usefulness of tomography is in the detection of organic lesions and anatomic changes associated with or causing the symptoms. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
All these diagnostic methods have shown sensory (lower sensory threshold, early fullness, early satiety, or epigastric pain) and motor (emptying delay or accommodation disorders) alterations more frequently in cases with dyspepsia, compared with controls.60–62 However, some of these methods have not been standardized, show no correlation between the different functional parameters, and no association with symptoms or treatment response has been established with them. Therefore, their routine use in daily clinical practice is not recommended. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
A biomarker is a normal functioning, objective, biologic indicator of pathogenic processes or pharmacologic responses to a therapeutic intervention and its potential and practical usefulness has been studied in other functional disorders.8 The main purpose of such research is to be able to directly diagnose functional dyspepsia, doing away with the long and costly diagnostic process of exclusion. Regarding dyspepsia, there are studies that have evaluated numerous biomarkers, such as pepsinogen, genetic polymorphisms, interleukins, visceral hypersensitivity, H. pylori infection, eosinophilia, and duodenal lymphocytosis, among others.63–68 Nevertheless, there are currently no biomarkers that allow a direct diagnosis of functional dyspepsia to be made. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Functional dyspepsia is a benign condition in which half of the patients show improvement or symptom disappearance over time.69 Functional dyspepsia is not associated with an increase in mortality in the community.70 Therefore, efforts should be directed at achieving adequate symptom control and improving quality of life, or reversing the deterioration that is frequently observed in those patients.26 GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Assuring the patient that the condition is benign has been shown to be useful in irritable bowel syndrome, but there are no studies confirming this in relation to functional dyspepsia. Modifying lifestyle has been suggested as a way to improve the symptoms of functional dyspepsia.71 A study conducted in Sweden showed there was a correlation between lifestyle changes and reduced symptoms over time, and one of the factors significantly associated with improvement was the suspension of smoking.72 A large number of patients with functional gastrointestinal disorders, including functional dyspepsia, state that their symptoms are triggered by the ingestion of certain foods. Even though dietary surveys have shown that food consumption is similar between patients with functional dyspepsia and controls, dyspeptic patients ingest a lower quantity of fat.73 This association suggests that diet plays a role in the treatment of functional dyspepsia, albeit the relation is difficult to define. Other factors involved in the development of symptoms are stressful situations, psychologic alterations, and psychiatric disorders. Epidemiologic studies have shown a greater prevalence of anxiety and depression in patients with functional dyspepsia, compared with healthy controls, as well as the important role of personality traits, previous stressful life events, and sleep alterations.74,75 A strong doctor-patient relationship is important as part of management to be able to go into details about personal events that could influence symptomatology. GRADE level of evidence and strength of recommendation: C1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Given the very complex pathophysiology of the functional gastrointestinal disorders, including functional dyspepsia, the possibility of placebo response is high. A Cochrane systematic review published in 2006 in patients in whom the term “non-ulcerous dyspepsia” was still applied, symptom response to placebo was from 30-40%.76 Later studies reported improvement in 19-49% of the cases of functional dyspepsia and a Cochrane review estimated a placebo response of 25%, slightly lower than that achieved in 34% of patients with proton pump inhibitors.77 Response factors have not been systematically analyzed and there are no controlled studies of placebo versus non-treatment. Endoscopy in which there are no abnormal findings is thought to have an apparent therapeutic effect.1,12 The consensus group does not recommend the administration of placebo in the treatment of dyspeptic patients, but it is important to know that some patients can report improvement without receiving active medication. This has special importance in the design of studies evaluating therapeutic options in this disease. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 85%. Partial agreement, 5%. Partial disagreement, 5%. Complete disagreement, 5%.
As described above, patients with one or several functional gastrointestinal disorders, very frequently state that their symptoms begin or worsen with the consumption of certain foods,71,73,78 particularly fat and spices, as well as foods with epinephrine or norepinephrine.79 The relation to caffeine, lactose, fructose, or wheat has not been established unequivocally, as it has been in irritable bowel syndrome. Some studies have shown an association between certain foods and a specific symptom. For example, the sensation of fullness has been associated with the consumption of beans, flour, chocolate, wheat, fried food, citrus fruits, and red meat. Bloating has been related to carbonated beverages, onions, and some fruits, such as bananas. Epigastric pain has been associated with chocolate, caffeine, pepper, and onions.78 In patients with dyspepsia, oral intake of fat significantly increases the presence of nausea and pain, compared with the ingestion of glucose, in comparison with healthy subjects, and it has been established that symptoms are associated with an increase in cholecystokinin levels.80 One study that evaluated changes in symptoms related to a specific diet, utilizing a questionnaire for a period of 7 days, showed improvement after reducing the daily consumption of fat and having a longer nocturnal fast.81 Despite this probable association, there is little scientific evidence of its usefulness.82 GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
There are numerous local action medications that include different types of antacids alone and combined, and others based on alginate, bismuth, and sucralfate, as well as medications that affect the formation of gas, such as simethicone and activated carbon. None of these has been shown to be more effective than placebo in the treatment of functional dyspepsia. No adequate studies have been conducted on antacids and alginate.1,12 In a meta-analysis of 5 studies that included 311 patients, bismuth was not better than placebo,83 nor was sucralfate, in the same meta-analysis with 2 controlled trials.83 There was also no difference upon comparing the two medications in 29 patients.84 A study that evaluated 276 patients with functional dyspepsia (Rome III) showed that the combination of simethicone, activated carbon, and magnesium oxide (called Carbosymag in France) was superior to placebo, reducing the intensity of epigastric pain, postprandial fullness, and subjective bloating (p < 0.05), with a NNT of 7, achieving an overall reduction of dyspeptic symptoms of 70%.85 However, there are no similar later studies supporting these findings, and therefore the evidence is insufficient for recommending their use. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%
Among the pathophysiologic mechanisms of functional dyspepsia, duodenal hypersensitivity to hydrochloric acid and its altered depuration have been described as capable of causing symptoms, particularly in epigastric pain syndrome. The antisecretory agents such as the H2 antagonists (H2As) and proton pump inhibitors (PPIs) block the normal secretion of gastric acid and reduce the associated symptoms. A Cochrane review with 10 studies and 3,347 patients showed superiority of both medication groups compared with placebo. PPIs obtained a symptomatic response of 34 vs 25% of the placebo, with a relative risk (RR) for symptom persistence of 0.87 (95% CI: 0.80-0.96) and a NNT of 10. The H2As had a RR for symptom persistence of 0.77 (95% CI: 0.65-0.92) and a NNT of 7. Most of the studies included in the meta-analysis used the Rome II criteria.77 A sub-analysis showed that PPIs improved only pain or symptoms that were similar to reflux. A second meta-analysis86 reported a reduction in the RR of 10.3% and a NNT of 14.6. Significant efficacy varied according to the dyspepsia subtype, so that “ulcer-type dyspepsia”, as it used to be called, had a RR reduction of 12.8%, the so-called “reflux-type dyspepsia” a RR reduction of 19.7%, and there was no benefit in the “dysmotility-type dyspepsia” or in nonspecific dyspepsia.87 A Japanese study with rabeprazole showed improvement, regardless of dyspepsia subtype,88 and subsequent studies reported an average improvement effect with PPIs of 32-68%.1,12 There are few later studies on H2As, but a more recent analysis with nizatidine described a therapeutic gain of 60% over placebo, including a beneficial effect on postprandial fullness, early satiety, and gastric emptying.89 GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
Another mechanism associated with symptoms in functional dyspepsia is the presence of abnormalities in gastric emptying and an abnormal receptive fundic relaxation after food consumption. Different prokinetics have been tested in functional dyspepsia. A meta-analysis that included 24 studies and that analyzed the effect of cisapride, domperidone, and itopride versus placebo found a 33% reduction of symptoms and a NNT of 6.76 Nevertheless, this study has been criticized for primarily including small studies, with dysmotility-type dyspepsia. A later Cochrane review that included 19 controlled trials with a total of 3,178 patients showed a RR reduction of symptom persistence of 33% (95% CI: 18-45%), but most of the trials that were included evaluated cisapride, which was taken off the market in the United States at the time of publication of the meta-analysis.77 Upon analyzing each prokinetic separately, short and medium-term effectiveness is good but long-term effectiveness is limited by side effects. Cisapride, a 5-HT4 agonist, was shown to be better than placebo, but was removed from the majority of the markets due to its effect on the QT segment of the electrocardiogram.90 Metoclopramide and domperidone are dopamine D2 receptor antagonists that block dopamine in the enteric nervous system, with a consequent increase in gastric emptying and motility. The two drugs also act at the central level, suppressing nausea and vomiting.79 Both metoclopramide and domperidone have been shown to be superior to placebo in controlled trials and meta-analyses with treatment durations of 4 to 6 weeks. However, their use is associated with side effects. Metoclopramide can cause various extrapyramidal symptoms by inhibiting the central dopaminergic system. In the short term it can cause trembling or acute dystonic reactions, in the mid term, hyperprolactinemia and galactorrhea, and in the mid and long term it can cause potentially irreversible effects such as late dyskinesia. Special permission is required to use domperidone in the United States,79 and like metoclopramide, it can cause hyperprolactinemia. Unlike metoclopramide, it does not cross the blood-brain barrier and therefore does not cause neurologic effects. Levosulpiride is another benzamide-derived dopamine D2 receptor antagonist whose effectiveness is similar to that of cisapride. It achieved symptomatic improvement of 33% in a randomized trial that included 140 patients.91 It has a mild antidepressant effect and thus has been included in the group of tricyclic antidepressants in some meta-analyses.77 In certain cases, it can also be associated with hyperprolactinemia. Itopride is a second generation prokinetic agent that showed progressive symptomatic improvement in the first 4 weeks of treatment in phase II studies, but with conflicting results in phase III studies.92 A meta-analysis that included 9 controlled clinical trials compared with placebo found itopride to be superior in relation to overall improvement (RR: 1.11, 95% CI: 1.03-1.19, p = 0.006), postprandial fullness (RR: 1.21, 95% CI: 1.03-1.44, p = 0.02), and early satiety (RR: 1.24, 95% CI: 1.01-1.53, p = 0.04).93 Mosapride was evaluated in a meta-analysis with 13 controlled clinical trials compared with placebo. Inconsistent results were observed, with no improvement using the Rome III criteria.94 Cinitapride and clebopride have not been evaluated in controlled trials on functional dyspepsia compared with placebo and they both have important extrapyramidal effects, especially clebopride. Erythromycin is a macrolide antibiotic that is a motilin-receptor agonist and is useful in gastroparesis, but it has not been shown to be efficacious in functional dyspepsia.95 Tegaserod, a 5-HT4 agonist, particularly used in chronic constipation, was associated with symptomatic improvement in women with functional dyspepsia,96 but its cardiovascular safety profile has limited its use. Acotiamide is an acetylcholinesterase inhibitor that accelerates gastric emptying and has been approved in Japan for its use in functional dyspepsia.97 It is involved in phase III studies in the United States, but has not yet been approved by the Food and Drug Administration.98 A study that evaluated the usefulness of acotiamide in functional dyspepsia showed symptomatic improvement compared with placebo (52 vs 35%, respectively, p < 0.001), elimination of food-related symptoms after 4 weeks (15.3 vs 9% respectively, p = 0.004), gastric accommodation reflex improvement (21.7 vs 4.4% respectively, p = 0.012), and increased gastric emptying rate (35 vs 11%, respectively).99 Acotiamide, like other prokinetics, has a greater effect on postprandial fullness and early satiety, but not on epigastralgia.100 The NNT is 6 for achieving symptomatic improvement and 16 for symptom elimination.98 Ghrelin is a gastrointestinal peptide related to motility and appetite regulation, and another line of research is being conducted on its analogs. Several ghrelin analogs have been evaluated in functional dyspepsia, including ulimorelin (TZP-101), TZP-102, and relamorelin (RM-131), but the evidence is conflicting and insufficient.79 GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Reports state that up to 5% of cases of dyspepsia in the community are due to H. pylori infection.101 Different clinical guidelines have suggested that the best strategy in uninvestigated dyspepsia is to “search for and treat” the bacterium, especially in young patients with no alarm symptoms, although the evidence comes from countries with low prevalences of this infection.102,103 In Mexico, where prevalence is greater than 70%, this strategy is controversial, given that only 10 to 15% of patients infected with H. pylori have been reported to develop symptoms. This strategy is equally as effective as endoscopy as a measure for reducing dyspepsia severity, but more cost-effective. Endoscopy can rule out organicity, but it can also have a placebo effect, particularly in anxious patients. This diagnostic and therapeutic strategy in functional dyspepsia is controversial. Evidence suggests that one out of every 14 patients with functional dyspepsia and H. pylori infection obtains symptomatic benefit after eradication. In a systematic review and meta-analysis of 17 studies that included 3,566 patients, the RR of system persistence with eradication therapy was 0.90 (95% CI: 0.86-0.94), with a 10% reduced risk and a NNT of 15.77 A more recent meta-analysis of 14 studies confirmed that eradication was superior to placebo and conventional management for obtaining symptomatic improvement.41 Four studies evaluated the effect of eradication on individual symptoms of functional dyspepsia and a significant effect on epigastric pain and burning sensation was observed in three of the four studies, but not on early satiety or postprandial fullness.104–107 Only one of the three studies reported improvement in dysmotility symptoms, as well as in pain.106 Studies with complete symptomatic response have reported its persistence for up to 12 months after treatment.41 The HEROES study, a more recent analysis that evaluated adults with functional dyspepsia (Rome III), compared triple therapy versus omeprazole and placebo.107 The main outcome measure (> 50% of symptomatic improvement at 12 months), was 49 vs 36.5% in favor of eradication. A higher percentage of the eradication group also had partial symptomatic improvement (78 vs 67.5%, respectively, p < 0.001). Upon analyzing population groups, a greater symptomatic response has been described in populations with high prevalence, and although the strains are usually different, a recent meta-analysis showed similar improvement in Asia, Europe, and America.41 In short, even though evidence shows that eradication treatment can be useful, predictive response factors that can detect the subgroup of patients that would benefit from this treatment alternative have not been identified. GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
An important role of the central nervous system-enteric nervous system axis has been described in the development of visceral hypersensitivity. Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) modulate serotonin levels, and therefore have an effect on motility and visceral nociception.108 They were considered second or third-line therapy for many years, especially in patients with anxiety or depression associated with dyspepsia. Today, their benefit is recognized, regardless of the presence or not of psychiatric comorbidity. A systematic review and meta-analysis of anxiolytics and antidepressants in functional gastrointestinal disorders concluded that antidepressants were superior to placebo in functional dyspepsia in 4 out of 13 articles. The studies included the evaluation of TCAs, levosulpiride, anticholinergics, and anxiolytics. The RR of symptom persistence with antidepressants in general was 0.55 (95% CI: 0.36-0.85).109 Another meta-analysis compared 7 studies that included antidepressants and anxiolytics with 11 studies that included antisecretory agents and prokinetics. Both medication groups were associated with the reduction of pain, but the benefit was borderline.110 Several subsequent studies have shown a modest benefit with TCAs, conflicting results with SSRIs, and no evidence in favor or against norepinephrine reuptake inhibitors. Upon analyzing the effect of different antidepressants separately on functional dyspepsia, venlafaxine,111 and sertraline112 were not better than placebo. Only one study has compared the effect of a TCA (amitriptyline), a SSRI (escitalopram), and placebo on functional dyspepsia.113 In that study, amitriptyline was superior to escitalopram and to placebo after 10 weeks, with an adequate response of 53 vs 38 vs 40% (amitriptyline, escitalopram, and placebo, respectively; p = 0.05), a trend toward epigastric pain improvement (67 vs 27 vs 39% amitriptyline, escitalopram, and placebo, respectively; p = 0.06), and with no significant effect on satiety. Both antidepressants improved quality of life.113 Mirtazapine has been shown to have an effect on the receptive relaxation of the gastric fundus, with significant improvement over placebo in early satiety, quality of life, tolerance to nutrients, and weight loss after 8 weeks of treatment.114 In addition to symptomatic improvement, another study with mirtazapine showed weight and body fat percentage recovery after 8 weeks of treatment.115 A recently published small study concluded that mirtazapine was superior to placebo for achieving improvement in symptom intensity, in the depression score, in the somatization score, and in the quality of life scores measured through the SCL-90 questionnaire in patients with functional dyspepsia (Rome II).116 Finally, 5-HT1A antagonists, such as buspirone and tandospirone, block the central serotoninergic response, thus improving the receptive fundic relaxation and gastric accommodation. Both drugs were effective in phase I and II studies. Buspirone was associated with improvement in subjective bloating, postprandial fullness, and fundic relaxation in a cross-over controlled phase III study for 4 weeks.117 Tandospirone was superior to placebo after 4 weeks in achieving improvement in discomfort (31.5 vs 12.7%, p = 0.002) and epigastric pain (p = 0.02), with a greater number of responders at weeks 3 (p = 0.017) and 4 (p = 0.016), when compared with placebo, and with no adverse effects.118 GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
There are no controlled trials with placebo that compare antisecretory agents or prokinetics with each other. A recently published study evaluated the effectiveness of PPI vs H2A combined with prokinetics.119 In that open study, 10mg of rabeprazole once a day was compared with the combination of 10mg famotidine twice a day plus 5mg of mosapride 3 times a day for 4 weeks. The primary outcome measure was the change in symptom score and the secondary outcome measure was treatment satisfaction. PPIs were superior to the famotidine/mosapride combination on day 28 in relation to symptomatic improvement (22.5 ± 29.2% vs 53.2 ± 58.6%, p < 0.0001) and this benefit was unrelated to the presence or absence of H. pylori. Greater treatment satisfaction was also observed in the group that received the PPI (87.7 vs 59.6%, p = 0.0012). Therapy with rabeprazole was the only predictor of treatment response. There are no studies that compare the combination of antisecretory agents or prokinetics with antidepressants. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
The therapeutic trial in uninvestigated dyspepsia with no alarm symptoms is usually 3 weeks before re-evaluating whether to change treatment or perform diagnostic tests. However, the appropriate treatment duration in functional dyspepsia has not been evaluated. The majority of studies with antisecretory agents and prokinetics show symptom changes at 4 and 8 weeks, whereas studies with antidepressants report improvement at 8 and 12 weeks. When dealing with a chronic disease, it is most likely that treatment can be longer in some cases or require intermittent cycles of one or several medications. At present, there is no evidence that evaluates treatment duration or intervals between treatments. GRADE level of evidence and strength of recommendation: D1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
The information on the usefulness of prebiotics, probiotics, or synbiotics in functional dyspepsia is found in the pediatric population and is scarce.120,121 Different brands of yoghurts are sold with added strains of probiotics and the popular belief is that these foods play a protective role in the stomach, but there is no quality information available for making a recommendation in relation to functional dyspepsia. Specific studies on this functional gastrointestinal disorder are required that evaluate the potential usefulness of these therapeutic options. GRADE level of evidence and strength of recommendation: D1 strong, in favor of the statement. Level of agreement: Complete agreement, 100%.
Mexico is one of the main chili pepper-consuming countries. Capsaicin is found in different foods, such as chili and red pepper. Capsaicin ingestion is popularly considered a cause of dyspeptic symptoms. There has been recent scientific evidence with controversial results, specifically in regard to functional dyspepsia. On the one hand, it has been reported that capsaicin consumption produces acute symptoms, associating its ingestion with dysmotility symptoms and epigastric burning sensation.122,123 On the other hand, repeated capsaicin administration for a prolonged period of time has been described to reduce symptoms in patients with functional dyspepsia.124–126 This effect is attributed to a dual desensitizing of chemoreceptors and mechanoreceptors by capsaicin.127 Some studies attribute a therapeutic effect specifically due to transient receptor potential vanilloid type 1 (TRPV1) desensitization with the ingestion of capsaicin for at least 3-5 weeks.128 It is not known whether this desensitizing is reversible or if it is maintained. Thus, more studies are required before its use can be recommended. GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 85%. Partial agreement, 10%. Complete disagreement, 5%.
These alternative treatments are increasingly gaining acceptance in the Western world. Clinical trials with good methodology and statistically significant results in favor of acupuncture have been conducted on patients with functional dyspepsia.129 There are even neuroimaging studies that have shown the areas of the brain that acupuncture stimulates.130 Its adverse effects include pain, fatigue, bleeding, and hematomas, and they present in 0.671 to 11.4% of the cases treated.131 Studies that have employed interferential electric stimulation have shown symptomatic improvement in patients with functional dyspepsia, but their study size is small and the long-term effect and safety of repeated application is not known.132 A Cochrane review analyzed the studies that compared manual acupuncture, electroacupuncture, and manual electroacupuncture versus medications (prokinetics), as well as true acupuncture versus sham acupuncture. The authors concluded that, even though the studies showed clinical improvement with significant differences, the evidence was low-quality, due to study design limitations (mainly blinding) and inaccuracy (small sample size for generalizing results). They could not reach a conclusion as to the efficacy and safety of these interventions in functional dyspepsia without better quality studies.133 The efficacy of transcutaneous electric stimulation has been demonstrated in gastroparesis, but not in functional dyspepsia in adults. Two studies on a pediatric and adolescent population have shown significant clinical improvement in functional dyspepsia, but more studies are needed to establish the efficacy and safety of these procedures in the long term.134,135 GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
Alternative medicine, especially herbal medicine, is popular among patients with functional gastrointestinal disorders, partially due to the limited therapeutic options of conventional medicine for controlling symptoms.136 Lahner et al.137 reported a 36.7% frequency of herbal consumption used in alternative medicine in relation to functional gastrointestinal disorders. There is no quality scientific evidence demonstrating the efficacy of the majority of herbal compounds.138 Iberogast (STW5) and rikkunshito are the two compounds that have greater quality scientific evidence and that have shown usefulness in the relief of functional dyspepsia symptoms.12 Comparative, controlled, randomized clinical trials have been conducted on both compounds and have reported dyspeptic symptom relief.139–141 GRADE level of evidence and strength of recommendation: A1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
The studies conducted up to now on the different psychologic therapies are not conclusive for making a recommendation in relation to functional dyspepsia. A 2005 Cochrane review that was updated in 2011 concluded that there is insufficient information for recommending psychologic interventions in non-ulcerous dyspepsia.142 Better-designed studies with a larger number of patients are required for this pathology.143–146 Compared with other functional gastrointestinal disorders, such as irritable bowel syndrome, in which recommendations have been made for the application of certain psychologic therapies,8 these treatments have been studied very little as an alternative therapeutic option in functional dyspepsia.147 GRADE level of evidence and strength of recommendation: B1 strong, in favor of the statement. Level of agreement: Complete agreement, 95%. Partial agreement, 5%.
Dyspepsia is a common motive for consultation in daily practice and one of the most frequently diagnosed digestive conditions. Thus, it is very important to communicate the changes and advances that have been made in the understanding of this disease in recent years. We present herein a consensus review that reaffirms, renews, and complements the knowledge of this condition contained in the Asociación Mexicana de Gastroenterología guidelines published in 2007.
Financial disclosureThis consensus was carried out with the financial support of Laboratorios Takeda México that partially financed the face-to-face voting session (transportation and accommodations). The authors received a fee for their participation.
Conflict of interestRamón Carmona-Sánchez is a Member of the Advisory Board of Mayoly-Spindler, a speaker for Mayoly-Spindler and Allegan, and participates in research protocols sponsored by Laboratorios Senosian and Asofarma.
Octavio Gómez-Escudero is a Member of the Advisory Board of Laboratorios Allergan and a Speaker for Laboratorios Allergan, Alfa-Wassermann, Asofarma, Astra-Zéneca, and Takeda and participates in a research protocol sponsored by Laboratorios Asofarma.
Mónica Zavala-Solares is a Speaker for Laboratorios Takeda.
María Victoria Bielsa-Fernández is a Speaker for Alfa-Wassermann and a Member of the Advisory Board of Alfa-Wassermann.
Enrique Coss-Adame is a Speaker for Laboratorios Takeda de México. He has been a Consultant for and collaborates with Laboratorios Asofarma de México and participates in a research protocol sponsored by Laboratorios Asofarma.
María Eugenia Icaza-Chávez is a Member of the Advisory Board of Laboratorios Mayoly-Spindler and Carnot, a Speaker for Mayoly-Spindler, Alfa-Wassermann, and Asofarma, and participates in a research protocol sponsored by Laboratorios Asofarma and another by Laboratorios Senosiain.
Francisco Huerta-Iga is a Speaker for Takeda and Asofarma.
Aurelio López-Colombo is a Speaker for Laboratorios Takeda de México.
Alejandra Noble-Lugo is a Speaker for Laboratorios Takeda de México, Alfa-Wassermann, and Astra Zéneca.
Ricardo Raña-Garibay is a Member of the Advisory Board and Speaker for Laboratorios Commonwealth.
José María Remes-Troche is a Member of the Advisory Board of Takeda Pharmaceuticals and Alfa-Wassermann, is a Speaker for Takeda, Asofarma, Alfa-Wassermann, and Astra-Zeneca and participates in a research protocol sponsored by Laboratorios Asofarma.
José Luis Tamayo is a Speaker and external advisor for Laboratorios Alfa-Wassermann, Asofarma, Mayoly-Spindler, and Takeda de México and participates in a research protocol sponsored by Laboratorios Asofarma.
Miguel A. Valdovinos is a Speaker for Laboratorios Takeda de México, Mayoly-Spindler, Carnot, Biocodex, and Danone. He is a Member of the Advisory Board of Mayoly-Spindler, Takeda, and Biocodex.
José Antonio Velarde Ruiz Velasco is a Speaker for Laboratorios Takeda México and Alfa Wassermann, is a Member of the Advisory Board of Laboratorios Carnot, and participates in a research protocol sponsored by Laboratorios Asofarma.
All the remaining participants declare that they have no conflict of interest.
Please cite this article as: Carmona-Sánchez R, Gómez-Escudero O, Zavala-Solares M, Bielsa-Fernández MV, Coss-Adame E, Hernández-Guerrero AI, et al. Consenso mexicano sobre la dispepsia. Revista de Gastroenterología de México. 2017;82:309–327.