Distal pancreatectomy is a frequent procedure and postoperative fistula, its most common complication, has an incidence of 30 to 60%. The aim of the present work was to study the role of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio, as indicators of inflammatory response in the setting of pancreatic fistula.
MethodsA retrospective observational study was conducted on patients that underwent distal pancreatectomy. The diagnosis of postoperative pancreatic fistula was made according to the definition proposed by the International Study Group on Pancreatic Fistula. The relation of postoperative pancreatic fistula to the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio was determined in the postoperative evaluation. SPSS v.21 software was utilized for the statistical analysis and a P<.05 was considered statistically significant.
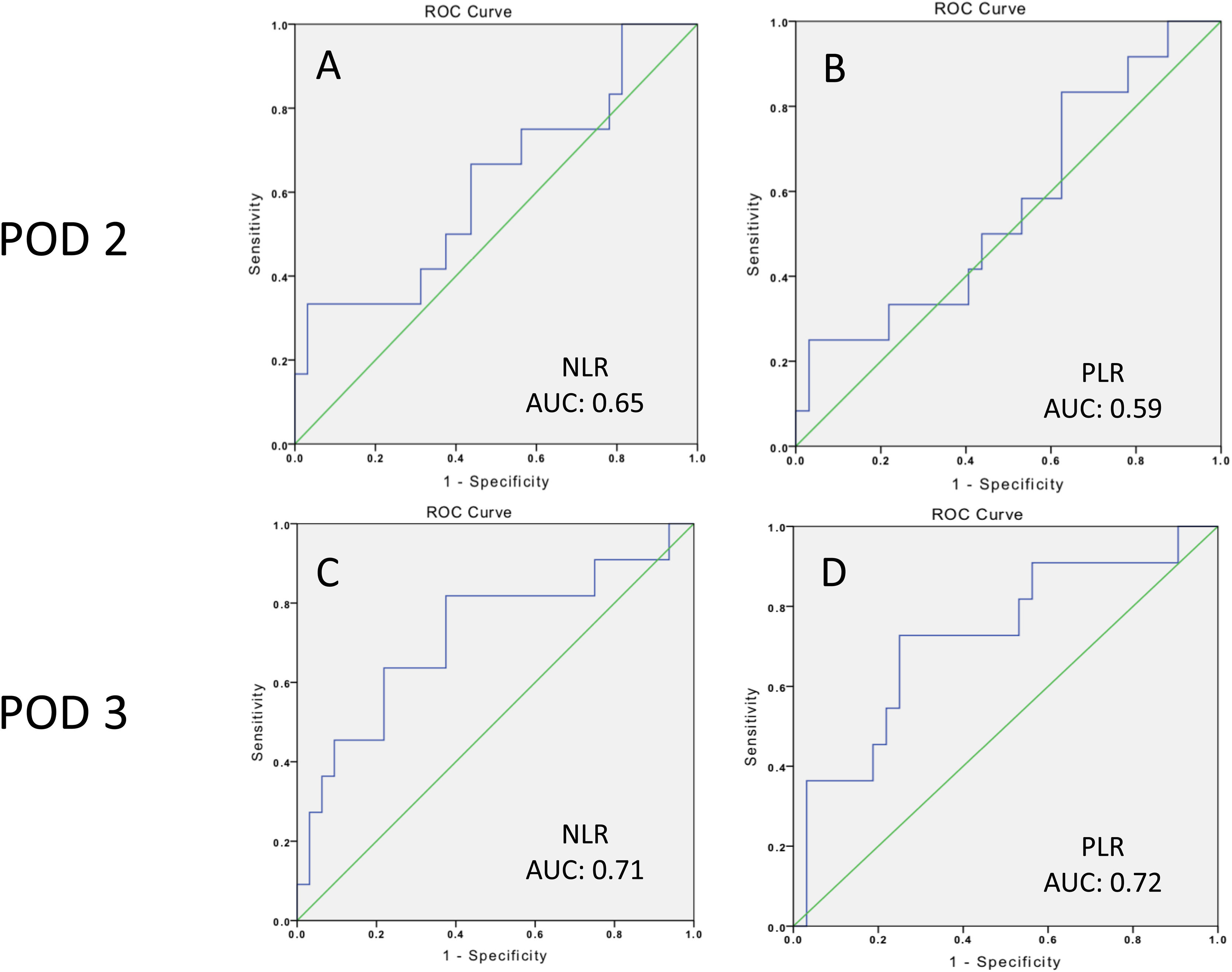
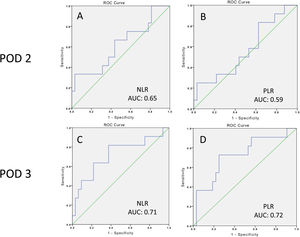
ResultsA total of 12 patients (27.2%) developed grade B or grade C postoperative pancreatic fistula. ROC curves were constructed and a threshold of 8.3 (PPV 0.40, NPV 0.86) was established for the neutrophil-to-lymphocyte ratio, with an area under the curve of 0.71, sensitivity of 0.81, and specificity of 0.62, whereas a threshold of 33.2 (PPV 0.50, NPV 0.84) was established for the platelet-to-lymphocyte ratio, with an area under the curve of 0.72, sensitivity of 0.72, and specificity of 0.71.
ConclusionThe neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio are serologic markers that can aid in identifying patients that will present with grade B or grade C postoperative pancreatic fistula, thus helping to provide an opportune focus on care and resources.
La pancreatectomía distal es un procedimiento frecuente y la fístula postoperatoria, su complicación más frecuente tiene una incidencia del 30 al 60%. El objetivo de este trabajo fue estudiar el papel de la relación neutrófilos/linfocitos y la relación linfocitos/plaquetas como indicadores de respuesta inflamatoria en el escenario de la fístula pancreática.
MétodosEste trabajo es un estudio observacional retrospectivo que incluye pacientes que fueron sometidos a pancreatectomía distal. El diagnóstico de fístula pancreática postoperatoria se estableció según la definición del Grupo Internacional de Estudio de Fístula Pancreática (ISGPF, por sus siglas en inglés). La asociación entre fístula pancreática posoperatoria, la relación neutrófilos/linfocitos y la relación plaquetas/linfocitos fue determinada en la evaluación posoperatoria. Se utilizó el software SPSS® v.21 para el análisis estadístico y un valor de p inferior a 0.05 se consideró estadísticamente significativo.
ResultadosUn total de 12 pacientes (27.2%) desarrollaron fístula pancreática postoperatoria (POPF, por sus siglas en inglés) grado B o C. Se construyeron curvas ROC y se estableció un umbral de 8.3 (VPP: 0.40; VPN: 0.86) para la relación neutrófilos/linfocitos con un área bajo la curva de 0.71, sensibilidad de 0.81 y especificidad de 0.62; por su parte, para la relación plaquetas/linfocitos se estableció un umbral de 33.2 (VPP: 0.50; VPN: 0.84), con un área bajo la curva de 0.72, sensibilidad de 0.72 y especificidad de 0.71.
ConclusiónLa relación neutrófilos/linfocitos y la relación plaquetas/linfocitos son marcadores serológicos que pueden ayudar a identificar a pacientes que presentarán fístula pancreática postoperatoria grado B o C, y así ayudar a enfocar la atención y los recursos de manera oportuna.
Distal pancreatectomy (DP) is a commonly performed procedure for the treatment of lesions located in the body or tail of the pancreas. Although the mortality rate in patients undergoing DP has decreased in recent years (reported between 0 and 5% at high-volume centers)1, the morbidity rate remains high (18 to 65%)2,3. Postoperative pancreatic fistula (POPF) is the most common complication after DP, with an incidence of 30-60%4, according to the definition proposed by the International Study Group on Pancreatic Fistula (ISGPF)5, which describes an output of drain fluid with an amylase content greater than 3 times the upper normal serum amylase value on or after postoperative day (POD) 3. The ISGPF also divided POPF into 3 grades: grade A is an asymptomatic fistula and the most common scenario in 31% of the patients, whereas grade B and grade C fistulas are clinically significant entities that require therapeutic intervention and can be associated with even more severe complications, such as intra-abdominal abscess, sepsis, pseudoaneurysm, hemorrhage, or death1,6,7.
Risk factors for POPF after DP are poorly studied, compared with POPF after pancreaticoduodenectomy. However, a number of nonsurgical and surgical factors have been described as predictors for POPF in DP patients, including older age, increased BMI, a history of diabetes, soft pancreas, blood transfusion, elevated intraoperative blood loss, longer operative time, pancreatic texture, types of stump closure, splenectomy, multiorgan resection, and extended lymphadenectomy2,8–12.
At the same time, systemic inflammation is a main component in the development of intra-abdominal complications. Hematologic cell counts are the most commonly used biochemical markers to identify the grade of inflammation within the abdominal cavity, specifically neutrophilia and lymphocytopenia13. In recent years, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) have been used as indicators of inflammatory response for different types of cancer14–18 and other intra-abdominal inflammatory diseases. Nevertheless, the use of those biochemical markers for postoperative complications after DP, mainly pancreatic fistula, are not well documented.
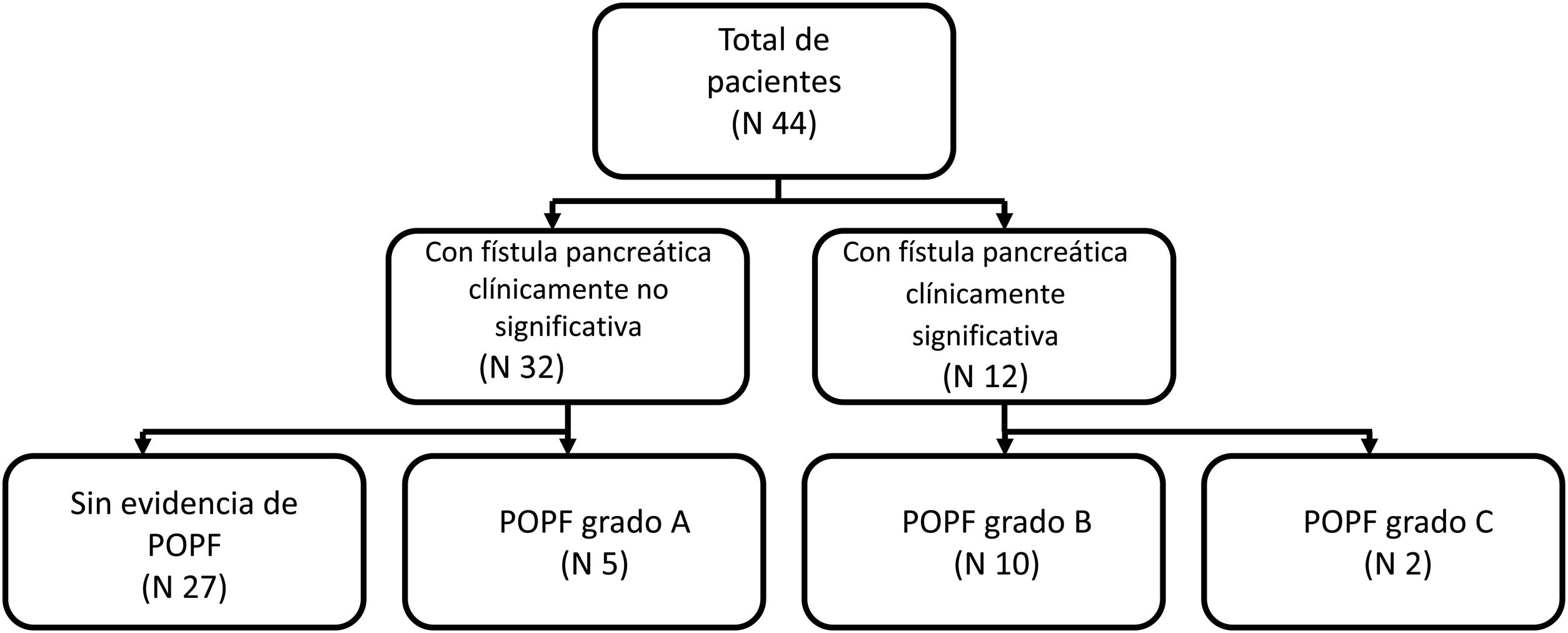
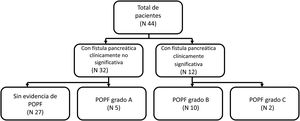
MethodsStudy designThe present work is a retrospective observational study on patients that underwent distal pancreatectomy within the time frame of 2014-2018. Electronic and physical records were reviewed to obtain the clinical and pathologic characteristics of each patient. Placing a closed suction drain in the surgical site at the time of surgery is routine practice at our hospital. We defined POPF according to the definition by the ISGPF: drain output with an amylase content > 3 times the upper normal serum amylase value, on or after POD 3. After POPF was documented, grading (A, B, or C) was established using the ISGPF classification as reference. Grade A was defined as a POPF that does not require percutaneous or endoscopic drainage, angiography, or antibiotics; grade B was defined as a POPF that requires percutaneous or endoscopic drainage, angiography, or antibiotics; and grade C was defined as a POPF that requires surgery or was associated with death. Patients were assigned to the group with clinically insignificant POPF (no evidence of POPF or grade A) or to the group with clinically significant POPF (grade B or grade C) (Fig. 1).
Inclusion and exclusion criteriaPatients were considered eligible for the study if they underwent DP during the study period and had a minimum postoperative follow-up time of 30 days. Exclusion criteria included DP with multiorgan resection, patients with concomitant hematologic diseases, chronic steroid use, and incomplete information.
Clinical/surgical characteristics and blood markerThe documented features were sex, age, BMI, comorbidities, surgical approach, presence of splenectomy, ASA classification, intraoperative blood loss, hospital length of stay (LOS), operative time, presence of POPF, and other postoperative complications. Cell count levels were recorded on POD 1, 2, and 3 and included hemoglobin, platelets, leukocytes, neutrophils, lymphocytes, NLR, and PLR.
The primary aim of the present work was to evaluate the association between POPF and the different biochemical markers, such as hematologic cell counts, NLR, and PLR, on POD 1, 2, and 3.
Statistical analysisMean and standard deviation were included for the quantitative variables, and frequency and proportions for the qualitative variables. The chi-square test was used to analyze the categorical variables. The Kolmogorov-Smirnov normality test was carried out for all the quantitative variables and the Student’s t test or Mann-Whitney U test were applied for the independent samples, according to the distribution data of the variables. The Wilcoxon test or Friedman test were used for the related samples. Diagnostic accuracy and the AUC were quantified using the ROC curve analysis and the Youden index was used to estimate the best cutoff values. The statistical analysis was performed using IBM® SPSS Statistics version 21 software. A p value ≤ 0.05 was considered statistically significant for a 2-tailed hypothesis test.
Ethical considerationsResearch involving human participants and/or animals: This article contains no studies on human participants or animals performed by any of the authors.
The present work was reviewed and approved by both the research committee and the ethics committee of the National Institute of Medical Sciences and Nutrition “Salvador Zubirán”. The authors declare that this article contains no personal information that can identify the patients. The authors declare informed consent was not necessary for this work.
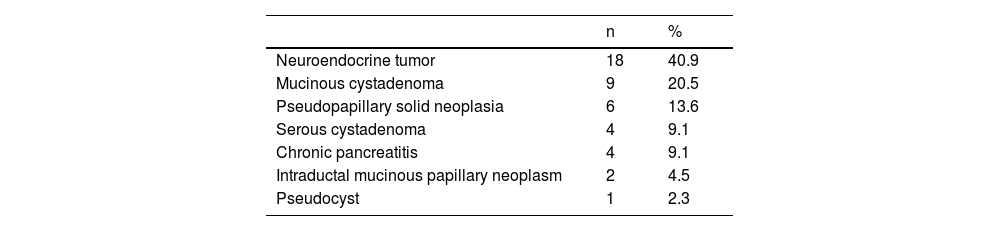
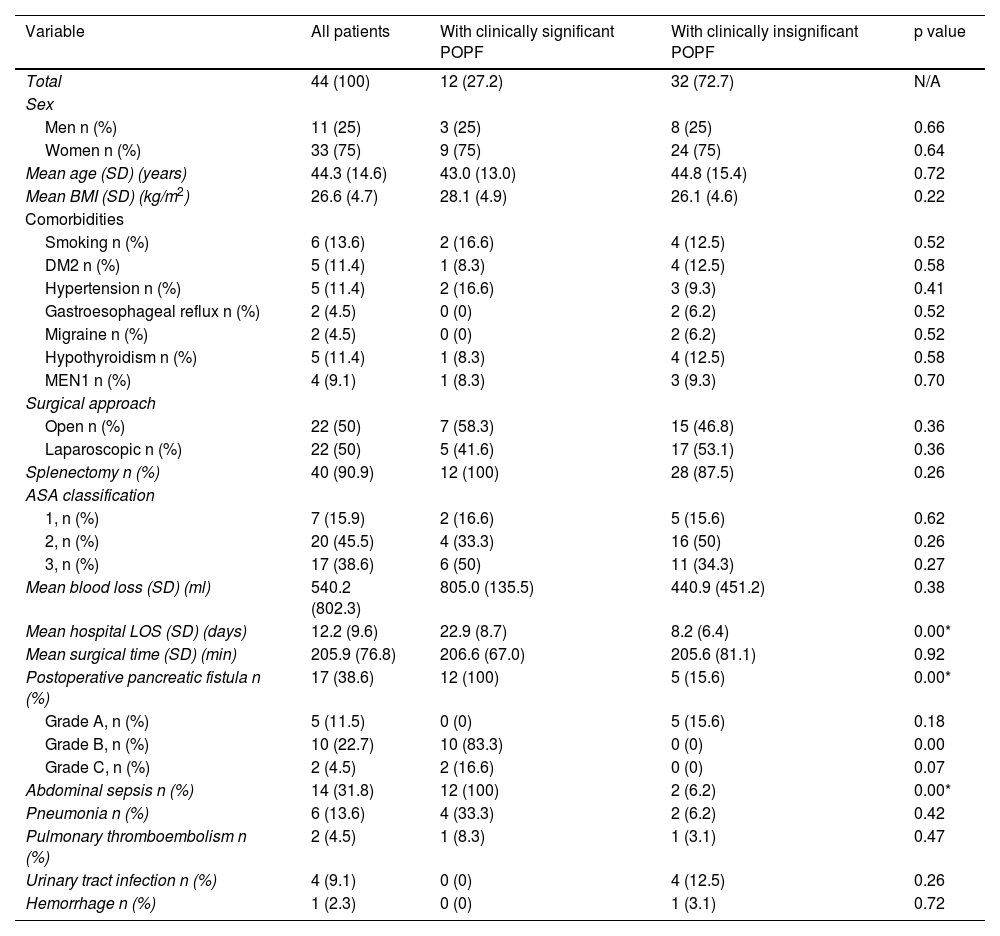
ResultsPopulation characteristics and clinical courseWithin the time frame of 2014 and 2018, a total of 44 patients underwent DP. Eleven (25%) of the patients were men and 33 (75%) were women. Mean patient age ± SD was 44.3 ± 14.6 years. The most frequent indication for DP was neuroendocrine tumor in 18 patients (40.9%), followed by mucinous cystadenoma in 9 patients (20.5%). Other indications included serous cystadenoma, chronic pancreatitis, pseudopapillary solid neoplasia, intraductal mucinous papillary neoplasm, and pseudocyst. Of the 44 patients, 17 (38.6%) developed POPF; 11.5% grade A, 22.7% grade B, and 4.5% grade C. There was only one reported death in our entire cohort, and it was secondary to postoperative early hemorrhage that was not related to POPF. Tables 1 and 2 summarize the indications for surgery and the surgical characteristics of the patients that underwent DP.
Overall characteristics and clinical course of the patients that underwent distal pancreatectomy.
| Variable | All patients | With clinically significant POPF | With clinically insignificant POPF | p value |
|---|---|---|---|---|
| Total | 44 (100) | 12 (27.2) | 32 (72.7) | N/A |
| Sex | ||||
| Men n (%) | 11 (25) | 3 (25) | 8 (25) | 0.66 |
| Women n (%) | 33 (75) | 9 (75) | 24 (75) | 0.64 |
| Mean age (SD) (years) | 44.3 (14.6) | 43.0 (13.0) | 44.8 (15.4) | 0.72 |
| Mean BMI (SD) (kg/m2) | 26.6 (4.7) | 28.1 (4.9) | 26.1 (4.6) | 0.22 |
| Comorbidities | ||||
| Smoking n (%) | 6 (13.6) | 2 (16.6) | 4 (12.5) | 0.52 |
| DM2 n (%) | 5 (11.4) | 1 (8.3) | 4 (12.5) | 0.58 |
| Hypertension n (%) | 5 (11.4) | 2 (16.6) | 3 (9.3) | 0.41 |
| Gastroesophageal reflux n (%) | 2 (4.5) | 0 (0) | 2 (6.2) | 0.52 |
| Migraine n (%) | 2 (4.5) | 0 (0) | 2 (6.2) | 0.52 |
| Hypothyroidism n (%) | 5 (11.4) | 1 (8.3) | 4 (12.5) | 0.58 |
| MEN1 n (%) | 4 (9.1) | 1 (8.3) | 3 (9.3) | 0.70 |
| Surgical approach | ||||
| Open n (%) | 22 (50) | 7 (58.3) | 15 (46.8) | 0.36 |
| Laparoscopic n (%) | 22 (50) | 5 (41.6) | 17 (53.1) | 0.36 |
| Splenectomy n (%) | 40 (90.9) | 12 (100) | 28 (87.5) | 0.26 |
| ASA classification | ||||
| 1, n (%) | 7 (15.9) | 2 (16.6) | 5 (15.6) | 0.62 |
| 2, n (%) | 20 (45.5) | 4 (33.3) | 16 (50) | 0.26 |
| 3, n (%) | 17 (38.6) | 6 (50) | 11 (34.3) | 0.27 |
| Mean blood loss (SD) (ml) | 540.2 (802.3) | 805.0 (135.5) | 440.9 (451.2) | 0.38 |
| Mean hospital LOS (SD) (days) | 12.2 (9.6) | 22.9 (8.7) | 8.2 (6.4) | 0.00* |
| Mean surgical time (SD) (min) | 205.9 (76.8) | 206.6 (67.0) | 205.6 (81.1) | 0.92 |
| Postoperative pancreatic fistula n (%) | 17 (38.6) | 12 (100) | 5 (15.6) | 0.00* |
| Grade A, n (%) | 5 (11.5) | 0 (0) | 5 (15.6) | 0.18 |
| Grade B, n (%) | 10 (22.7) | 10 (83.3) | 0 (0) | 0.00 |
| Grade C, n (%) | 2 (4.5) | 2 (16.6) | 0 (0) | 0.07 |
| Abdominal sepsis n (%) | 14 (31.8) | 12 (100) | 2 (6.2) | 0.00* |
| Pneumonia n (%) | 6 (13.6) | 4 (33.3) | 2 (6.2) | 0.42 |
| Pulmonary thromboembolism n (%) | 2 (4.5) | 1 (8.3) | 1 (3.1) | 0.47 |
| Urinary tract infection n (%) | 4 (9.1) | 0 (0) | 4 (12.5) | 0.26 |
| Hemorrhage n (%) | 1 (2.3) | 0 (0) | 1 (3.1) | 0.72 |
ASA: American Society of Anesthesiologists; BMI: body mass index; DM2: type 2 diabetes mellitus; LOS: length of stay; MEN1: Multiple endocrine neoplasia type 1; POPF: postoperative pancreatic fistula; SD: standard deviation.
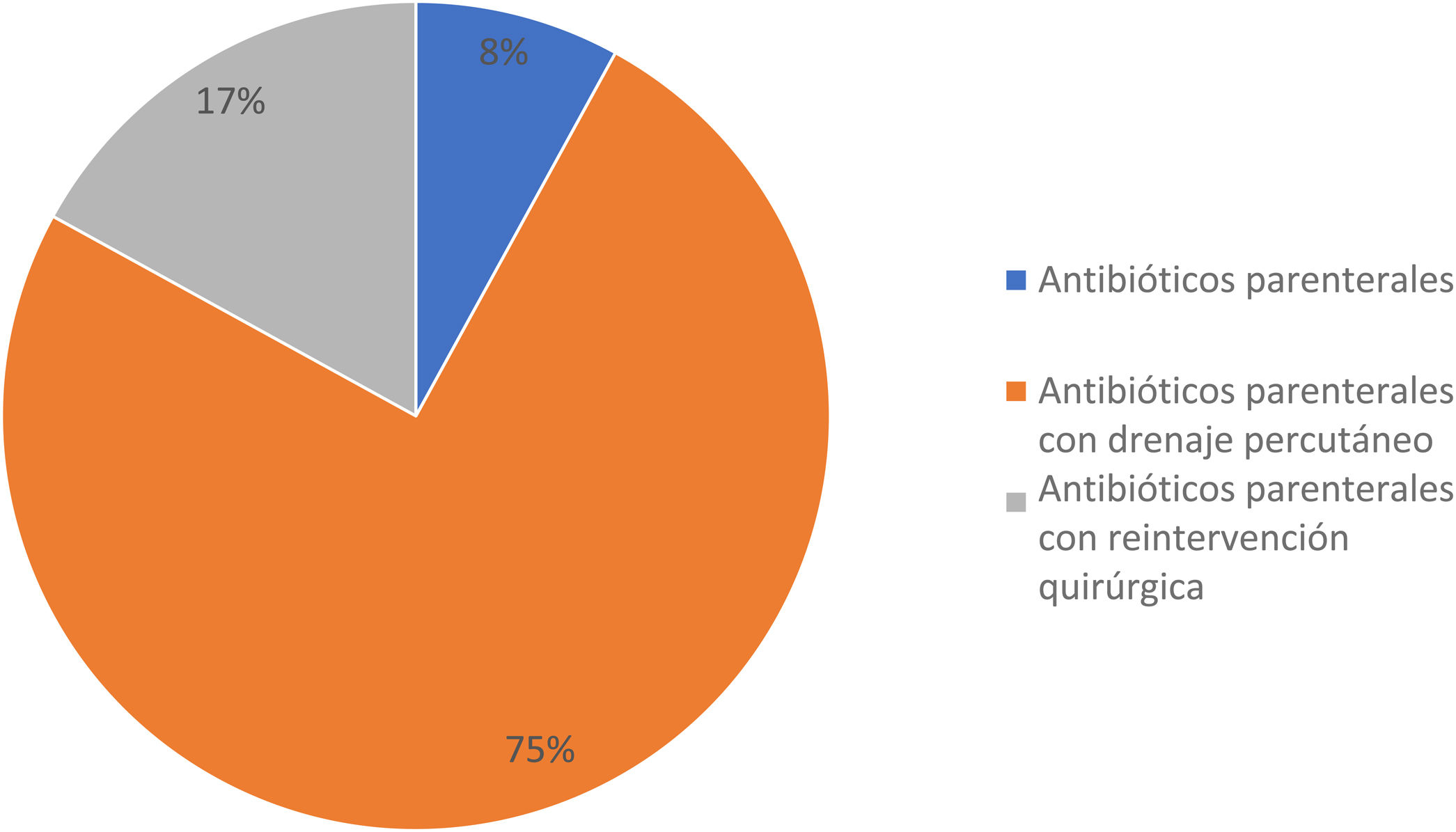
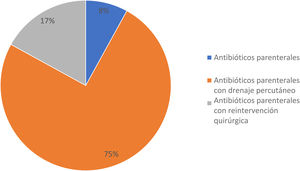
A total of 12 patients (27.2%) developed clinically significant POPF; 3 of them (25%) were men and 9 (75%) were women, with a mean age ± SD of 43.0 ± 13.0 years. In the comparison of the 2 study groups, there were no differences regarding sex, age, BMI, comorbidities, and preoperative laboratory tests; with respect to the surgical and postoperative features, there was a statistically significant difference in hospital LOS: the patients with clinically significant POPF had a mean of 22.9 ± 8.7 days and the patients with clinically insignificant POPF had a mean of 8.2 ± 6.4 days (p < 0.001; Mann-Whitney U test). Treatment for the patients with clinically significant POPF included parenteral antibiotics in one patient (8.3%), parenteral antibiotics with percutaneous drainage in 9 patients (75%), and parenteral antibiotics with surgical reintervention due to abdominal sepsis in 2 patients (16.6%) (Fig. 2). Relevantly, 5 cases of DP (41.6%) were performed laparoscopically, whereas 7 cases (58.3%) were open procedures, with a mean surgical time of 206.6 ± 67.0 min (range 140-350 min) and mean blood loss of 805.0 ± 135.5 ml. All the procedures included splenectomy at the time of surgery, and none required blood transfusion (Table 2). In the clinically insignificant POPF group, there were 2 cases of abdominal sepsis due to an infected fluid collection and amylase level below the POPF criterion. There was no difference between groups regarding pneumonia, pulmonary thromboembolism, urinary tract infection, and hemorrhage.
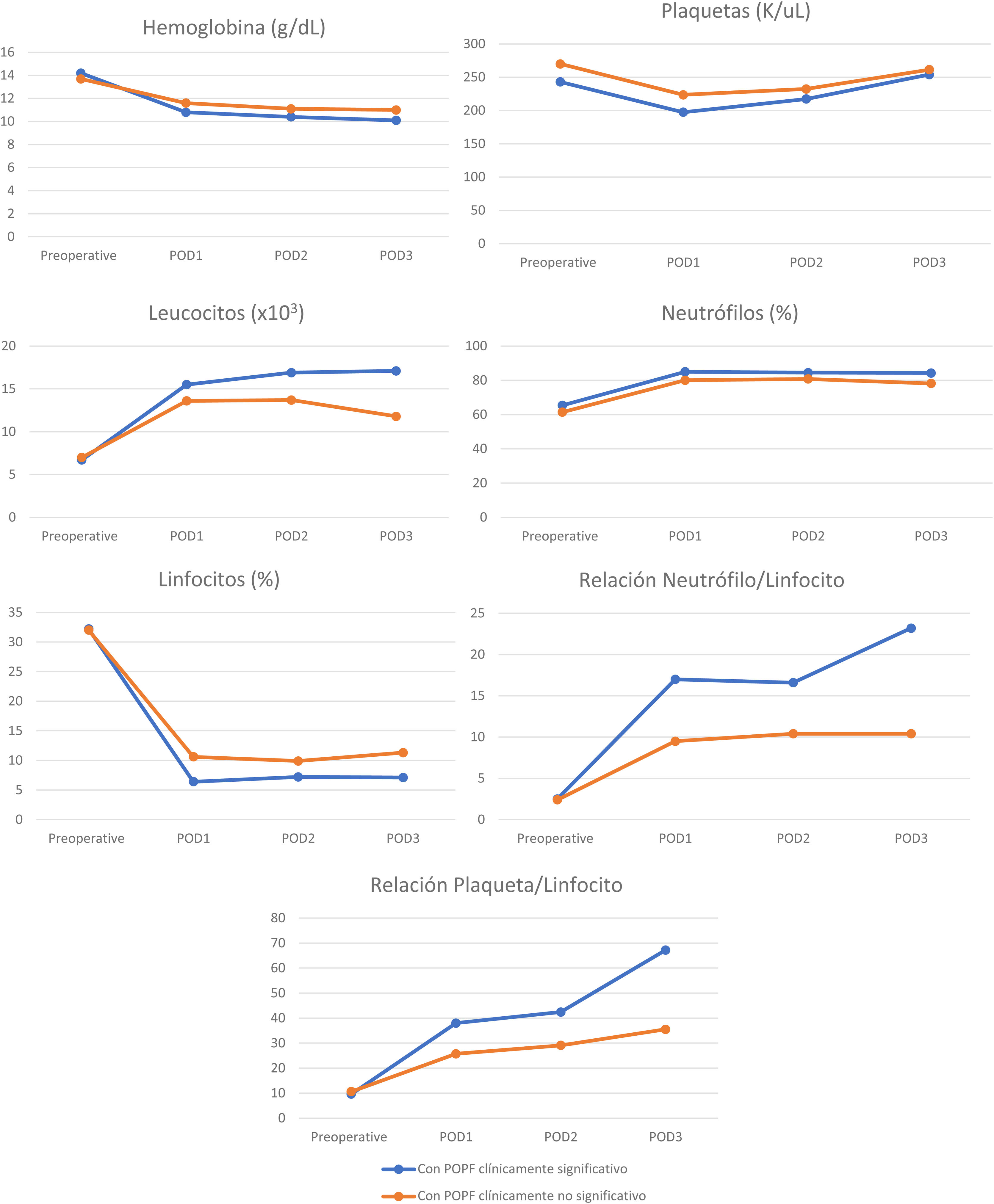
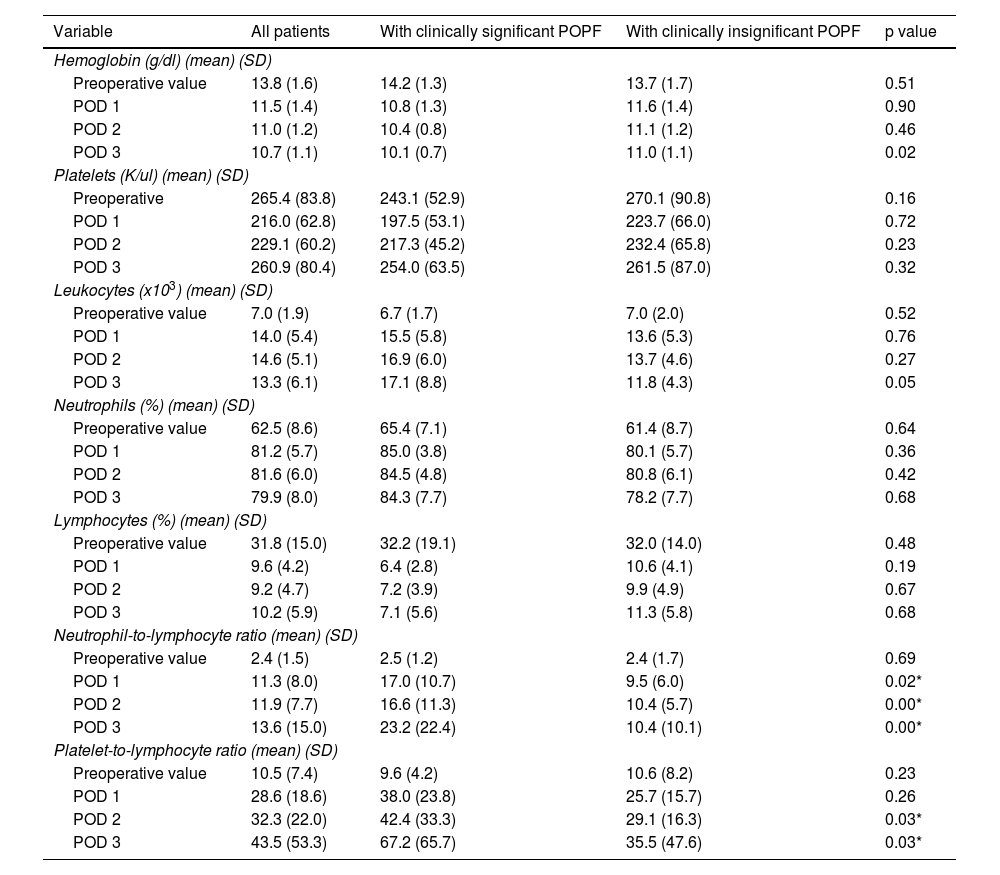
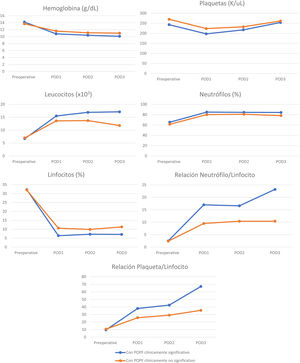
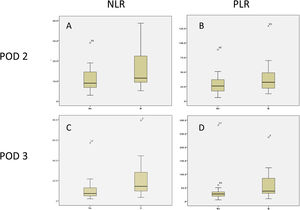
Perioperative blood marker levelsThere was no significant difference between preoperative and postoperative hemoglobin, platelet, leukocyte, neutrophil, and lymphocyte levels, in patients with and without clinically significant pancreatic fistula on POD 1, 2, and 3. However, the NLR and PLR were significantly increased when the 2 groups were compared (Table 3). Cell counts normally increase after surgical trauma and are expected to normalize after day 3, but there was an evident difference in the NLR and PLR between the patients with clinically significant and clinically insignificant POPF (Figs. 3 and 4).
Cell counts on POD 1, 2, and 3 of patients that underwent distal pancreatectomy, with and without POPF.
| Variable | All patients | With clinically significant POPF | With clinically insignificant POPF | p value |
|---|---|---|---|---|
| Hemoglobin (g/dl) (mean) (SD) | ||||
| Preoperative value | 13.8 (1.6) | 14.2 (1.3) | 13.7 (1.7) | 0.51 |
| POD 1 | 11.5 (1.4) | 10.8 (1.3) | 11.6 (1.4) | 0.90 |
| POD 2 | 11.0 (1.2) | 10.4 (0.8) | 11.1 (1.2) | 0.46 |
| POD 3 | 10.7 (1.1) | 10.1 (0.7) | 11.0 (1.1) | 0.02 |
| Platelets (K/ul) (mean) (SD) | ||||
| Preoperative | 265.4 (83.8) | 243.1 (52.9) | 270.1 (90.8) | 0.16 |
| POD 1 | 216.0 (62.8) | 197.5 (53.1) | 223.7 (66.0) | 0.72 |
| POD 2 | 229.1 (60.2) | 217.3 (45.2) | 232.4 (65.8) | 0.23 |
| POD 3 | 260.9 (80.4) | 254.0 (63.5) | 261.5 (87.0) | 0.32 |
| Leukocytes (x103) (mean) (SD) | ||||
| Preoperative value | 7.0 (1.9) | 6.7 (1.7) | 7.0 (2.0) | 0.52 |
| POD 1 | 14.0 (5.4) | 15.5 (5.8) | 13.6 (5.3) | 0.76 |
| POD 2 | 14.6 (5.1) | 16.9 (6.0) | 13.7 (4.6) | 0.27 |
| POD 3 | 13.3 (6.1) | 17.1 (8.8) | 11.8 (4.3) | 0.05 |
| Neutrophils (%) (mean) (SD) | ||||
| Preoperative value | 62.5 (8.6) | 65.4 (7.1) | 61.4 (8.7) | 0.64 |
| POD 1 | 81.2 (5.7) | 85.0 (3.8) | 80.1 (5.7) | 0.36 |
| POD 2 | 81.6 (6.0) | 84.5 (4.8) | 80.8 (6.1) | 0.42 |
| POD 3 | 79.9 (8.0) | 84.3 (7.7) | 78.2 (7.7) | 0.68 |
| Lymphocytes (%) (mean) (SD) | ||||
| Preoperative value | 31.8 (15.0) | 32.2 (19.1) | 32.0 (14.0) | 0.48 |
| POD 1 | 9.6 (4.2) | 6.4 (2.8) | 10.6 (4.1) | 0.19 |
| POD 2 | 9.2 (4.7) | 7.2 (3.9) | 9.9 (4.9) | 0.67 |
| POD 3 | 10.2 (5.9) | 7.1 (5.6) | 11.3 (5.8) | 0.68 |
| Neutrophil-to-lymphocyte ratio (mean) (SD) | ||||
| Preoperative value | 2.4 (1.5) | 2.5 (1.2) | 2.4 (1.7) | 0.69 |
| POD 1 | 11.3 (8.0) | 17.0 (10.7) | 9.5 (6.0) | 0.02* |
| POD 2 | 11.9 (7.7) | 16.6 (11.3) | 10.4 (5.7) | 0.00* |
| POD 3 | 13.6 (15.0) | 23.2 (22.4) | 10.4 (10.1) | 0.00* |
| Platelet-to-lymphocyte ratio (mean) (SD) | ||||
| Preoperative value | 10.5 (7.4) | 9.6 (4.2) | 10.6 (8.2) | 0.23 |
| POD 1 | 28.6 (18.6) | 38.0 (23.8) | 25.7 (15.7) | 0.26 |
| POD 2 | 32.3 (22.0) | 42.4 (33.3) | 29.1 (16.3) | 0.03* |
| POD 3 | 43.5 (53.3) | 67.2 (65.7) | 35.5 (47.6) | 0.03* |
POD: postoperative day; POPF: postoperative pancreatic fistula; SD: standard deviation.
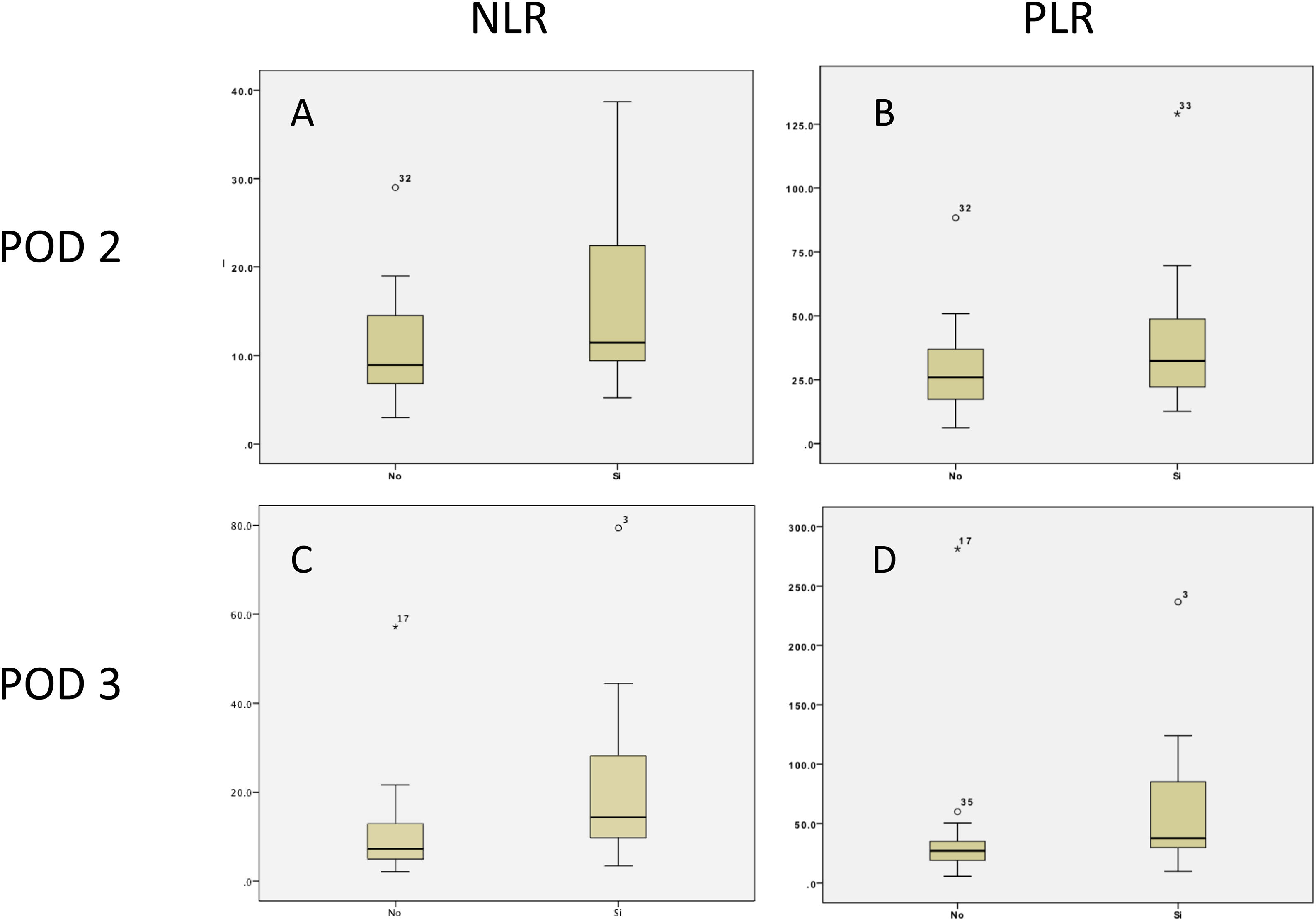
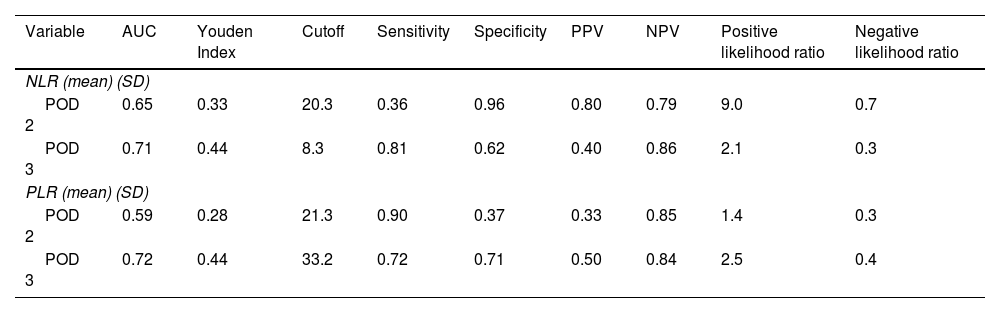
Regarding clinically significant POPF, after the ROC curve analysis, a threshold of 8.3 (PPV 40%, NPV 86%) was established for the NLR on POD 3, with an area under the curve of 0.71, sensitivity of 81%, and specificity of 62%, whereas the threshold for the PLR on POD 3 was 33.2 (PPV 50%, NPV 84%), with an area under the curve of 0.72, sensitivity of 72%, and specificity of 71% (Table 4 and Fig. 5).
Area under the curve, Youden Index, cutoff values, sensitivity, specificity, positive predictive value, and negative predictive value of the NLR and PLR on POD 2 and 3, for clinically significant POPF.
| Variable | AUC | Youden Index | Cutoff | Sensitivity | Specificity | PPV | NPV | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|
| NLR (mean) (SD) | |||||||||
| POD 2 | 0.65 | 0.33 | 20.3 | 0.36 | 0.96 | 0.80 | 0.79 | 9.0 | 0.7 |
| POD 3 | 0.71 | 0.44 | 8.3 | 0.81 | 0.62 | 0.40 | 0.86 | 2.1 | 0.3 |
| PLR (mean) (SD) | |||||||||
| POD 2 | 0.59 | 0.28 | 21.3 | 0.90 | 0.37 | 0.33 | 0.85 | 1.4 | 0.3 |
| POD 3 | 0.72 | 0.44 | 33.2 | 0.72 | 0.71 | 0.50 | 0.84 | 2.5 | 0.4 |
AUC: area under the curve; NLR: neutrophil-to-lymphocyte ratio; NPV: negative predictive value; PLR: platelet-to-lymphocyte ratio; POD: postoperative day; POPF: postoperative pancreatic fistula; PPV: positive predictive value.
Pancreatic fistula remains a clinical problem following DP, and the delay in its diagnosis and treatment can lead to serious morbidity and even death. Patients with risk factors for surgical complications require special attention during the postoperative period. At our hospital, the percentage of POPF after DP is 38.6%, similar to the 27.2% for grade B or grade C POPF previously reported in the scientific literature18.
When POPF is identified, the clinical scenarios involved cover a broad spectrum. In the most frequent situation associated with a grade A POPF, there is no need for further intervention. However, a considerable number of patients with grade B or grade C POPF will require further interventions, such as parenteral antibiotic therapy, percutaneous/endoscopic drainage, angiographic procedures, and/or surgery, with cost implications for the healthcare system. The rise in costs was demonstrated by the increase in hospital LOS from 8.2 to 22.9 days, between patients with clinically significant and clinically insignificant POPF, in our study population.
The only published meta-analysis on POPF after DP, by Peng et al., indicates that soft pancreatic texture (OR 1.8, 95% CI 1.08-3.02; p = 0.03), higher BMI (OR 2.19, 95% CI 1.35-3.56, p < 0.001), blood transfusion (OR 1.5, 95% CI 1.11-2.16; p = 0.02), intraoperative blood loss (OR 2.25, 95% CI 1.54-3.29; p < 0.0001), and prolonged operative time (OR 1.67, 95% CI 1.08-2.58, p < 0.0001) are significant risk factors for POPF18. However, many of the studies in that meta-analysis did not include the grade of POPF, reflecting that markers for clinically significant pancreatic fistula after DP remain poorly studied. Additionally, it is common practice to place a closed suction drain at the time of surgery, to then postoperatively evacuate the pancreatic juice and reduce the incidence of intra-abdominal collections, abscesses, or bleeding, as well as to earlier detect pancreatic leaks and hemorrhage. Nevertheless, some studies and clinical trials have demonstrated that routine drain placement after DP is not associated with an increase or decrease in the complication rate, or a reduction in the need for postoperative intervention.5,19,20. Moreover, other authors have suggested that drains might favor POPF and the development of intra-abdominal abscesses21. Thus, a proper serologic marker for identifying patients that will present with a grade B or grade C POPF would help to focus care and resources, in a timely manner, and distinguish the patients that would benefit from early drainage removal from those that would not. The NLR and PLR could aid in such patient stratification, given that systemic inflammation is a main component of intra-abdominal complications after surgery, and peripheral blood tests could reflect inflammatory conditions within the abdominal cavity, such as pancreatic fistula development. The association with intra-abdominal complication, between the NLR and PLR, reflects a response to local tissue damage22, in which neutrophilia inhibits the immune system by suppressing lymphocytes, activated T cells, and natural killer cells23, and lymphopenia decreases the anti-tumor cellular immune response24. Therefore, both neutrophilia and lymphopenia lead to a systemic inflammatory response. In our present work, the NLR and PLR were studied as serologic markers for clinically significant POPF, establishing a threshold of 8.3 (PPV 40%, NPV 86%) for the NLR, with an AUC of 0.71, sensitivity of 81%, and specificity of 62%, whereas a threshold of 33.2 (PPV 50%, NPV 84%) for PLR was established, with an AUC of 0.72, sensitivity of 72%, and specificity of 71%. To the best of our knowledge this is the first study to evaluate the prognostic value of the NLR and PLR, for clinically significant POPF.
ConclusionBased on our results, the NLR and PLR have good prognostic value as serologic markers for clinically significant POPF at POD 3, with a threshold of 8.3 (PPV 40%, NPV 86%) for the NLR, with an AUC of 0.71, sensitivity of 81%, and specificity of 62%; and a threshold of 33.2 (PPV 50%, NPV 84%) for the PLR, with an AUC of 0.72, sensitivity of 72%, and specificity of 71%. In the proper surgical scenario, we recommend that patients with the abovementioned NLR and PLR cutoff values at POD 3 should be closely monitored, avoiding early drain removal, given that those markers could reveal an inflammatory state that is not clinically evident at POD 3.
Financial disclosureNo financial support was received in relation to this study/article.
Author contributionsConception and design of the study: Sánchez-Morales and Domínguez-Rosado.
Data recollection: Cisneros-Correa and Lanzagorta-Ortega.
Data analysis and interpretation: Sánchez-Morales and Cisneros-Correa
Drafting of manuscript: Sánchez-Morales and Pérez-Soto.
Manuscript approval: Domínguez-Rosado and Chan.
Conflict of interestThe authors declare that there is no conflict of interest.