Colorectal cancer (CRC) is one of the most prevalent cancers worldwide, and significantly contributes to cancer-related deaths. Most cases arise from adenomatous polyps. Biomarkers currently play an important role in tumor progression. Our aim was to identify perivascular mast cells and analyze the expression of laminin-332, MMP-9, and VEGF in cases of adenoma and CRC in humans.
Materials and methodsPatients were selected at the Coloproctology Service and samples were obtained through biopsies. Adenoma and CRC slides were examined, utilizing immunohistochemistry to detect molecules, and were processed, using 1% Alcian Blue (pH 0.5) for mast cell staining.
ResultsHigher density of perivascular mast cells was observed in adenomas. Laminin-332 expression revealed basement membrane discontinuity associated with tumor invasion in CRC. MMP-9 immunostaining in adenoma was detected in glandular epithelium and lining epithelium, in areas close to the basement membrane, whereas in CRC, the enzyme was found in the cytoplasm of invasive clusters. VEGF expression was associated with cell atypia in adenoma and in areas of disorganization of the epithelium-connective tissue interface in CRC. VEGF has also been detected in endothelial cells from microvessels.
ConclusionsWe demonstrated the different patterns of perivascular mast cells and molecular expression in colorectal neoplasms. Those analyses favor the recognition of the predisposition to the disease, or its early stage, and have the potential to define the molecular profile of the lesions.
El cáncer colorrectal (CCR) es uno de los más prevalentes en el mundo y contribuye significativamente a las muertes relacionadas con el cáncer. La mayoría de los casos surgen de pólipos adenomatosos. Actualmente, los biomarcadores juegan un papel importante en la progresión tumoral. Nuestro objetivo fue identificar mastocitos perivasculares y analizar la expresión de laminina-332, MMP-9 y VEGF en casos humanos de adenoma y CCR.
Materiales y métodosLos pacientes fueron seleccionados en el Servicio de Coloproctología y las muestras se obtuvieron mediante biopsias. Las laminillas de adenoma y CCR se examinaron mediante inmunohistoquímica para detectar moléculas y se procesaron con azul Alcian al 1% (pH 0,5) para la tinción de mastocitos.
ResultadosSe observó una mayor densidad de mastocitos perivasculares en los adenomas. La expresión de laminina-332 reveló discontinuidad de la membrana basal asociada con la invasión tumoral en el CCR. Se detectó inmunotinción de MMP-9 en adenoma en epitelios glandulares y de revestimiento en áreas cercanas a la membrana basal, mientras que en CCR la enzima se encontró en el citoplasma de grupos invasivos. La expresión de VEGF se asoció con atipia celular en adenoma y en áreas de desorganización de la interfaz entre el epitelio y el tejido conectivo en CCR. Se ha detectado VEGF en células endoteliales de microvasos.
ConclusionesDemostramos los diferentes patrones de mastocitos perivasculares y expresión molecular en neoplasias colorrectales. Estos análisis favorecen el reconocimiento de la predisposición o estadio temprano de la enfermedad y tienen el potencial de definir el perfil molecular de las lesiones.
Colorectal cancer (CRC) is the third most common type of cancer in women and men1. Most cases arise from adenomatous polyps, and in addition to clinical and nutritional aspects, the expression of different molecules and changes in the microenvironment play an important role in the tumorigenesis of CRC2,3.
Vascular endothelial growth factor (VEGF) plays a critical role in angiogenesis, which is responsible for the nutritional supply of proliferating neoplastic cells, that in turn, provides favorable conditions for metastatic dissemination4,5. In the immune system, mast cells are related to neoplastic progression, including the promotion of angiogenesis6. When released by cancer cells, VEGF stimulates the migration of mast cells to hypoxic areas, and production factors stimulate the infiltration of more mast cells7.
Matrix metalloproteinases (MMPs) are proteinases involved in the remodeling and degradation process of the extracellular matrix (ECM). They are released by several defense cells and by tumor cells, in response to a variety of stimuli8. MMPs (such as MMP-2 and MMP-9) are involved in the initiation of cancer, in addition to invasion and metastasis. Degradation of the basement membrane, with the generation of laminin-332 and type IV collagen fragments, results in cell adhesion loss and metastasis formation9,10. MMP-9 expression is considerably increased in most neoplasms, showing an important proteolytic action11,12.
Laminin-332 is a glycoprotein composed of three chains (α3β3γ2) and provides support for cells that are a source of cytokines and growth factors. Laminin-332 performs several functions in normal epithelial homeostasis, such as inducing and maintaining cell polarity, adhesion, proliferation, and differentiation, as well as supporting the repair of epithelial tissue13. Studies demonstrated the expression of laminin-332 fragments in human cancers, as an indication of poor prognosis and induction of migration14,15.
Thus, the aim of the present study was to evaluate the presence of perivascular mast cells and the expression of VEGF, MMP-9, and the laminin-332 γ2 chain in human adenomas and CRCs within the process of colorectal tumorigenesis.
Materials and methodsA retrospective, descriptive and comparative study was carried out. Patients seen at the Coloproctology Service of a hospital in Brazil, between 2014 and 2016, were selected. All patients underwent a colonoscopy procedure and there were no changes in the care protocol. Samples were obtained from excisional and incisional biopsies of colonic lesions suspected of adenoma or CRC. Slides were prepared and assigned by the Laboratory of Pathological Anatomy connected with the Coloproctology Service. The following inclusion criteria were adopted: clinical profile, availability of lesion data (histopathological diagnosis), and sufficient tissue for analysis.
Histochemical and immunohistochemical analysesMouse anti-γ2 chain laminin-332 antibody (clone B-2: sc-25341, Santa Cruz Biotechnology, Santa Cruz, CA), at 1:100 dilution; rabbit anti-MMP-9 (whole molecule-ab 38898, Abcam), at 1:500 dilution; and rabbit anti-VEGF (ab46154, Abcam), at 1:500 dilution, were used for detecting the γ2 chain of laminin-332, MMP-9, and VEGF, respectively. 3 µm slides underwent deparaffinization and rehydration, after which a citrate buffer (pH 6.0) was used for 30 min at 93 °C for heat-induced antigen retrieval for the γ2 chain of laminin-332 and MMP-9 from the incubated slides. VEGF slides were pretreated in Tris-EDTA pH 9.0 buffer at 95 °C for 20 min. The sections were then treated with protein block solution (DPB-125) (Spring Bioscience, California, USA) for 10 min at room temperature and incubated with the respective primary antibody in a humidified chamber overnight at 4 °C. Endogenous peroxidases were blocked with hydrogen peroxide block (DHP-125) (Spring Bioscience) for 10 min. Immunodetection was performed with a biotin-free immunoenzymatic antigen detection system (Reveal System, SPB-999) (Biogen, Sao Paulo, Brazil). Briefly, the sections were incubated with complement reagent (DCMT-999) (Spring Bioscience) for 10 min and then with horseradish peroxidase (HRP) conjugate for 15 min. Immunoreactivity was visualized using 3.3′-diaminobenzidine (DAB) (DABC-004 and DABS-125) (Spring Bioscience) as the chromogen. Lastly, the slides were lightly counterstained with Mayer’s hematoxylin. After being washed with water and dehydrated in alcohol series, the slides were cleaned using xylene and mounted with dibutylphthalate polystyrene xylene (DPX) (Sigma-Aldrich, Darmstadt, DE). The slides were thoroughly washed with PBS during the immunohistochemistry process, and negative controls were performed omitting the primary antibody. One percent Alcian Blue (pH 0.5) staining was performed for perivascular mast cell analysis. For the positive control, we used normal colorectal mucosa tissue obtained during colonoscopy.
Microscopic analysisThe sections were analyzed for clinical information and scored blindly by a single investigator using a PrimoStar microscope with a Zeiss AxioCam ERC5s digital camera (Carl Zeiss Vision GmbH0) (Carl Zeiss, Oberkochen, DE) and AxioVision 4.2 Release 4.8.2 imaging software (Carl Zeiss Vision GmbH) (Carl Zeiss). For mast cell staining, only cells near vessels (<0.5 mm distance) were quantified. Mast cell density per vessel was established (M/V) and groups were defined as <1 M/V and ≥1 M/V. A total of 5 and 10 fields were observed in adenoma and CRC, respectively. Expression of the γ2 chain of laminin-332 was evaluated according to tissue sites: basement membrane and epithelial compartment. In basement membrane staining, absence and continuity were considered. Regarding MMP-9 and VEGF, the labelling index was defined in the parenchyma (lining or glandular epithelium) and stroma cells. All slides were evaluated at ×40 and ×100 magnification.
Statistical analysisThe chi-square test and Fisher’s exact test were used for the perivascular mast cell analysis of the positive/negative cells close to the vessels and M/V. The results were considered significant with a p < 0.05. GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) was used for the statistical analysis.
Ethical considerationsThe present work met all the human research ethics codes, according to the Declaration of Helsinki, and was approved by the Hospital Ethics Committee (process number 406.002). All patients agreed to participate and signed statements of informed consent.
ResultsA total of 66 patients were selected: 58 cases of adenoma (87.88%) and 8 cases of CRC (12.12%). Concerning tumor grade and dysplasia degree, the majority of adenoma lesions were classified as tubular (82.92%) and had low-grade dysplasia (85.36%); only 9.75% were classified as tubulopapillary and 7.33% as serrated.
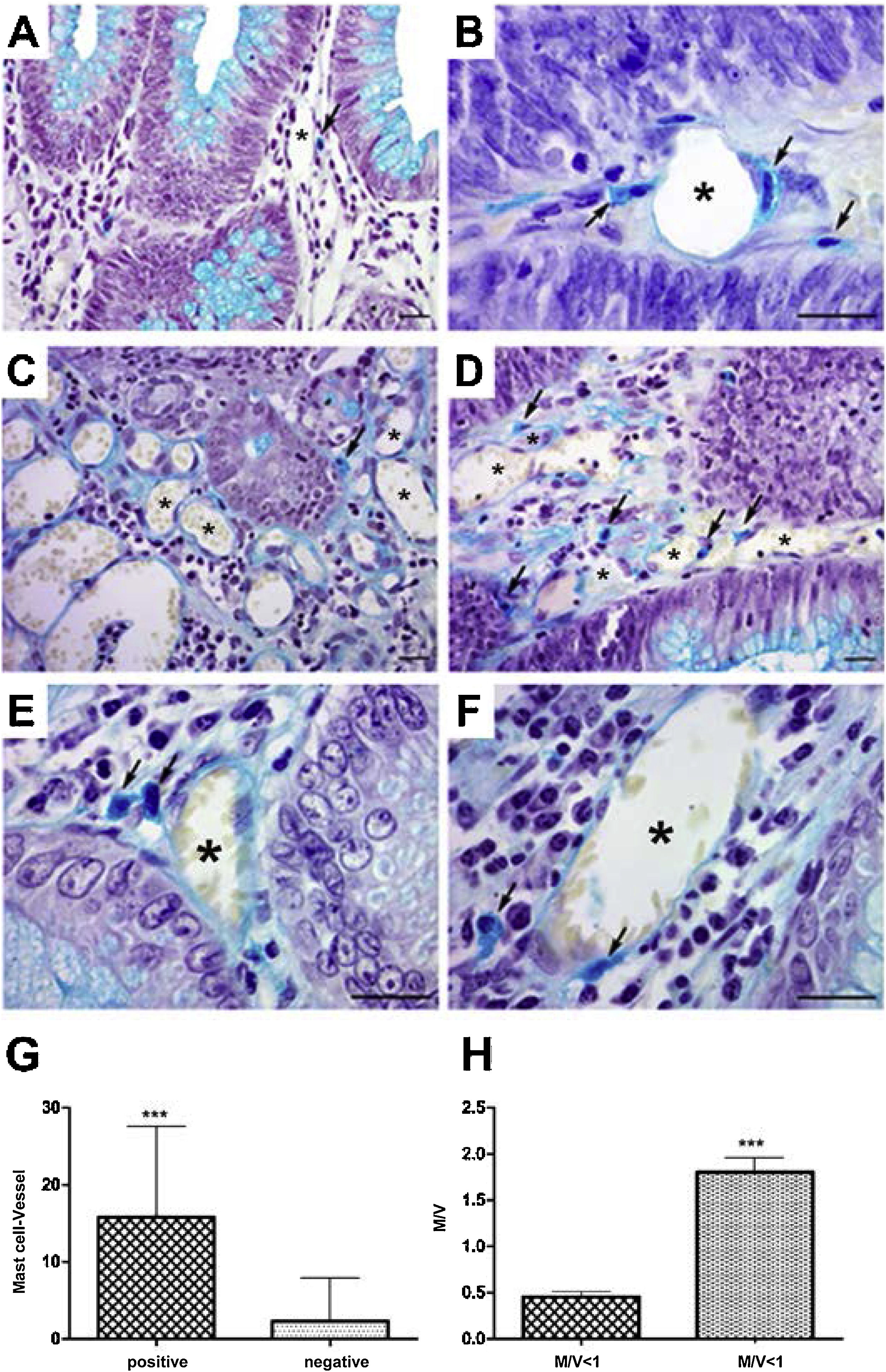
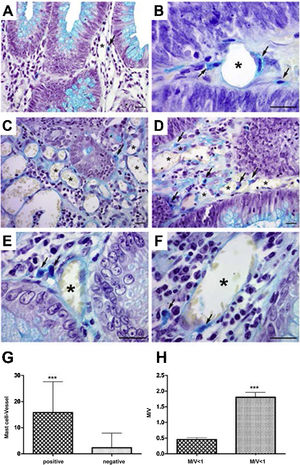
In the analysis of perivascular mast cells, samples of adenomas showed isolated cells in the vascular wall (Fig. 1A), as well as groups of cells around the vessel (Fig. 1B [arrows]). In the CRC, mast cells could be seen as isolated cells (Fig. 1C [arrow]), in groups (Fig. 1D [arrows]), and in the vascular wall (Fig. 1E and F [arrows]). Quantitative analysis showed a positive correlation between the presence of mast cells close to the vessels (p < 0.001) (Fig. 1G) and high cell density (M/V) (p < 0.001) in the adenoma samples (Fig. 1H).
Perivascular mast cells in the neoplasms. A) Mast cells in small vessels near the glandular epithelium. B) Mast cells near and in the vessel wall (arrows) in adenoma. C) Distribution of mast cells in colorectal cancer, isolated (arrow) and D) grouped (arrow). E) Presence of mast cells close to vessels (arrows) and F) in the wall (arrow).
Staining: Alcian Blue; scale bar = 20 μm.
G) Quantitative analysis showing positive correlation between mast cells close to vessels and H) mast cell density in adenoma samples.
M/V: mast cell/vessel; mean: 0.44 (<1 M/V), 1.90 (≥1 M/V); standard deviation: 0.25 (<1 M/V), 0.91 (≥1 M/V).
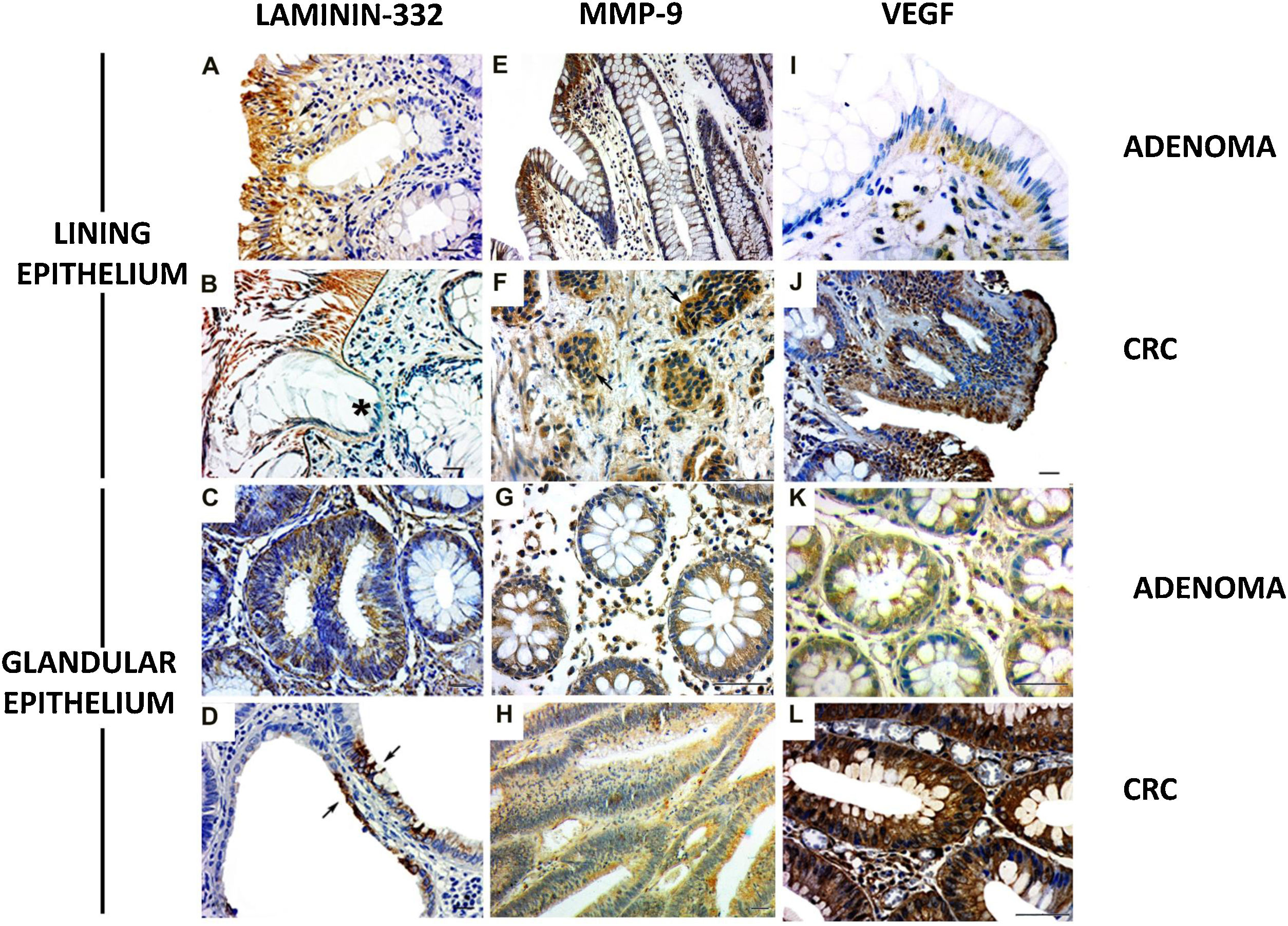
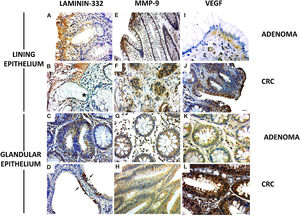
The γ2 chain of laminin-332 has been observed in benign and malignant lesions. The expression was present in the basement membrane and in the cytoplasm of the lining epithelium (Fig. 2A and B) and the glandular (Fig. 2C and D [arrows]) epithelium, although greater expression was observed in the former. In addition, there was discontinuity of the basement membrane at the tumor invasion sites (Fig. 2B [*]). Regarding MMP-9, the cases of adenoma showed cytoplasmic expression in the lining epithelium and in the stromal cells close to the basement membrane (Fig. 2E). The CRC lesions showed MMP-9 expression in the cytoplasm of invasive clusters of malignant cells (Fig. 2F [arrows]). There was MMP-9 expression in the glands (Fig. 2G). Enzyme expression was also detected in the glandular epithelium and in vessels of the CRC cases (Fig. 2H).
Expression of laminin-332, MMP-9, and VEGF in colorectal neoplasms. A) Presence of γ2 laminin-332 in cytoplasm of lining epithelium and basement membrane in adenoma sample. B) In CRC, laminin expression demonstrated the discontinuity of the basement membrane at the tumor invasion sites (*). C) Discrete laminin expression in the cytoplasm of adenoma glands. D) Intense laminin expression in glandular epithelium of CRC (arrows). E) MMP-9 expression in cytoplasm of lining epithelium and stroma, close to the epithelium. F) In CRC, MMP-9 expression was in the cytoplasm of invasive clusters of malignant cells (arrows). G) The cytoplasm of adenoma glands showed MMP-9 expression in a few cells. H) Most of the altered CRC glands had cytoplasmic expression of MMP-9. I) VEGF expression in adenomas was observed in the cytoplasm of the lining epithelium and stromal cells. The endothelium of the stromal vessels also showed immunostaining. J) In CRC, VEGF expression was found in areas of disorganization of the epithelium-connective tissue interface. K) VEGF was discretely expressed in the glands in adenoma when compared with the glands in CRC. L) The malignant neoplasm also had vessels with VEGF expression.
Immunohistochemistry: scale bar = 20 μm.
VEGF expression in adenomas was observed in the cytoplasm of the lining epithelium and stromal cells. The endothelium of the stromal vessels also showed immunostaining (Fig. 2I). In the CRC, there was VEGF expression in the areas of epithelium-connective tissue interface disorganization (Fig. 2J). In the adenoma glands, expression was present in the cytoplasm, but not evident in the stroma (Fig. 2K). With respect to the glands in the CRC samples, VEGF was also detected in the cytoplasm (Fig. 2J), and the stroma showed important immunostaining in microvessel endothelial cells (Fig. 2L).
Discussion and conclusionsIn the study described herein, we evaluated the presence of perivascular mast cells and the expression of the γ2 chain of laminin-332, MMP-9, and VEGF, in benign and malignant lesions at the colorectal site. Our data showed that adenomas had a higher density of mast cells close to vessels than CRC did. Although the study molecules were found in both groups, the γ2 chain protein was highly expressed in the cytoplasm of malignant tumor cells. Basement membrane disruption in CRC lesions, an association between VEGF expression and cell atypia, and MMP-9 expression at the invasive fronts were also observed.
CRC is the third most widely diagnosed cancer, and the third most lethal, in both sexes1. The majority of CRCs develop from the normal epithelium-adenoma-carcinoma sequence2. Certain processes then precipitate or influence local invasion and metastasis, which are features related to poor prognosis16. The tumor microenvironment is formed by different stromal cells, fibroblasts, and the tumor itself. In addition to the cellular component, there are also ECM and vascular structures. The biological events that participate, both in the progression of the tumor and in the attempts to eliminate the altered cells, are coordinated by signals from that microenvironment17. The role of microsatellite instability (MSI) in cancer is well defined as a CRC screening tool in patients with Lynch syndrome, but the role of MSI in sporadic cancer has not yet been accurately described18. Some studies have indicated that tumors with the MSI phenotype are less aggressive, predict sensitivity to immunotherapy, and are associated with good prognosis, but said phenotype is not found in the majority of cancers18,19.
Mast cells are best known for their participation in allergy. Nevertheless, in the context of carcinogenesis, they have been reported to be an important source of proinflammatory and angiogenic mediators17. In our samples, we observed a higher density of perivascular mast cells and a close association between those cells and blood vessels in adenomas. Mast cells play an important role in the tumor microenvironment, stimulating pro-angiogenic cytokines and facilitating tumor vascularization and growth6,20–22. Heijmans et al.20 reviewed the influence of mast cells on the growth of the premalignant polyp as it progresses to CRC. Rios et al.23 demonstrated that the content of certain mast cell mediators undergoes variation, which suggests that the release of molecules related to angiogenesis and metastasis is heterogeneous, with different biological properties for each type of cancer. Mast cells could thus be associated with events that would guarantee the establishment and survival of cancer cells, such as angiogenesis. In addition, mast cells signal to other cells, including tumor cells, and act as a trigger for the expression of signaling pathways and growth factors.
Regarding laminin-332, overexpression of that glycoprotein was reported to be a marker of the mesenchymal epithelium transition process and acts as a facilitator of cancer migration24. It is also an important poor prognosis factor in breast cancer and gastrointestinal cancer14,15. The CRC samples showed a discontinuity of the basement membrane at the sites of tumor invasion, demonstrated by γ2 laminin-332 expression. On the other hand, a continuous basement membrane was described for adenomas. γ2 laminin-332 expression in the cytoplasm of the lining epithelium and glandular epithelium clearly demonstrated the change in laminin deposition, upon malignant transformation. Laminin-332 expression in the migrating edge of tumor cells and immunostaining in the cytoplasm at the invasive front could represent the initial process of mobility, invasion, and metastasis25,26. ECM has become increasingly important as an inducer of cancer development and progression3.
MMPs act in the remodeling and degradation of ECM8. Those enzymes cleave different components of the matrix and thus regulate the tumor microenvironment. That process has been associated with invasion and metastasis in several neoplasms, including CRC8,10. A study that used MMP-9 inhibitors demonstrated a decrease in tumor growth and incidence of metastasis at the colorectal site9. Our analysis showed MMP-9 expression in the cytoplasm of the glandular epithelium and lining epithelium in adenoma and CRC, particularly in areas close to the basement membrane. Similar aspects have been described by other authors10,11. Furthermore, it is important to highlight that MMP-9 expression was found in the cytoplasm of invasive malignant clusters. The correlation between that expression pattern and distant metastasis has been reported in other studies10–12. Another aspect associated with MMP-9 is the ability to induce VEGF concentration15. Secreted VEGF is a key factor in the formation of new blood vessels, an event known as neoangiogenesis4. Said process is related to invasiveness, metastasis, and poor prognosis of the disease because the tumor cells use the new vessels to grow27. In fact, experimental models in which VEGF was inhibited resulted in progression-free survival in several types of cancer, including CRC.27 In our adenoma samples, VEGF expression was detected in the lining epithelium cytoplasm and in stromal cells adjacent to the basement membrane, which was thickened. In CRC samples, the expression was found in the disorganized areas of the epithelium-connective tissue interface. VEGF overexpression in the cytoplasm of tumor cells may be associated with poor prognosis5,28, and we believe that the presence of that factor is essential, first for favoring cell proliferation when cancer sets in, and then for its metastatic spread.
The study of malignant transformation involves analyzing the expression of several molecules that are activated or inhibited, leading to changes not only in the tissue of cancer origin, but also in the stroma, which will support the growth of the lesion and serve as a route for future metastasis. The mast cells and all the molecules described in the present study play a role in the process of malignant transformation and may be related to growth, survival, invasion, and metastasis. Therefore, we consider that the histological and immunohistochemical investigation of adenomas and CRC lesions is a key tool in predicting a potential malignant transformation or determining the prognosis of an already established CRC. Such information is valuable for guiding the best therapeutic approach.
Even with a limited number of cases, our data demonstrated an increase in the density of perivascular mast cells in adenoma. Furthermore, it was possible to observe a close association between the cell and the vessels present in the stroma, in both groups. The expression of laminin-332 found in the cells of the invasion fronts and the discontinuity aspect of the basement membrane demonstrate the change in the functions of that molecule in the presence cancer. Regarding MMP-9, its epithelial expression close to the basement membrane in both neoplasms suggests a role of that enzyme in the alteration of ECM components. Lastly, the expression of VEGF in the lining epithelium, endothelial cells, and stroma may be related to the formation of new vessels. All those findings reinforce the presence of numerous changes that occur in the process of malignant transformation.
Conflict of interestThe authors declare that there are no conflicts of interest.
Financial disclosureThis work was supported by grants from the Brazilian National Council for Scientific and Technological Development (CNPq), process number: 479694 2013-3, and by the State of Espirito Santo Research Foundation (FAPES), process number: 67659870 006/2014.
Author contributionsAll authors contributed to the study conception and design. Material preparation was carried out by Luciano Pinto Nogueira da Gama de Souza, Willian Grassi Bautz, and Flavya da Silva Souza Ribeiro. Data collection and analysis were performed by Luize Meloti Fiorio, Izabela Silva Sinara Alves, Franciele Rohor de Souza, and Letícia Nogueira da Gama-de-Souza. The first draft of the manuscript was written by Luize Meloti Fiorio and Letícia Nogueira da Gama-de-Souza. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The Histotechnical Laboratory of the Federal University of Espirito Santo played an important role in the histopathological experiments. We also thank Dr. Giovanni José Zucoloto Loureiro and Dr. Rossini Cipriano Gama, from the Vitória Apart Hospital Coloproctology Service.
Please cite this article as: Meloti-Fiorio L, Silva-Sinara-Alves I, Rohor-de-Souza F, Grassi-Bautz W, Silva-Souza-Ribeiro F, Pinto-Nogueira-da-Gama L, et al. Los mastocitos perivasculares y la expresión de VEGF, laminina-332 y MMP-9 en neoplasias colorrectales humanas. Rev Gastroenterol Méx. 2023;88:361–368.