Antecedente: El desarrollo y la validación de los cuestionarios para evaluar calidad de vida (QoL) se han transformado en un área importante de la investigación. Sin embargo, la literatura revela una proliferación de instrumentos de medición no validados en el escenario de la salud, los cuales, no contribuyen al perfeccionamiento del conocimiento científico.
Objetivo: Presentar mediante el análisis de los cuestionarios validados disponibles, una lista de verificación sobre los aspectos prácticos de cómo llevar a cabo la adaptación transcultural de los cuestionarios de calidad de vida (genéricos, o específicos de una enfermedad) para que no sea olvidado ningún paso en el proceso de evaluación, evitándose de esta manera, validacione sin suficientes o incompletas.
Metodología: Hemos consultado los libros de texto básicos, la base de datos de Pubmed, usando las siguientes palabras claves: calidad de vida, cuestionarios y gastroenterología, con límites en «estudios de validación», tanto en inglés, como en español y portugués, sin límite de tiempo, con el objetivo de analizar la traducción y validación de los cuestionarios disponibles en Mapi Research y en el sitio PROQOLID.
Resultados: Se presenta una lista de verificación, para ayudar en la planificación y ejecución dela adaptación transcultural de los cuestionarios de calidad de vida, acompañado de un glosario de términos clave en esta área del conocimiento. Se utilizó el acrónimo DETAC que son 5 pasos, cada uno en referencia a una fase del procedimiento a seguir.
Conclusiones: Este artículo brinda información acerca de cómo debe realizarse la adaptación cultural de cuestionarios sobre calidad de vida y cómo minimizar errores comunes.
Background: The development and validation of questionnaires for evaluating quality of life (QoL) has become an important area of research. However, there is a proliferation of non-validated measuring instruments in the health setting that do not contribute to advances in scientific knowledge.
Aims: To present, through the analysis of available validated questionnaires, a checklist of the practical aspects of how to carry out the cross-cultural adaptation of QoL questionnaires(generic, or disease-specific) so that no step is overlooked in the evaluation process, and thus help prevent the elaboration of insufficient or incomplete validations.
Methods: We have consulted basic textbooks and Pubmed databases using the following key-words quality of life, questionnaires, and gastroenterology, confined to «validation studies» in English, Spanish, and Portuguese, and with no time limit, for the purpose of analyzing the translation and validation of the questionnaires available through the Mapi Institute and PROQOLID websites.
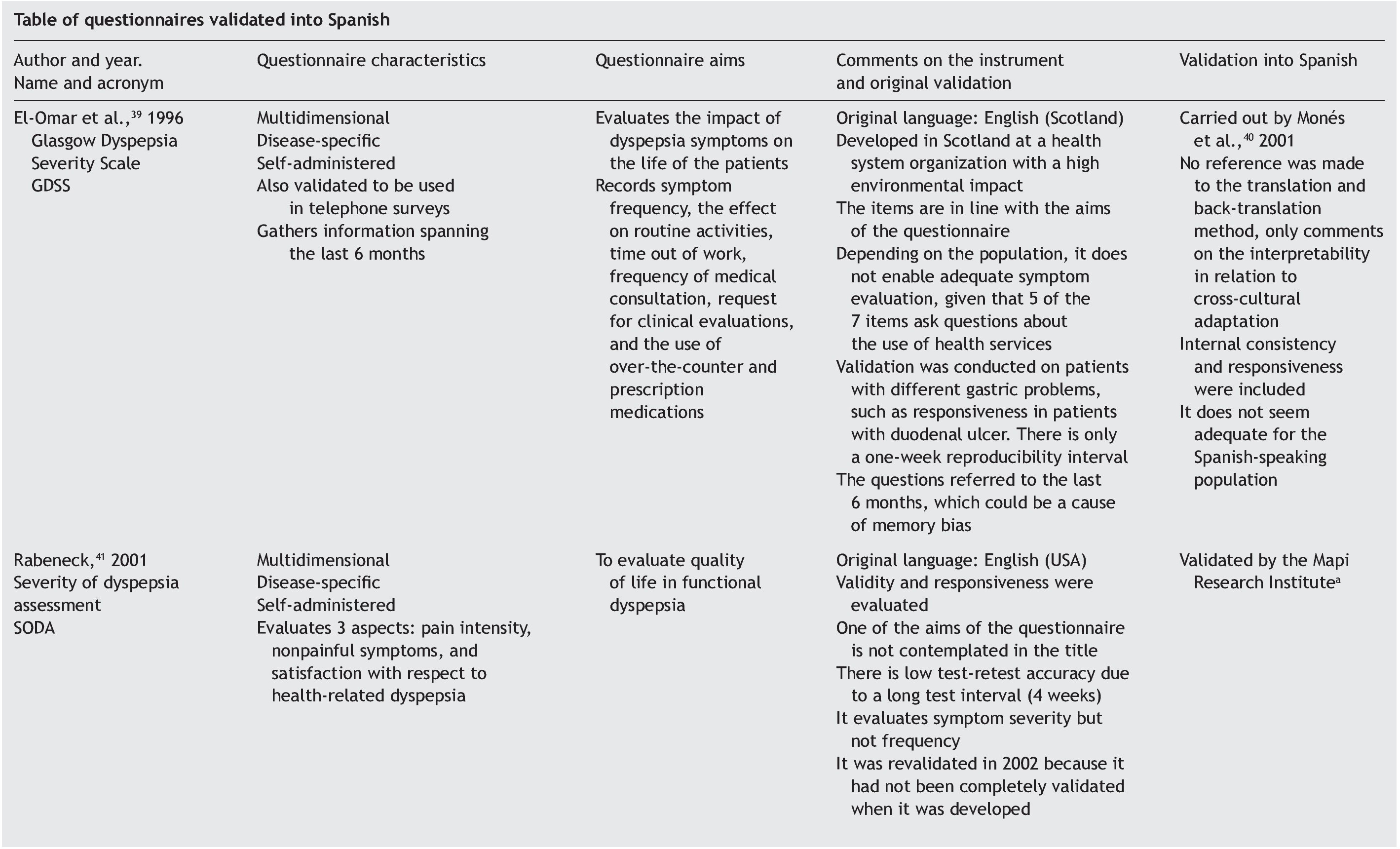
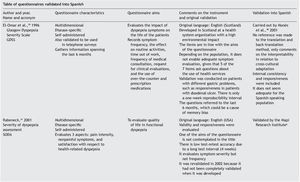
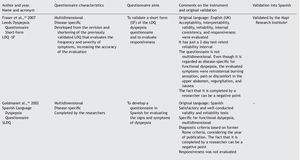
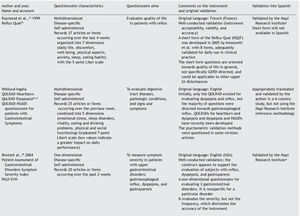
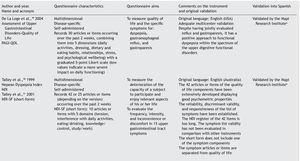
Results: A checklist is presented to aid in the planning and carrying out of the cross-cultural adaptation of QoL questionnaires, in conjunction with a glossary of key terms in the area of knowledge. The acronym DSTAC was used, which refers to each of the 5 stages involved in therecommended procedure. In addition, we provide a table of the QoL instruments that havebeen validated into Spanish.
Conclusions: This article provides information on how to adapt QoL questionnaires from a cross-cultural perspective, as well as to minimize common errors.
Introduction
Cross-cultural research has been carried out for years in the social sciences and its importance has been recognized in the health sciences, especially in the development of the concept of health-related quality of life1 (HRQOL).
In phase III clinical trials for the development of new drugs, quality of life measures have been almost systematically incorporated as one of the aims to be evaluated so that these drugs can be considered adequate for their proposed clinical application. The concept of quality-adjusted life years (QALY) is relatively new and is used to evaluate cost-benefit studies for the development of strategies in the area of health. On the other hand, it is often seen that these clinical trials use non-validated instruments for measuring quality of life, limiting the interpretation of their study data.2
Physicians and researchers that do not have adequate instruments for measuring quality of life in their languages have 2 options: to develop a new instrument or to modify one that has been previously validated into another language, known as the process of cross-cultural adaptation.2,3
In the health setting, there is a proliferation of instruments that are neither valid nor accurate, and therefore do not contribute to the advancement of scientific knowledge.3
Given this reality, the aim of the present article is to review the practical aspects of how to carry out the cross-cultural adaptation process of quality of life questionnaires, both general and HRQOL, developing a checklist that attempts to ensure that no step in this process is left out, thus avoiding the elaboration of insufficient or incomplete validations of these tools. A glossary of terms is also presented to aid in understanding the terminology used in this area of knowledge.
Methodology
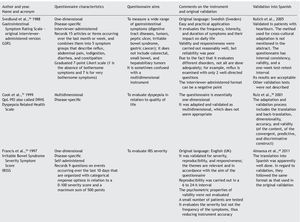
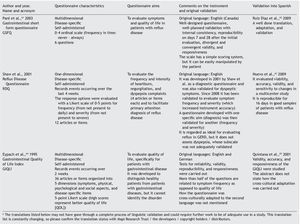
We have consulted basic textbooks and the Pubmed database, using the following keywords: quality of life, questionnaires, and gastroenterology, limiting them to "validation studies" in English, Spanish, and Portuguese, with no time restrictions, for the purpose of finding and analyzing validated general or disease-specific questionnaires in the field of gastroenterology.
The articles that resulted from the search were selected according to the study aim and analyzed with respect to the methodology used for their translation and cross-cultural validation. In addition, the questionnaires were analyzed on the available PROQOLID and Mapi Research Institute websites. After analyzing the translations and validations, common errors that impede the use of these questionnaires in clinical trials were identified; they are necessary mainly in the follow-up and evaluation of patients with functional digestive disorders. In regard to elaborating the checklist, the guidelines of the Mapi Research Institute were followed.
The initial process of questionnaire selection and study design
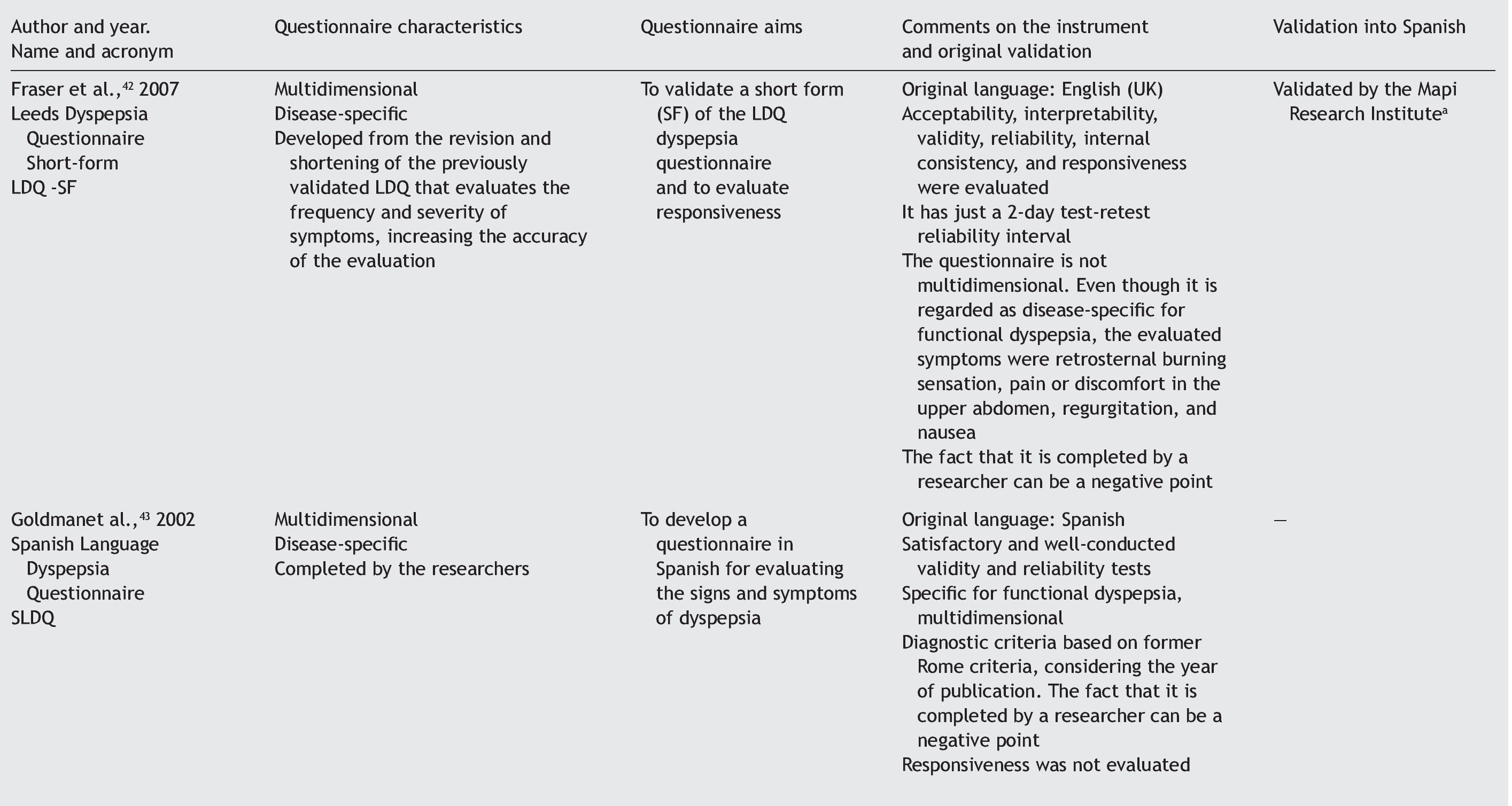
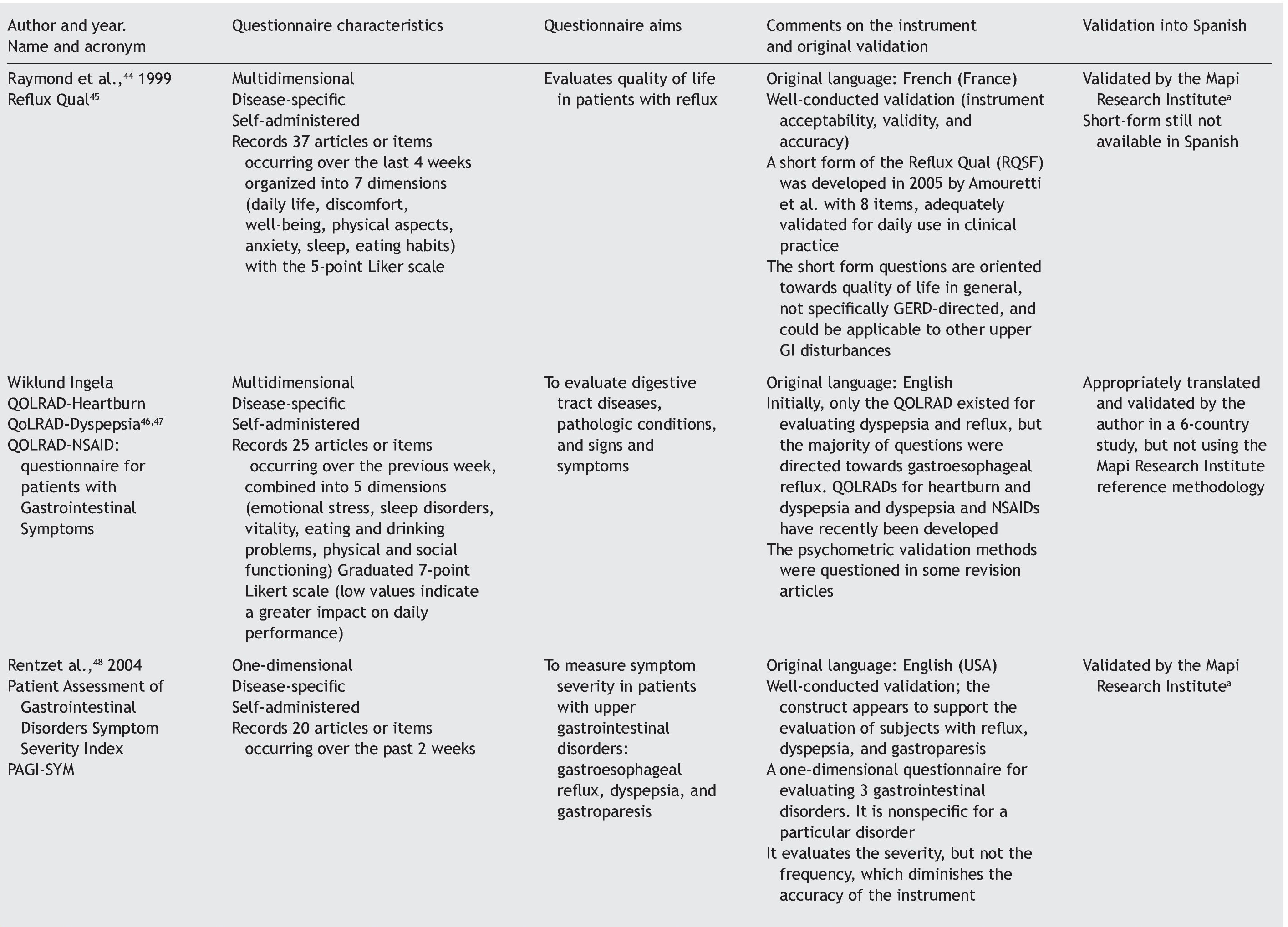
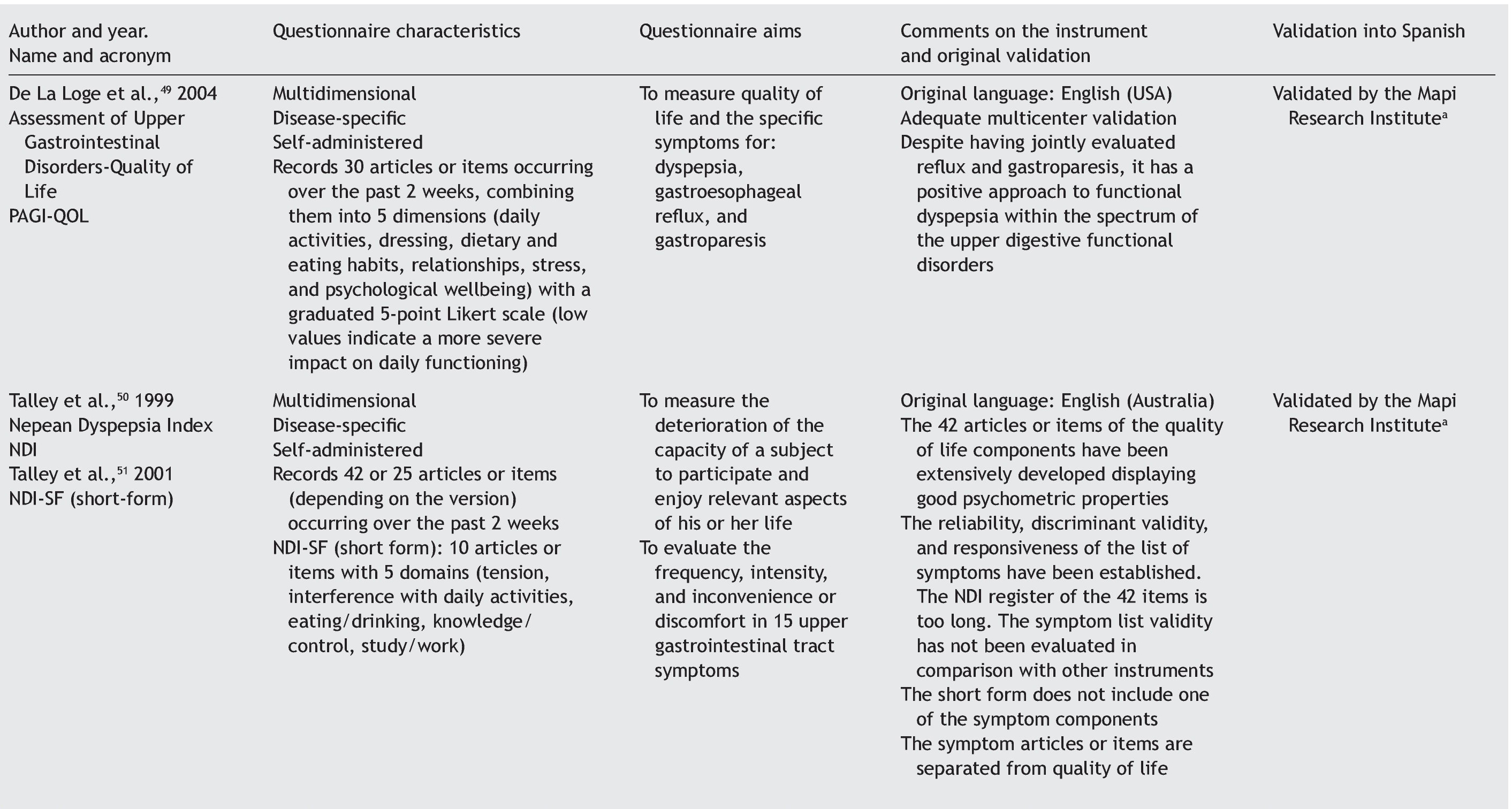
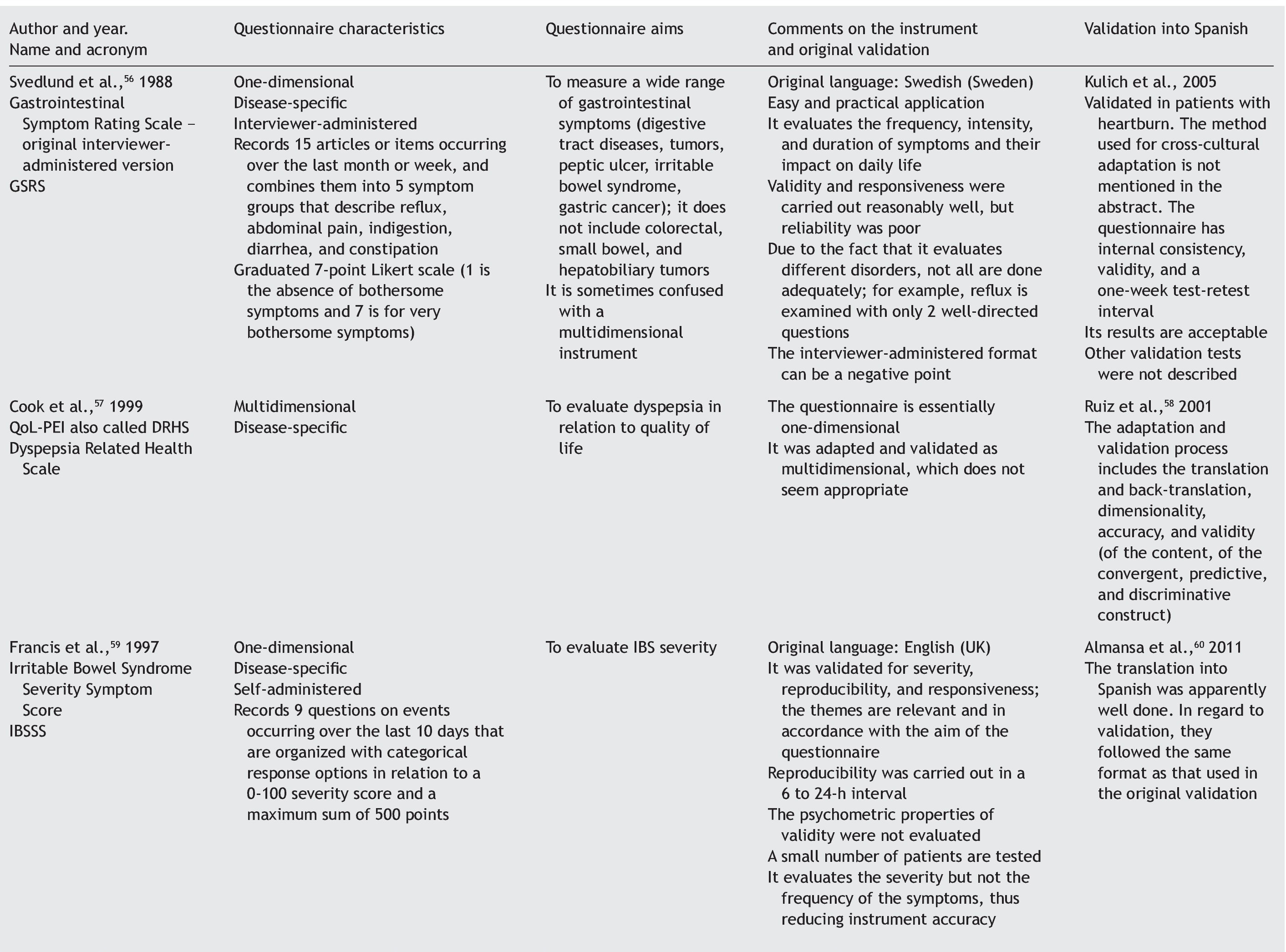
Quality of life can be evaluated by means of 2 basic approaches: through general questionnaires or through disease-specific questionnaires.4-6 When the study aim is to evaluate quality of life in general, the generic questionnaire can be chosen. When this aim is solely to evaluate the frequency, intensity, and duration of the symptoms of a given disease, then a one-dimensional disease-specific questionnaire7 should be selected. If the study aim is to evaluate HRQOL, the best choice is a multidimensional disease-specific questionnaire that examines aspects beyond the physical symptoms, such as the effect on the patient's social and emotional life and/or the impact on daily activities.
Scale selection should be determined by the content and context of its use, given that there is no single evaluation tool; because every instrument has its advantages and disadvantages, the one that best adapts to the desired aim must be found.7 The questionnaire that is to be translated and validated into another language should be one that is practical to apply and that has the capacity to be generalized, so it can be used in different populations without losing its basic characteristics.8
The translation process
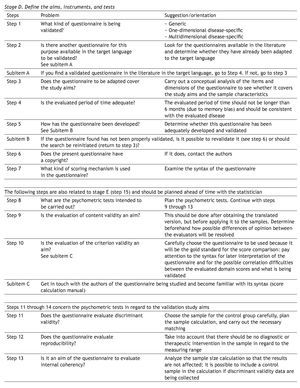
The first step in a formal translation process implies that 2 or more translators work either separately or together to produce a concordant version of the questionnaire. Another approach is the back-translation or inverse translation method in which at least 2 persons competent in the source language of the process concur.1,2,7
Questionnaire translation recommendations from the Mapi Research Institute9 and Guillemin et al.2 have been widely used in validation studies8,10-14 and employ steps that are similar to those recommended by other authors.1,7,15
The process of translation and inverse translation (back-translation), as well as the work of a review committee, should mainly focus on the semantic equivalence evaluation.7,16 But there are other equivalence aspects that must also be evaluated, such as idiomatic equivalence and experimental or cultural equivalence that involve slang expressions, sayings, or words that are particular to a given culture.2,15,16 Some translators are not aware of the strict requisites involved in the translation of cross-cultural studies, and thus waste time making a literal translation, rather than paying proper attention to cultural meanings.
The need to make adjustments to the measuring instruments is not limited to situations of different countries and/or languages; local and regional adjustments are also required. It is difficult to decide whether the translated text is in accordance with the cultural characteristics of the population on which the version will be used. When choosing terminology, how much is gained with the cultural approximation and how much is lost in terms of generalization and comparison possibilities, has to be taken into account. Linguistic changes are produced in the same population with the passage of time, sometimes making transitory adjustments necessary.16
It is advantageous to use words that are applicable to large geographic areas and cultural regions; experience has shown that an instrument is rarely used only in the country or cultural region in which it was created or for which it was adapted.7
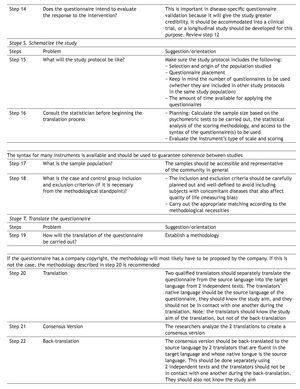
Applying the questionnaire: practical aspects
Important methodological problems are related to the decision of whether a healthy control group is necessary. One alternative is to use blood donors, because this group is naturally regarded as one of healthy volunteers. Even though it may not be the ideal control group, from a methodological viewpoint, it is the best that has been used in various studies.11,17,18 Blood donors are loyal to their centers and are considered healthy, and they tend to care about others.19
The subjects should always answer the questionnaires before their medical consultation or treatment in order to avoid measurement bias, except when response to the intervention is being analyzed; this is the test that evaluates the sensitivity of the instrument for detecting individual symptom variation.
Care should be taken that the clinical examination, diagnosis, test results, or medical rulings do not influence the answers to the questionnaire.
Linguistic validation of the questionnaires: practical aspects
The validation process of a questionnaire must also follow well-defined stages so that its usefulness and safety in clinical research is confirmed. This is produced through the gauging of clinical measurement properties known as "psychometric properties".20,21 Different psychometric requisites should be covered in the process of linguistic validation, such as reliability, validity, and response to the intervention. Reliability can be evaluated by internal consistency, reproducibility, and discriminant validity. Validity can be evaluated by content value, criterion value, and construct value.20,21Response to the intervention is regarded as a separate and distinct property from the psychometric properties of validity and reliability in quality of life questionnaires because it evaluates questions related to the sensitivity of the instrument.4,22
The majority of researchers evaluate and/or publish response to the intervention results separately or after the validity and reliability results.23-26
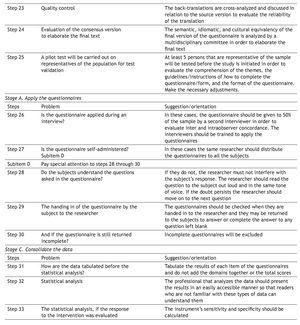
Statistical analysis and result presentation: practical aspects
To advance the statistical analysis, the score of each question and answer (item) of the questionnaire should be tabulated, as opposed to the result of the sum of the domains or the total score.
The score of added dimensions derived from the individual items should be simply expressed; for example, as a percentage in relation to the maximum score of the results. This manner of expression enables direct interpretation, even when the reader is not familiar with the instrument. When percentages are used, in a hypothetical situation, a patient could reach 60% of the possibilities of the ideal score established at the beginning of the study, and show positive or negative score variations after the procedure being studied.27
Another precaution when presenting results is whether the dimensions should be combined into a single score or not. Results of specific dimensions can better reflect the possible interactions between the intervention and the dimensions evaluated in relation to the total gross result.27
Final considerations
The development and validation of instruments for evaluating quality of life and their specific components have become an important area in medical research. However, in order to demonstrate their measurement properties, these instruments must be evaluated and re-evaluated in different situations, in different research centers, and by many researchers in different populations.15
In our analysis, we have found the following problems: questionnaires whose aims are not in accordance with the themes and questions; questionnaires originally developed for a given disease, but validated and used for others, thus laying open the risk for bias; questionnaires that evaluate long periods of time before the medical history, which is conducive to memory bias in the patients; reproducibility tests carried out in short periods of time that can also lead to patient memory bias; questionnaires validated specifically for one disease, when in reality their design is multidimensional or vice versa; scoring systems that are too complex, that make interpretation and statistical analysis difficult; questionnaires based on outdated diagnostic criteria; questionnaires designed for specific health systems that often do not state their aims in their titles; translations without back-translations and/or incomplete validations (psychometric properties).
With this scenario in mind, the present article attempted to analyze information as to how the cultural adaptation of quality of life questionnaires should be carried out and how to reduce the possibility of committing the common errors that are seen in this field of investigation.
A checklist with the acronym DSTAC was proposed to help in the planning and implementation of these types of studies, along with a glossary of key terms in this area of knowledge.
Financial disclosure
No financial support was received in relation to this article.
Conflict of interests
The authors declare that there is no conflict of interest.
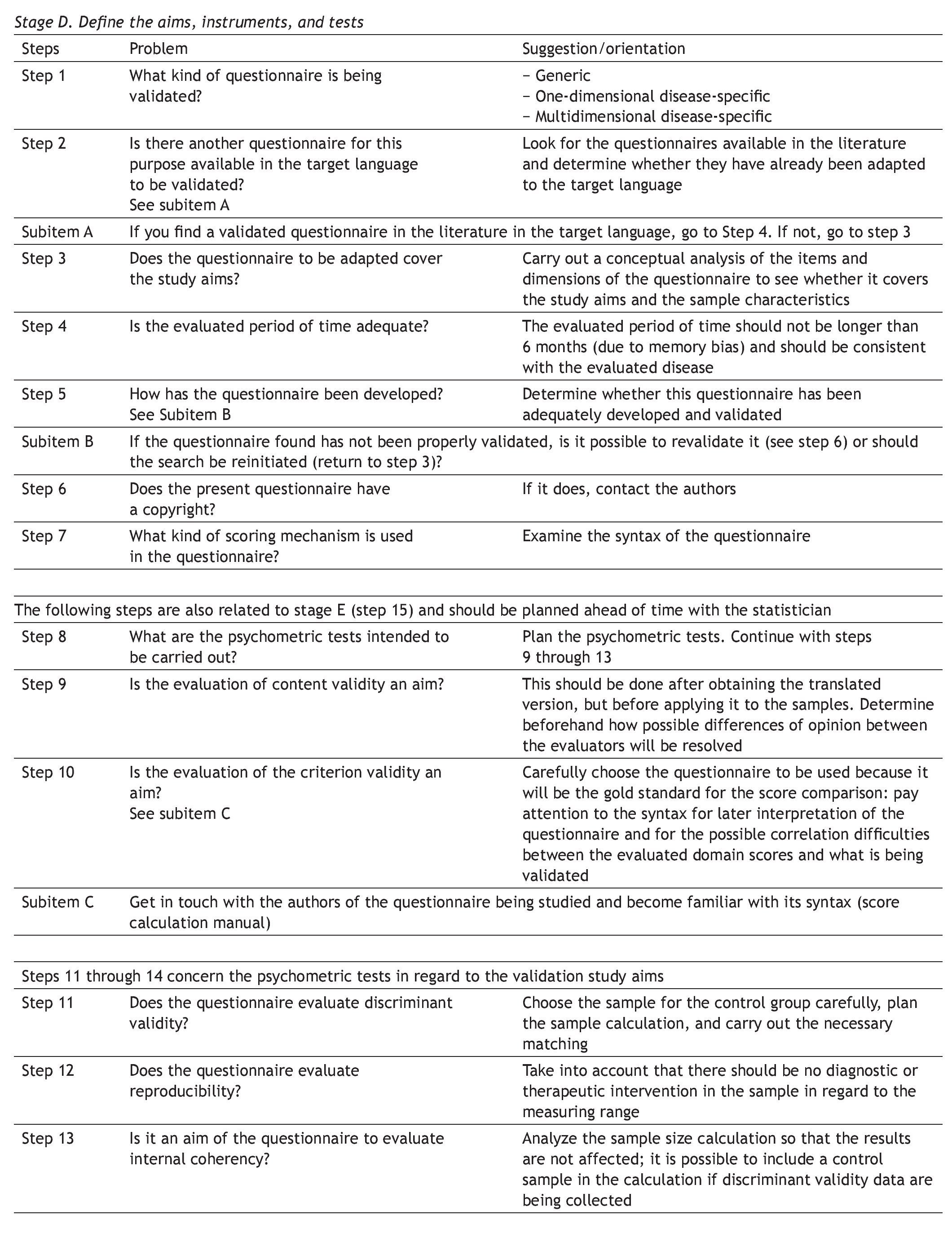
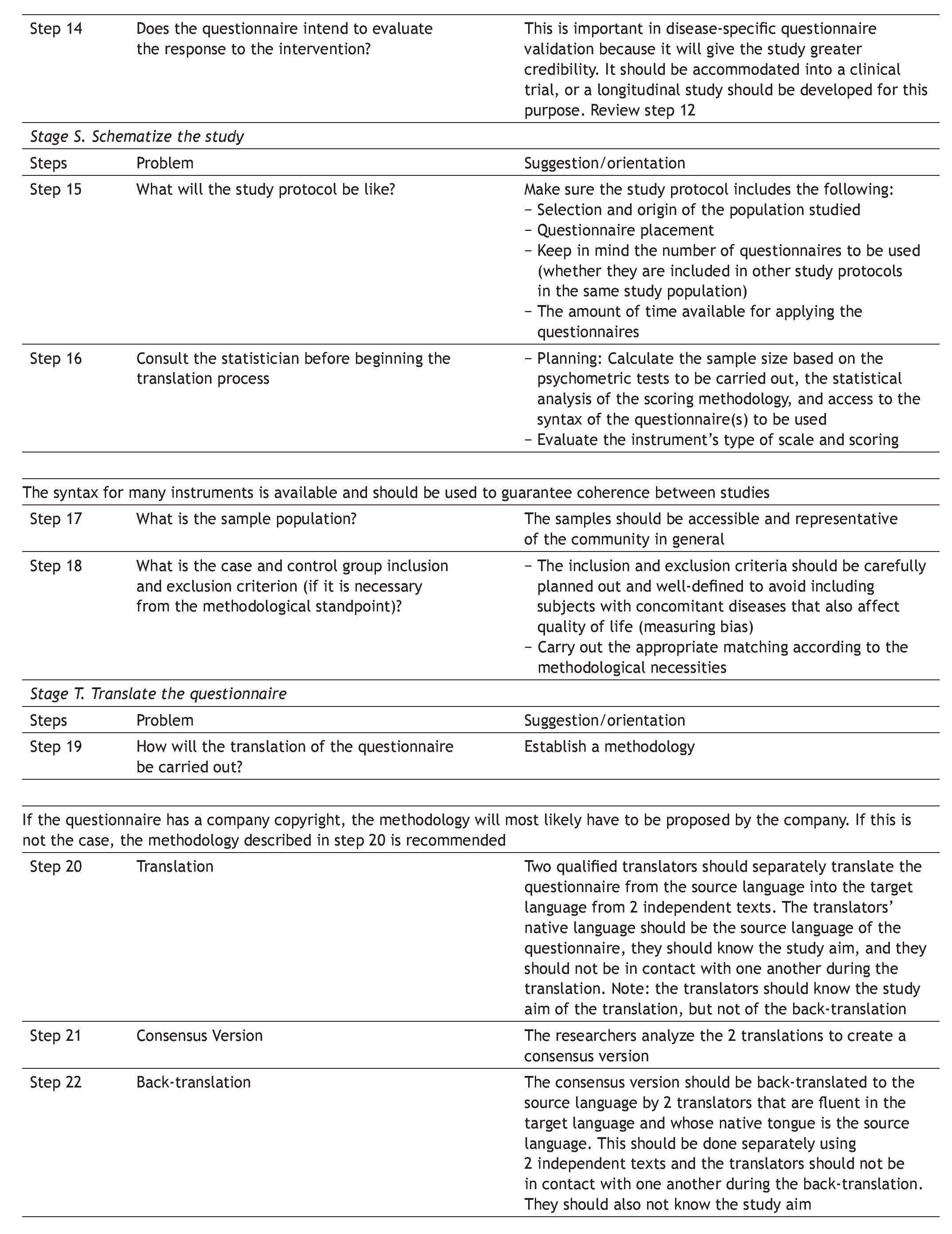
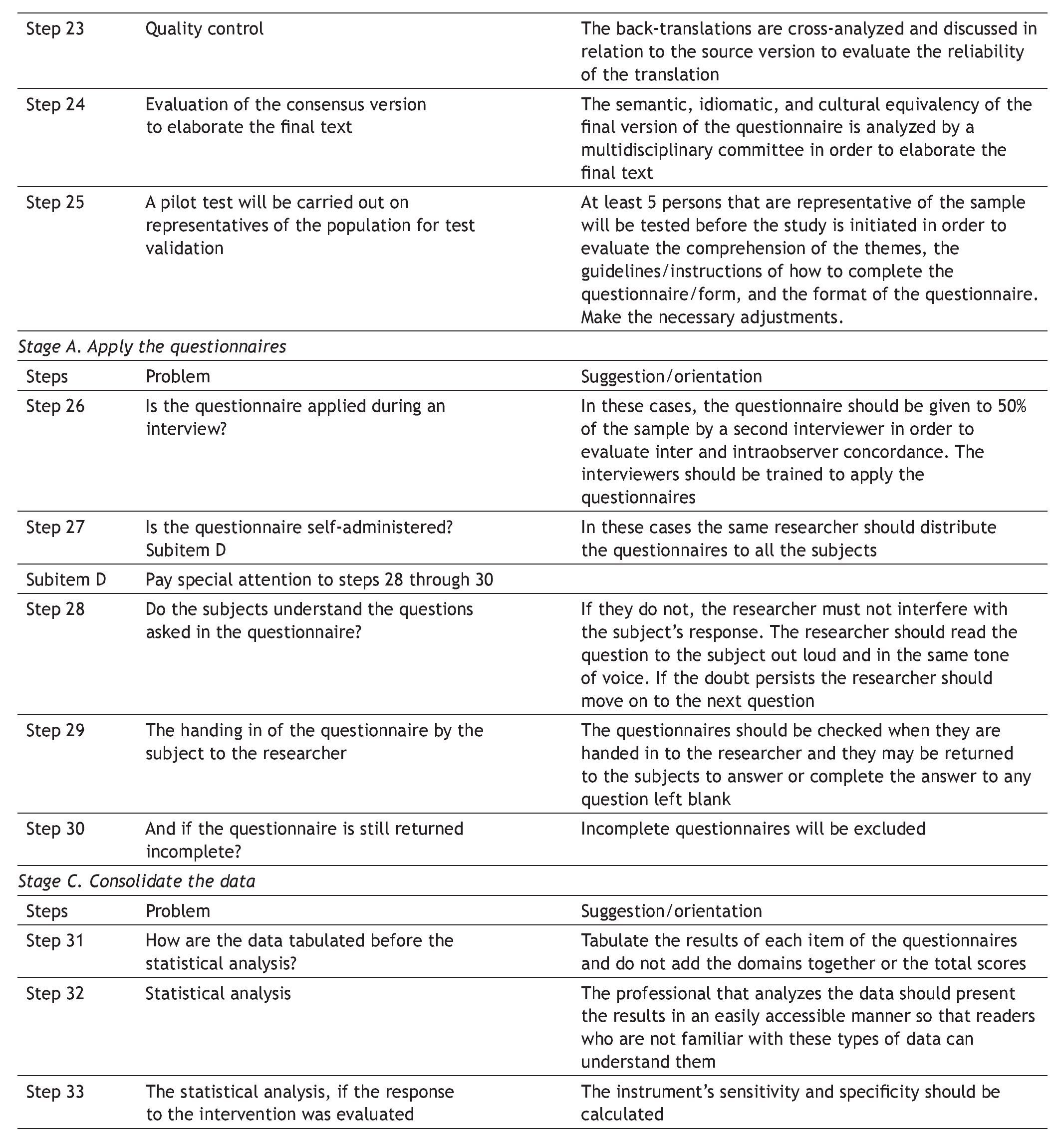
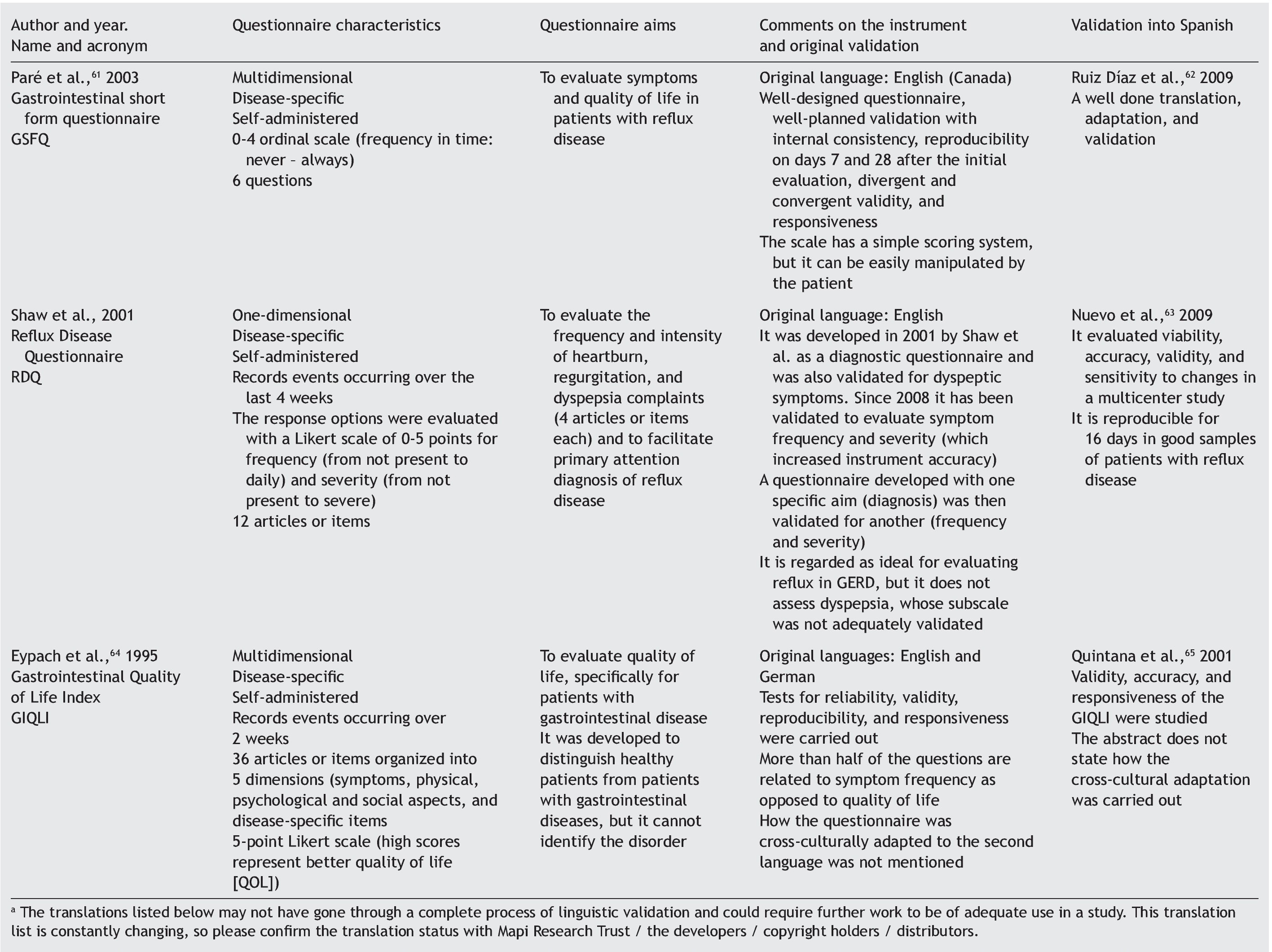
Appendix 1. DSTAC protocol for the cross-cultural adaptation of quality of life questionnaires
The aim of this protocol is to aid in the planning and culmination of cross-culturally adapted and validated generic or disease-specific quality of life questionnaires. A glossary of principal terms used in this area of research is listed at the end of the protocol.
The protocol is divided into 5 stages, each stage referring to a research phase, as follows:
D Define the aims, instruments, and tests of the study
S Schematize the study protocol
T Translate the questionnaire
A Apply the questionnaires
C Consolidate, analyze, and present the study data
Appendix 2. Glossary of common terms
Cross-cultural adaptation: the production of an equivalent instrument adapted to another culture.7
Quality of life (QOL): A subjective parameter by which direct questioning is a simple and appropriate way to gather information about how a patient feels and lives,30 improving the comprehension of the disease and treatment impact31; the perception of the individual as to his or her position in life in the context of the culture and value system in which he or she lives in relation to that individual's objectives, expectations, standards, and concerns10,32; it is the existing relation between how the individual actually lives and the desired life pattern, which represents the comparative parameter within the community itself of those persons that feel they have or do not have quality of life.17
Health-related quality of life (HRQOL): the elements of quality of life directly related to individual health. It examines how the individual feels and perceives the manifestations of disease in daily life; it is a disease severity marker.7,17,33
Generic questionnaires: these instruments attempt to measure the important aspects of quality of life, making a health profile analysis.7
Disease-specific questionnaires: instruments that attempt to measure HRQOL, focusing on the health aspects that are within the field of interest. The questionnaire can be specific for a disease, population, or for a health-related problem or function.7
One-dimensional disease-specific questionnaires: they deal with HRQOL measurements that evaluate the frequency, intensity, and duration of disease symptoms.7
Multidimensional disease-specific questionnaires: these instruments deal with measurements for evaluating domains that go beyond symptoms, such as the impact on the patient's social and emotional life and/or daily or routine activities.7
Internal consistency: a measurement used to evaluate accuracy through examining the coherence among the items and the homogeneity of the instrument; it is the most widely used measurement for estimating accuracy.7,28,29
Construct: these are abstractions, such as anxiety, pain, and fear - theoretical constructions whose aim is to organize and give meaning to our environment. Their formation is based on the relations between the measured variables that, in turn, are incalculable, and it is believed that they are responsible for the relation between the measured variables.3,7
Culture: values, beliefs, norms, and practices of a particular group of persons that direct their thoughts, decisions, and actions in a standardized way.1
Dimensions / domains: used synonymously, they signify the fragmentation of the global concept of quality of life into various components that represent the elements of the questionnaire, simplifying its evaluation; the fractioning of the overall concept of quality of life into various components, which are the dimensions, and taking the simplest evaluations.17,27
Concept and item equivalence: the exploration of the different items and areas covered by the source instrument for determining if they are relevant and pertinent to the culture to which they are to be adapted.9,16
Cultural equivalence: the evaluation of the use of terms, so that they are coherent with the experiences lived by the study population within its cultural context.2,15,16
Idiomatic equivalence: the translation of idiomatic and colloquial expressions that can rarely be translated and in these cases have to be substituted with equivalent expressions.2,15,16
Semantic equivalence: the evaluation of the equivalence of the grammar, vocabulary, and words of the source instrument with the one being adapted; it implies the capacity to transfer the meaning of the concepts contained in the source instrument to the produced version, having a similar effect on the subjects in the setting of the two cultures that participate in this process.2,7,15,16
Ethnicity: it is regarded as a measurement of cultural heritage.1
Reliability: it is the certainty and confidence that the score represents the true score, which is a question of stability during the time of result repetition; the capacity of the test to be repeated several times and produce the same result; it provides information on the stability of the construct and whether it can be generalized.7
Disease-specific instruments: they focus on measuring the HRQOL, and are centered on specific aspects of health status for the area of interest.7
Generic instruments: they evaluate all the important aspects of quality of life, making a health profile analysis.7
Multidimensional instruments: they evaluate social and emotional aspects, the impact of disease on daily life, as well as the severity, frequency, and duration of symptoms at a given period of time.30
One-dimensional instruments: they evaluate the frequency, severity, and duration of symptoms at a given period of time.30
Cross-cultural research: a term that is frequently applied to prevalence and incidence studies of different diseases or of psychosocial variables in different countries or distinct ethnic or social groups.1,7
Psychometrics: this refers to the discipline related to psychological or mental tests and to any quantitative analysis of the psychological traits or attitudes of an individual, as well as to his or her mental processes31; it is a branch of statistics that studies and measures psychological phenomena through tests that analyze the accuracy and validity of the questionnaire.3,20,21
Race: it refers to the phenotype, for example, the color of the skin.1
Reproducibility: also called test-retest, it is the test that attempts to show the accuracy of data obtained through the application of the instrument at different points in time; the obtention of similar data in repeated evaluations (temporary data repetition); the correlation between the points obtained by the same person on different occasions, usually within a 15-day interval, in the search for similar results to demonstrate data accuracy.34
Response to the intervention: a test that evaluates the sensitivity of the instrument for detecting variations in an individual's symptoms.4,35
Back-translation: to translate a document from the source language into the target language and then back to the source language.1,2,7,36
Validation: a test that evaluates whether the instrument actually measures what it is supposed to and if it maintains the measurement value when the hypotheses about the relation between the scale score and a particular criterion are confirmed.7
Discriminant validity: a test that evaluates the capacity of the instrument to distinguish between extremes, such as patient groups and control groups; it evaluates specificity, as well as the discrepancy between the questionnaire results and the variables, that theoretically, should not correlate.37
Content validity: a test through which the instrument is analyzed by reviewers that are experts in the construct's area of research, that did not participate in the experiment, for the purpose of evaluating whether the translation and the instrument content are representative of the condition intended to be analyzed (semantic equivalence and construct validity)37; it is an interview-based qualitative evaluation.7
Construct validity: a test that analyzes whether the instrument is representative of the theoretic referential of the construct being evaluated37 through statistical processes for supporting the hypothesis of the structure of the questionnaire's scale.33
Criterion validity (concurrent): a test that analyzes the quality of the construct of the instrument that is being evaluated in relation to the established and widely accepted criterion standard.34
Validity of outcome measures: the psychometric validation of a measure based on the symptoms incorporating various components such as content validity, construct validity, reliability, response capacity, and criterion validity. The participation of the patients in developing their result measurement is stressed by the Food and Drug Administration. This can be fostered by structured interview sessions, focal groups, and quality research methods. The measuring of the result must have an effective measurement range so that the instrument can detect changes in the results during the clinical trial.38
Received 8 Mar 2012;
accepted 23 January 2013
* Corresponding author at:
Servicio de Gastroenterología. Hospital de Clínicas de Porto Alegre, Brazil,
Rua Ramiro Barcelos, 2350, BomFim, Porto Alegre/RS, CEP 90035 003.
Tel. and fax: +55 51 33599874.
E-mail address:AdrianaLauffer@terra.com.br (A. Lauffer).