Even though the term hepatocellular carcinoma designates the most common type of primary liver cancer, the disease has a high level of heterogeneity due to its etiology, geographic variation, behavior, and association with specific genetic alterations. The aim of the present study was to establish, through a cluster analysis, the clinical characteristics that enable homogeneous conglomerates to be defined.
Materials and methodsAn exploratory cluster analysis was developed utilizing the K-means method for sub-classifying 119 cases of patients with hepatocellular carcinoma. Sixty-two of those patients met the inclusion criteria, as well as none of the exclusion criteria. For the cluster analysis, an n-dimensional space was defined, in which n was equal to the number of variables included in the study (n = 17). The spatial coordinates corresponded to any possible magnitude between the minimum and maximum values of the variables analyzed (age, sex, tumor volume, AFP, AST, DB, Alb, Na, INR, Cr, HBV, HCV, OH, NASH, cirrhosis, multiple tumors, and neotumor).
ResultsFour patterns with homogeneous clinical characteristics were identified, in which age at presentation, history of hepatitis B virus infection, altered liver profile with cholestatic dominance, and low albumin levels were associated with an apparently worse outcome.
ConclusionsHow heterogeneity in hepatocellular carcinoma could be reduced was shown through utilizing an unsupervised learning method to define specific subgroups, in whom known pathophysiologic mechanisms could better explain tumor behavior and define the determining prognostic factors related to the subgroups.
A pesar de que el término hepatocarcinoma se utiliza para designar tumores primarios hepáticos, su etiología, variación geográfica, comportamiento y asociación con alteraciones genéticas específicas hace de esta una enfermedad con elevada heterogeneidad. El objetivo de esta investigación es establecer características clínicas que permitan definir conglomerados homogéneos utilizando análisis de clúster.
Materiales y métodosEsta investigación desarrolló un análisis exploratorio de clúster utilizando el método K-means para subclasificar a 119 casos de pacientes con hepatocarcinoma de los cuales 62 cumplieron con criterios de inclusión y no de exclusión. Para el análisis de clúster se definió un espacio N dimensional donde N fue igual al número de variables incluidas en el studio (N = 17), las coordenadas espaciales correspondieron a cualquier magnitud posible entre el valor mínimo y el máximo de las variables incluidas (edad, volumen tumoral, AFP, AST, BD, Alb, Na, INR, Cr, HBV, OH, NASH, tumor Múltiple, Neo, HCV, Sexo, Cirrosis).
ResultadosSe identificaron cuatro patrones con características clínicas homogéneas en los cuales la edad de presentación, el antecedente de infección por virus de hepatitis B, la alteración del perfil hepático con dominancia colestásica y los bajos niveles de albúmina se asociaron con un aparente peor pronóstico.
ConclusionesSe demuestra cómo la heterogeneidad en hepatocarcinoma puede reducirse utilizando métodos de aprendizaje no supervisado para definir subgrupos específicos, en los cuales los mecanismos fisiopatológicos descritos puedan explicar mejor el comportamiento tumoral y definir determinantes pronósticos relacionados con los subgrupos.
Hepatocellular carcinoma is the most frequent malignant tumor of the liver1. Its behavior varies greatly, and its causes are numerous1. In regard to risk subtypes, screening studies for hepatocellular carcinoma in patients with cirrhosis2–3 have not taken into account causes other than hepatitis B.
The most widely used subclassification method across the globe is the Barcelona Clinic Liver Cancer (BCLC) system, which is based on three clinical characteristics: tumor size, performance status, and Child-Pugh grade of liver dysfunction4. Nevertheless, that strategy is only applicable in patients with cirrhosis of the liver and does not consider the behavior of the tumor that develops in the liver with an inflammatory burden secondary to hepatitis B virus infection or the fibrotic phenomena secondary to cirrhosis, or an apparently healthy liver. In 2018, Llovet et al., and in 2019, Harding et al. underlined the importance of subclassifying hepatocellular carcinoma, based on molecular conglomerates, to offer targeted therapies utilizing precision medicine5–6. However, the definition of conglomerates that are not molecular, but rather clinical, can aid in distinguishing the scenarios in which the determining management factors are either molecular or covariable.
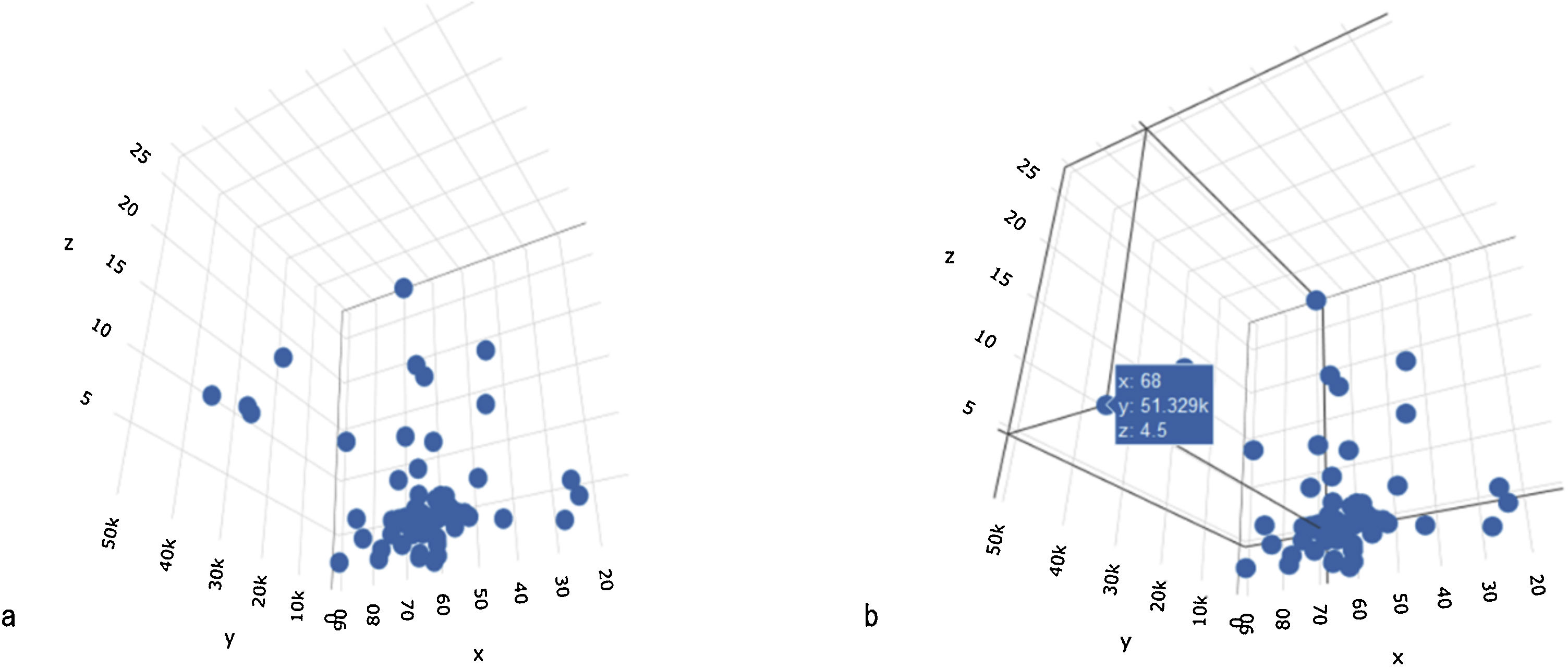
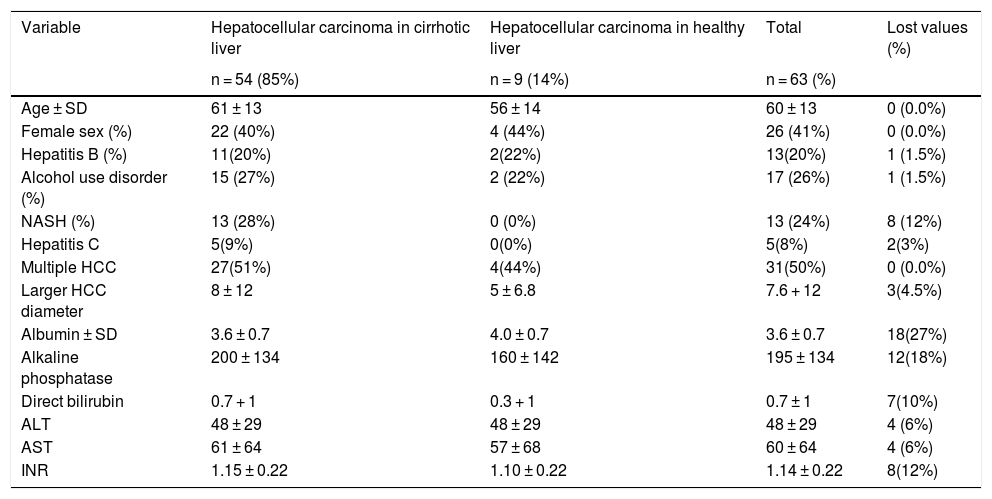
The subclassification of clinical problems, utilizing magnitude vectors, is unconsciously carried out in daily clinical practice. For example, by using the time variable as a magnitude term, we can subclassify abdominal pain as acute or chronic, which is an essential fact for the approach to that pathology. Such an example is valid for illustrating a method of univariate subclassification. The introduction of more variables can improve classification. In those cases, each magnitude vector is a dimension in which each element can be situated, according to its attributes (Fig. 1A–B).
A. N-dimensional space, configured by three variables: x = age, y = AFP levels, and z = tumor size. The three variables configure the three-dimensional space in which each case is spatially located. B. Location of a case in the n-dimensional space, according to specific values of the x, y, and z variables (age, AFP, tumor diameter).
A cluster analysis is a multidimensional evaluation, in which each dimension is defined by all possible values of a variable and the multidimensional space is defined by the quantity of variables included in the analysis.
The elevated heterogeneity in hepatocellular carcinoma led Liu et al. to carry out a cluster analysis7 on 256 patients, published in 2016, looking for genomic correlations and defining five tumor subgroups, each with a specific genomic identity. That study enabled the variability in the behavior of hepatocellular carcinoma to be defined, as well as the possibility of the existence of subgroups based on specific genomic profiles. Nevertheless, their analysis only considered the determining genomic factors and did not take into account the environment in which the disease developed, which can significantly influence the biologic behavior of the tumor8. There are presently no subclassifications of hepatocellular carcinoma, according to established tumor behavior9.
Regarding the loco-regional environment, there are no descriptive studies that aid in understanding the behavior of hepatocellular carcinoma in those patients, signifying a knowledge gap that is especially related to the identification of subgroups of patients already diagnosed with hepatocellular carcinoma. Therefore, we carried out an analysis by conglomerates, based on the presentation of hepatocellular carcinoma, its clinical characteristics, tumor behavior, and the liver status in which the tumor developed.
Materials and methodsAn analytic, cross-sectional, exploratory study was conducted. Patients with hepatocellular carcinoma that were seen at the participating healthcare institutions during the study period were the reference population.
The inclusion criteria were patients diagnosed with hepatocellular carcinoma treated at the participating hospitals.
The exclusion criteria were pregnant patients, patients with incomplete follow-up, and patients with inconsistencies in the recorded clinical history and/or incomplete studies.
Because a cluster analysis does not involve a pre-established hypothesis test, a sample size for the study was not defined.
The SAP system, which collected clinical and surgical data on the patients treated at the San Vicente Fundación university hospital during the study period, was utilized as the information source. Due to availability limitations regarding the number of cases, the validation process used in the study was not specified.
The diagnosis of hepatocellular carcinoma and/or cirrhosis of the liver was made based on magnetic resonance imaging criteria, and only selected cases were confirmed through biopsy.
Statistical analysisA cross-sectional study with a sequential analysis plan was conducted. Each variable was initially evaluated separately, determining its distribution and whether it met with the normality supposition, as well as establishing the proportion of absent data and whether there was a pattern of lost data. A correlation analysis was then performed between the continuous variables and the categorical variables. Finally, a preliminary bivariate analysis was carried out, evaluating the correlation matrix of the variables. The variables that demonstrated independence in the correlation, and those that were relevant due to biologic plausibility, were included in the cluster analysis.
To establish the presence of significant differences between groups, the statistical tests for hypothesis tests, according to the nature and distribution of the variables, were performed. The Kolmogorov–Smirnov test was employed for the continuous variables with non-normal distribution and the Mann–Whitney U test was used for testing the hypothesis. The Fisher’s exact test or the chi-square test were utilized to compare the categorical variables.
The association measures, with their respective 95% confidence intervals, were calculated for outcomes related to tumor progression time. With that information, the compound frequency tables were constructed, showing the chi square and p values of the hypothesis tests and crude ORs, as well as the Pearson correlation coefficients for continuous variables.
The lost values underwent a statistical analysis to determine the pattern of data loss, considering that, if it was above 5% and the pattern of data loss was random, a multiple imputation would be carried out for the statistical analyses.
The cluster analysis sought to classify the individuals with hepatocellular carcinoma, forming groups or conglomerates in such a way that the patients within each conglomerate were homogeneous in relation to the values adopted for each type of variable. For that purpose, an n-dimensional space was defined, in which n represented the number of variables included in the analysis. Within that space, the location of each case was determined by the magnitude of each variable, by which the spatial coordinates were defined. Fig. 1A shows the spatial representation of the cases of hepatocellular carcinoma on a plane defined by 3 variables (x = age of 68 years; y = alpha-fetoprotein level of 51,329; and z = tumor volume of 68 cm3).
The term distance in said space defined the degree of similarity or dissimilarity in each of the groupings. In other words, one observation was similar to another, to the degree that the distance separating them was the least possible. The cluster analysis method was non-hierarchical, utilizing K-means, considering that the aim of the work was exploratory. That grouping method was initially defined by an a priori cluster number between two and four, due to the anticipated limitations of sample size.
A Euclidian distance was defined in the present work to evaluate grouping patterns, more than types of displacement or magnitude between variables.
The processing and analysis of information was performed utilizing the foreign, cluster, and factoextra R statistical package. The data processing did not include the identification data of the participants and informed consent was not required, given the retrospective study design and the fact that no experimental interventions were performed.
Ethical considerationsThe present research was observational and no type of intervention or experiments on humans was performed.
Informed consent was not requested because no personal data that could identify the patients were published in the present study.
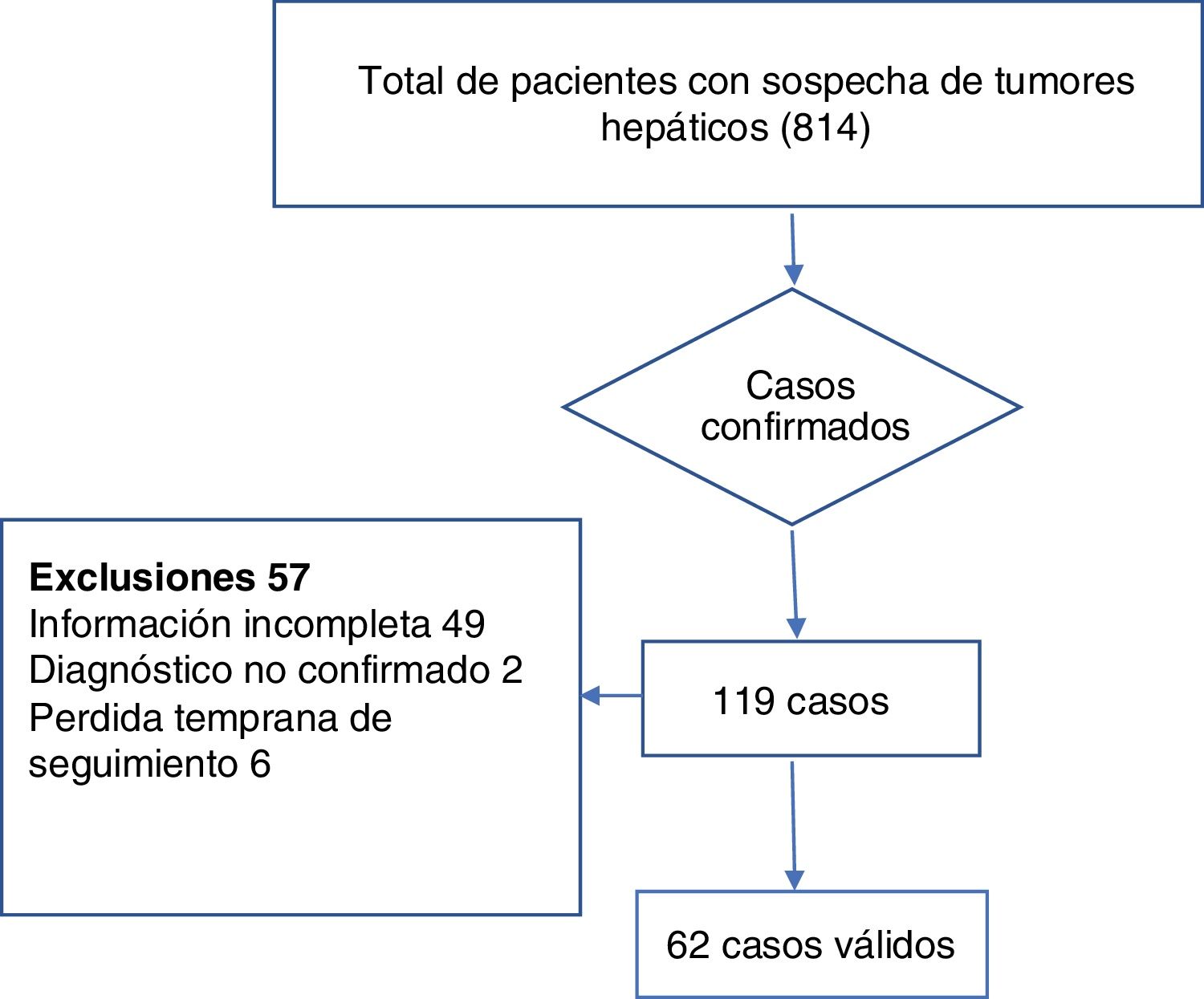
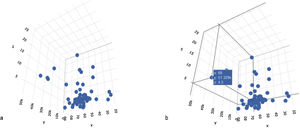
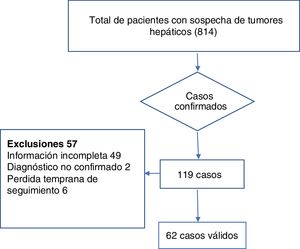
ResultsAfter excluding patients with metastasis or non-neoplastic tumors from the total of 814 patients with liver tumors, there were 119 cases of hepatocellular carcinoma, 62 of which met the inclusion criteria. Fig. 2 provides a detailed view of case selection.
Table 1 summarizes the characteristics of the study participants that developed hepatocellular carcinoma, with and without cirrhosis of the liver.
Clinical and laboratory test characteristics of the participants.
| Variable | Hepatocellular carcinoma in cirrhotic liver | Hepatocellular carcinoma in healthy liver | Total | Lost values (%) |
|---|---|---|---|---|
| n = 54 (85%) | n = 9 (14%) | n = 63 (%) | ||
| Age ± SD | 61 ± 13 | 56 ± 14 | 60 ± 13 | 0 (0.0%) |
| Female sex (%) | 22 (40%) | 4 (44%) | 26 (41%) | 0 (0.0%) |
| Hepatitis B (%) | 11(20%) | 2(22%) | 13(20%) | 1 (1.5%) |
| Alcohol use disorder (%) | 15 (27%) | 2 (22%) | 17 (26%) | 1 (1.5%) |
| NASH (%) | 13 (28%) | 0 (0%) | 13 (24%) | 8 (12%) |
| Hepatitis C | 5(9%) | 0(0%) | 5(8%) | 2(3%) |
| Multiple HCC | 27(51%) | 4(44%) | 31(50%) | 0 (0.0%) |
| Larger HCC diameter | 8 ± 12 | 5 ± 6.8 | 7.6 + 12 | 3(4.5%) |
| Albumin ± SD | 3.6 ± 0.7 | 4.0 ± 0.7 | 3.6 ± 0.7 | 18(27%) |
| Alkaline phosphatase | 200 ± 134 | 160 ± 142 | 195 ± 134 | 12(18%) |
| Direct bilirubin | 0.7 + 1 | 0.3 + 1 | 0.7 ± 1 | 7(10%) |
| ALT | 48 ± 29 | 48 ± 29 | 48 ± 29 | 4 (6%) |
| AST | 61 ± 64 | 57 ± 68 | 60 ± 64 | 4 (6%) |
| INR | 1.15 ± 0.22 | 1.10 ± 0.22 | 1.14 ± 0.22 | 8(12%) |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; HCC: hepatocellular carcinoma; INR: international normalized ratio; NASH: nonalcoholic steatohepatitis; SD: standard deviation.
In the bivariate analysis, the dichotomous outcomes related to early loss to follow-up, defined as under six months, were viewed an as indirect indicator of poor prognosis.
None of the dichotomous variables were related to the early loss to follow-up in the chi-square test result.
A correlation analysis of continuous variables was conducted that produced low correlation levels for transaminases. Only aspartate aminotransferase (AST) was included in the conglomerate analyses due to the lack of independence between the AST and alanine aminotransferase (ALT) variables. Compound variables, such as the MELD or Child-Pugh scores, were not included. The graphic exploration of the continuous variables by category subgroups, utilizing box and whisker plots, found a tendency of hepatocellular carcinoma to appear at earlier ages in patients with hepatitis B virus infection, than in those without it. Cholestasis was also more frequent in that subgroup.
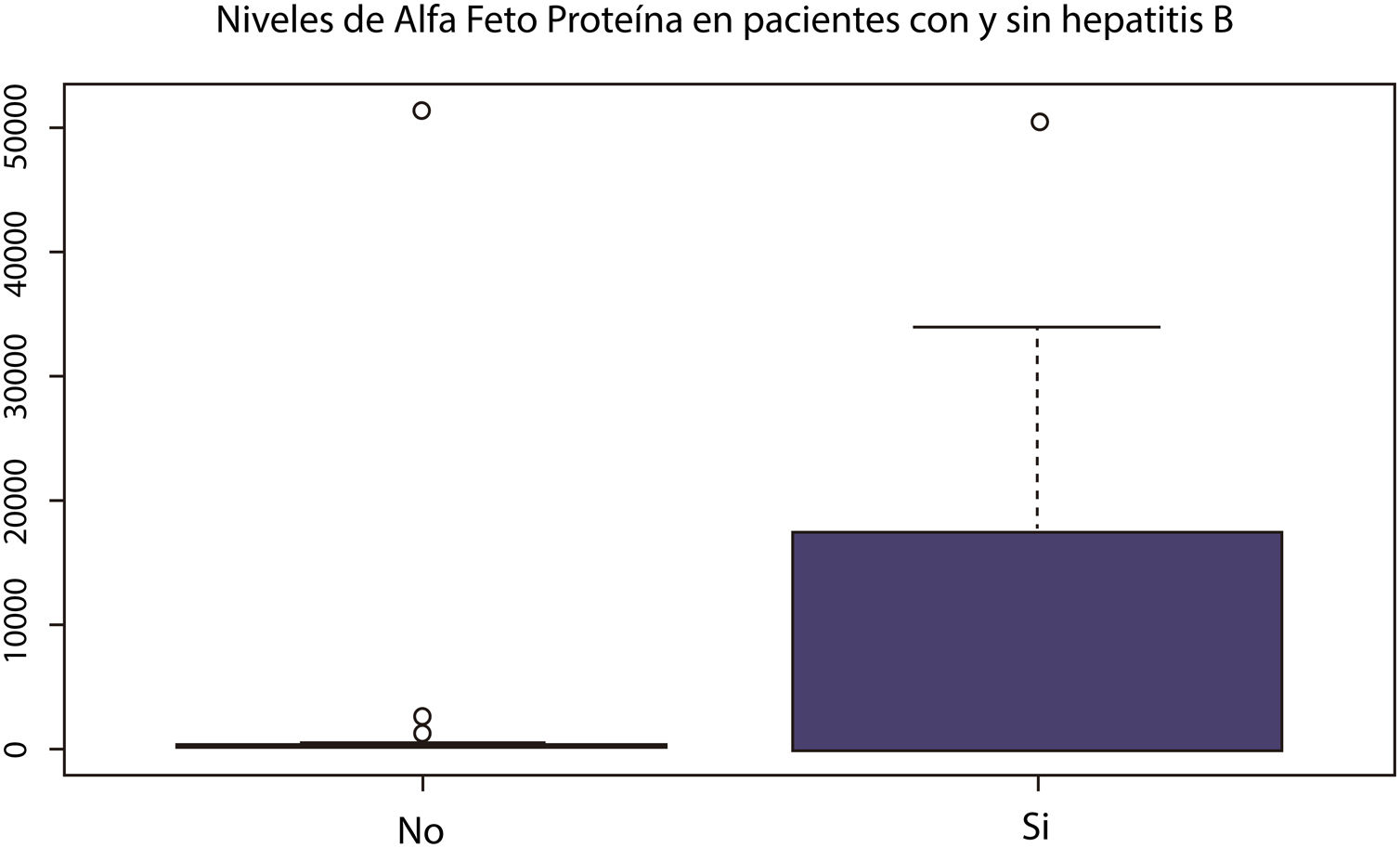
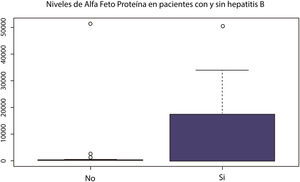
When alpha-fetoprotein behavior was evaluated in patients with or without hepatitis B virus infection, there were marked differences in the distribution of values, reaching higher levels and a more heterogeneous distribution in the patients with hepatitis B (Fig. 3). The patients with hepatitis C virus infection showed a tendency toward higher transaminase and alkaline phosphatase levels, but they had no differences in follow-up duration or alpha-fetoprotein levels.
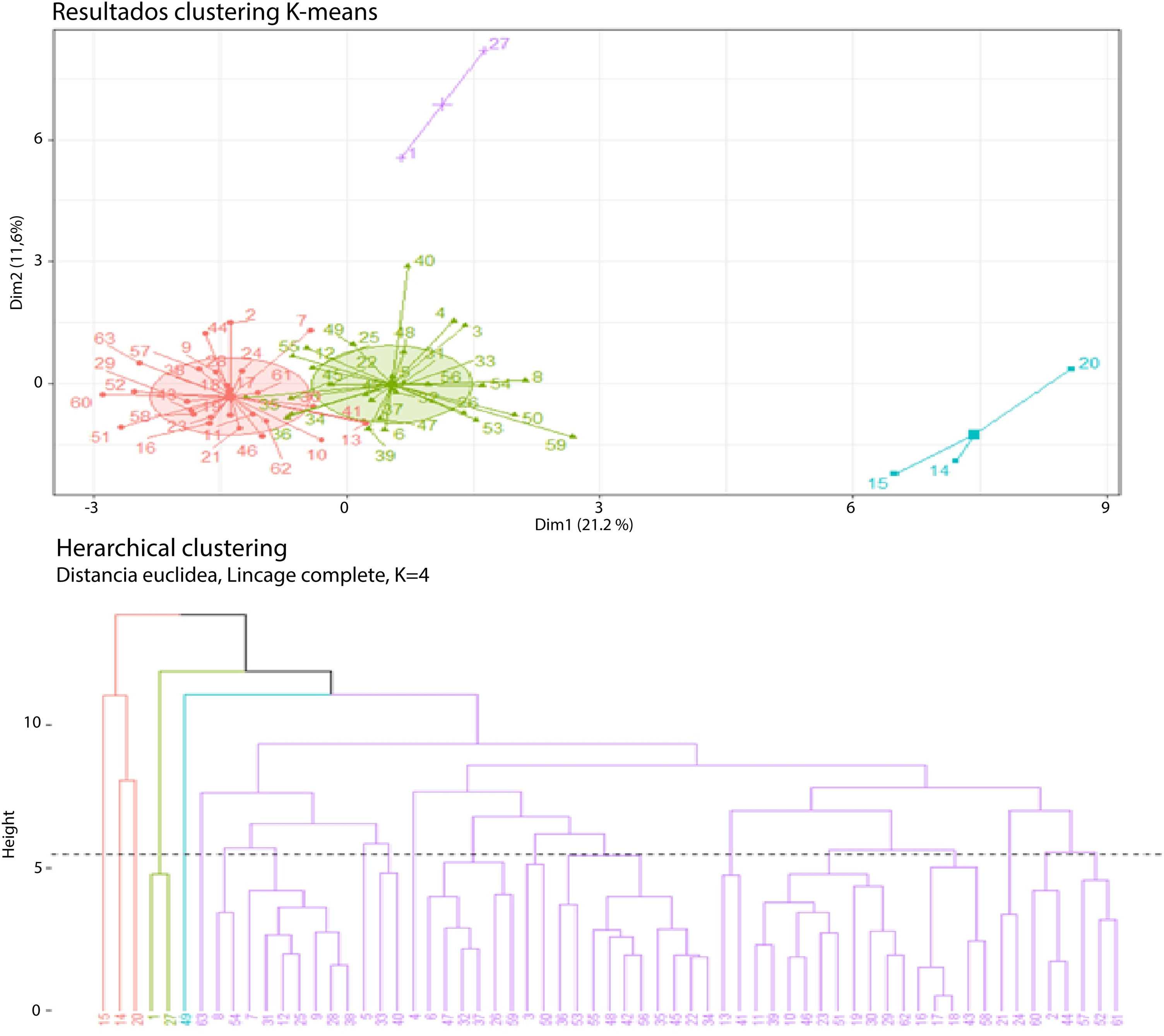
The K-means method was used for establishing the conglomerate patterns, defining a priori cluster groups 2, 3, and 4 because of limitations that could have presented due to the sample size for a higher number of conglomerates.
For the cluster analysis, an n-dimensional space was defined, in which n was equal to the number of variables included in the study (n = 17). The spatial coordinates corresponded to any possible magnitude between the minimum and maximum values of the variables included in the analysis (age, sex, tumor volume, alpha-fetoprotein [AFP], AST, direct bilirubin [DB], albumin [Alb], sodium [Na], international normalized ratio [INR], creatinine [Cr], hepatitis B virus [HBV], hepatitis C virus [HCV], alcohol use disorder [OH], nonalcoholic steatohepatitis [NASH], cirrhosis, multiple tumors, other neoplasias [Neo]). The variables of complications associated with cirrhosis of the liver were not included because of limitations due to sample size.
The analysis of two conglomerates defined two groups: one with a total of 38 cases and one with 24. When the grouping pattern for three conglomerates was evaluated, the formation of three patterns was found: one with 27 cases, one with 3 cases, and one with 32 cases.
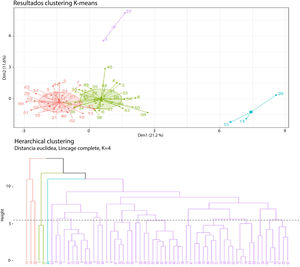
When the conglomerate pattern for four clusters was analyzed, the formation of two groups that dominated the grouping pattern was found: one with 33 cases, one with 24 cases, and two small groups of 3 and 2 cases each (Fig. 4).
First cluster (cases 15-14-20): Cluster 1 consisted of young patients, with a mean age of 27 years (standard deviation of 12 years), hepatitis B, a cirrhotic liver, tumor involvement in numerous segments, mean tumor diameter of 6 cm (standard deviation of 4 cm), tendency toward thrombocytopenia (mean platelets of 59,000 (standard deviation of 7000), elevated transaminases (mean AST of 275 U/L; standard deviation of 107 U/L), and dominance of a cholestatic pattern (mean alkaline phosphatase of 564 mg/dl; standard deviation of 229 mg/dl). The marked variability in the alpha-fetoprotein levels was striking in this group of patients (mean 25,717 ng/mL; standard deviation of 24,762 ng/mL), creatinine levels were normal (creatinine 0.6 mg/dl), and the mean INR was 1.4, standard deviation of 0.1. The mean follow-up period was 7 months, with a standard deviation of 4.
Second cluster (cases 1-27): Cluster 2 consisted of older adult patients (mean age of 66 years, standard deviation of one year), with a cirrhotic liver, history of alcohol use disorder, no hepatitis B, a tendency to develop large tumor masses (mean tumor diameter of 23 cm; standard deviation of 4 cm), and multiple liver segment involvement. More modest transaminase levels were observed (AST 63 U/L; standard deviation of 0 U/L), there was a predominantly cholestatic pattern in the liver profile (mean alkaline phosphatase of 362 mg/dl; standard deviation of 65 mg/dl), normal albumin, no alterations in kidney function, and no hyponatremia. Alpha-fetoprotein levels were not consistent with tumor mass. The mean follow-up period was 8 months, with a standard deviation of 6.5 months.
Third cluster: Cluster 3 consisted of older adult patients with a mean age of 64 years, with a standard deviation of 8.9 years. The majority of patients had cirrhosis (84%), no hepatitis B (7% in the cluster), and a low level of alcohol use disorder (18%). The mean tumor diameter was 4 cm (standard deviation of 2 cm) and the majority of cases presented as single masses (64%). Transaminase levels were significantly lower than those in clusters 1 and 2 (mean AST of 40 U/L; standard deviation of 23 U/L), as were the alkaline phosphatase levels (mean of 160; standard deviation of 79). The alpha-fetoprotein levels (mean of 148; standard deviation of 214) had no correlation with tumor diameter (Pearson correlation coefficient of 0.005), mean albumin level was 4.1, standard deviation of 1.9, and there were no alterations in the levels of sodium or creatinine or in the INR. There was no preference in relation to sex in this grouping pattern. The mean follow-up period was 36 months, with a standard deviation of 34.
Fourth cluster: Cluster 4 consisted of adult patients, with a mean age of 58 years (standard deviation of 8.9 years), a majority of men (80%), with cirrhosis (80%), no hepatitis B (30% with hepatitis B), and no history of alcohol use disorder (35% with alcohol use disorder in the cluster). There was a tendency to present with tumors with a mean diameter of 6 cm (standard deviation of 4 cm) and a high level of multiple presentations (65% with more than one liver segment involved). Transaminase levels were higher than those in cluster 3 (mean AST of 63 U/L; standard deviation of 41 U/L), slightly elevated levels of alkaline phosphatase (mean of 184 mg/dl; standard deviation of 81 mg/dl), and mild thrombocytopenia (mean platelets of 134,000; standard deviation of 118,000). Those patients had normal creatinine, sodium, and INR levels but their nutritional profile and/or low liver synthesis was expressed by low levels of albumin (mean of 3 g/dl; standard deviation of 2 g/dl). Alpha-fetoprotein levels were significantly higher than those in cluster 3 (mean of 8081 ng/mL; standard deviation of 15,639 ng/mL). The mean follow-up duration was 16 months, with a standard deviation of 15 months.
ConclusionsDespite being a neoplastic expression of liver tissue, hepatocellular carcinoma can have a very different clinical behavior, depending on the clinical context in which the tumor develops. The first cluster consisted of young patients with a history of hepatitis B infection. The pathophysiology of hepatocellular carcinoma in patients with hepatitis B has the related factor of an altered regulation of epigenetic mechanisms, in which non-encoded endogenous microRNA regulates the post-transcriptional expression of target genes, possibly with future prognostic and diagnostic utility.10 The particular clinical characteristics of cluster 1 and their distance from other groups of patients with hepatocellular carcinoma may be due to the clinical expression of alterations in the regulation and expression of epigenetic mechanisms by endogenous micro RNA. The second cluster consisted of older adult patients with alcoholic hepatopathy, whose genetic susceptibility has been the target of the pathophysiologic explanation of this type of condition. The genetic variants in PNPLA3 and TM6SF2 have been associated with the development of hepatocellular carcinoma in individuals with alcohol-related cirrhosis.11 The differential feature of the third cluster was advanced age. In 2017, the results of the study by Guo et al.12 on hepatocellular carcinoma in older adults were published. Those authors utilized a propensity score and demonstrated that comorbidities and the lack of opportunity for complete surgical treatment were key determining factors in the progression and outcome of the patients they analyzed.
The fourth cluster consisted of adult patients, the majority of whom were men, with a mean age of 58 years, that had strikingly low levels of albumin and an apparently worse outcome.
The present study shows the elevated clinical variability that hepatocellular carcinoma can express, in which genetic conditions, environmental influences, viral infection expressions, and general decline due to aging or poor nutrition become essential determining factors of the clinical characteristics in those patients.
Some of the limitations of our study were its retrospective design, with the consequently high risk for bias, and the high number of lost data, as well as the fact that the indirect prognostic marker evaluated in months of follow-up was a variable exposed to numerous confounding variables. In addition, the study was developed at a liver transplantation referral center, which could have induced bias through the concentration of patients with good liver reserve that were candidates for liver transplant. The exploratory nature of the study, which attempted to identify the hypothesis of the relation of the clinical behavior of hepatocellular carcinoma to the clinical context in which the lesion developed, could likely result in confounding factors due to the size of the sample and the number of variables included in the analysis. Despite those aspects, conglomerate patterns could be identified in which the nutritional profile, age at appearance (with onset at an early age in patients with hepatitis B), and an altered liver profile with cholestatic predominance were associated with forms of hepatocellular carcinoma expression with an apparently worse outcome.
It should be underlined that the present study is an exploratory analysis showing that the phenotypic expression of hepatocellular carcinoma in the Mexican environment can have special conditioning elements that are important to take into account.
Prospective studies with a larger number of patients are needed to validate the present findings and combine them with the markers established in the works of Llovet and Harding, to incorporate clinical and genetic markers as determining prognostic factors in hepatocellular carcinoma.
Conflict of interestThe authors declare that there is no conflict of interest.
Financial disclosureNo financial support was received in relation to this study.
Please cite this article as: Niño-Ramírez S, Jaramillo-Arroyave D, Ardila O, Guevara-Casallas LG. Disminuyendo la heterogeneidad en hepatocarcinoma. Análisis de clúster por variables clínicas en pacientes atendidos en una institución de cuarto nivel de complejidad. Revista de Gastroenterología de México. 2021;86:356–362.