Strongyloides stercoralis (S. stercoralis or Ss) is an intestinal nematode that is highly prevalent in tropical regions of Africa, Asia, and South America1. Approximately 50-100 million individuals are infected worldwide, particularly in Brazil and Thailand, with a prevalence of 13% and 23.7%, respectively2. The majority of infected persons are asymptomatic, but some, especially immunocompromised individuals, have severe manifestations3–5.
A 16-year-old male from Tabatinga, in the Brazilian state of Amazonas, sought medical attention at a hospital in Leticia, due to the 3-week progression of fever, epigastric abdominal pain radiating to the right iliac fossa, excessive vomiting, bloody diarrhea, and weight loss (20 kg). Laboratory work-up results were leukocytosis (20,600 mm3), neutrophilia (17,922/µl), and thrombocytosis (610,000/µl); human immunodeficiency virus was negative; and Ss was detected in stool, for which the patient received ambulatory treatment with a subtherapeutic dose of albendazole.
His fever persisted and the patient had bilious vomiting and increased abdominal pain at one week of treatment. He went to the emergency room, where signs of peritoneal irritation were documented. An exploratory laparotomy was performed, revealing an indurated retrocecal appendix that was then resected.
Nevertheless, the patient continued to present with fever, excessive vomiting, and a lack of bowel movements for one week. He was referred to the Hospital Internacional de Colombia (HIC) on day 10 of his hospitalization.
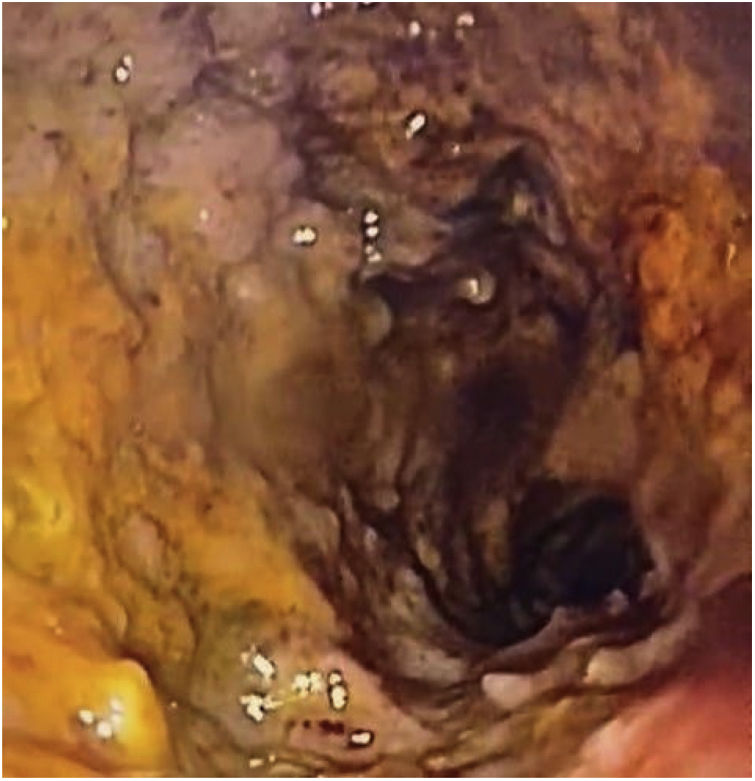
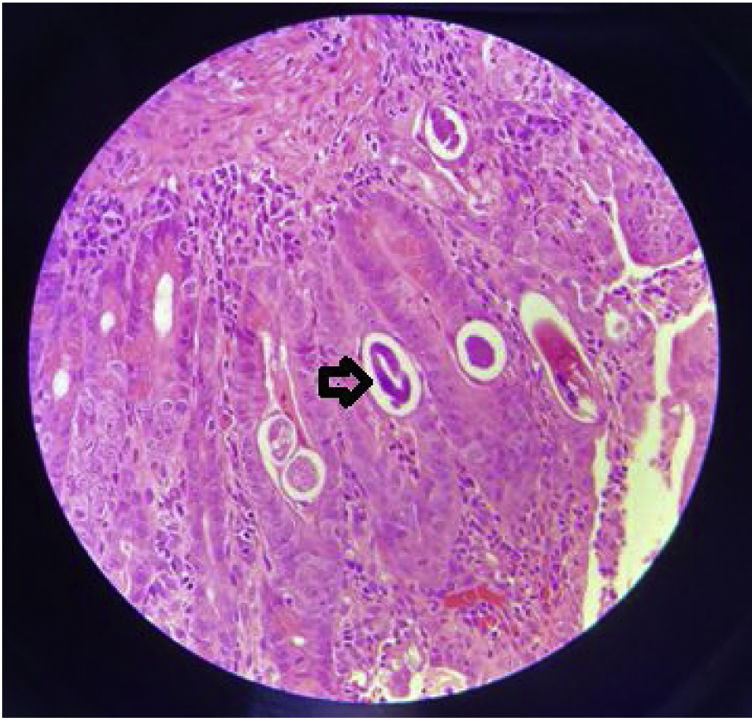
The patient was in poor general condition and hemodynamically unstable. He also presented with high bilioenteric output through the nasogastric tube and had signs of peritoneal irritation. Anthropometry revealed weight of 39 kg (P0, –3.10 SD), height of 150 cm (P0, –2.9 SD), and BMI of 17.3 kg/m2 (P6, –1.5 SD). The patient required vasoactive support (norepinephrine). Severe anemia was reported (Hb: 7 g/dl) and he underwent red blood cell transfusion. Contrast abdominal tomography identified generalized distension of the small bowel segments and thickening of the duodenal wall. Panendoscopy revealed severe erosive gastroduodenitis, pseudomembranes, and multiple inflammatory pseudopolyps in the duodenal bulb (Fig. 1). The histologic study reported severe gastroduodenitis due to Ss (Fig. 2). Management with ivermectin 200 µ/kg/day, oral albendazole 800 mg/day, and endovenous piperacillin/tazobactam 80 mg/kg/dose every 6 hours, all for 14 days, was indicated. Larvae were expelled during 13 treatment days. Stool exam was positive for Ss. Tests for hepatitis B, hepatitis C, cytomegalovirus, and Epstein-Barr virus were negative. Human-T lymphotropic virus type 1 (HTLV-1) had a signal-to-cutoff (S/CO) value of 173.210 (normal: < S/CO value of 1). No ocular involvement was found in the ophthalmologic evaluation. An echocardiogram showed mild dysfunction of the left ventricle and dyskinesia of the basal septum. Blood culture at admission and the control culture at 2 weeks were negative.
The patient presented with improved signs of ileus on day 10 and complete tolerance of oral diet on day 14. Stool exams were negative after 2 weeks of treatment, with nutritional recovery (reaching 44 kg). He was discharged from the hospital after 2 months.
Ss has a complex life cycle, with the capacity to exist and replicate in the host for decades because the larvae mature in the gastrointestinal tract and invade the perianal skin, completing the autoinfection cycle6.
Ss has 4 clinical presentations: a) acute strongyloidiasis: presentation includes rash at the site of penetration of the larvae, cough, wheezing, low-grade fever, epigastric tenderness, diarrhea, nausea or vomiting7; b) chronic strongyloidiasis: it is usually asymptomatic but can cause abdominal pain, nausea, vomiting, diarrhea, malabsorption syndrome, paralytic ileus, rash or larva currens (serpiginous, erythematous papules that rapidly progress) in the perianal area and thighs8; c) hyperinfection: it occurs when there is a high parasite load and is predominantly seen in endemic zones. It is limited to the gastrointestinal tract and lungs (accelerated autoinfection cycle). Its main presentation is diarrhea that is generally high output and occasionally dysenteric, associated with abdominal pain and vomiting. Paralytic ileus, malabsorption, hypoalbuminemia, edema, weight loss and/or massive alveolar hemorrhage are seen in advanced phases7,8; and d) disseminated disease: evidence of larval migration to sites outside of the parasitic replication cycle, finding larvae in the central nervous system, liver, kidney, and other organs4–6.
The main risk factor for hyperinfection or dissemination is cellular immunosuppression that is generally secondary (e.g., steroid use). Our patient presented with symptoms of intestinal obstruction secondary to severe gastroduodenitis due to a hyperinfection syndrome. He had not received steroids, but nevertheless presented with several risk factors, such as place of residence, malnutrition, and HTLV-1 infection (said virus decreases Th2 lymphocytes, eosinophils, mastocytes, and several interleukin mediators of the immune response to parasites)8.
Treatment should be aggressive, because without it, the mortality rate approaches 100%. Ivermectin, at a dose of 200 mcg/kg/day, is the medication of choice9. The definitive length of treatment duration has yet to be defined, but prolonged regimens of one to 2 weeks are indicated for immunocompromised patients, to prevent relapses9. Thiabendazole or albendazole is utilized as second-line treatment10. The combination of ivermectin and thiabendazole, until the absence of parasites in stool exams is achieved or there is improvement of ileus, has been suggested10. The addition of antibiotics to act against Gram-negative or anaerobic microbes that cause sepsis due to bacterial translocation is also recommended. Due to the severity of our patient’s clinical picture, we combined ivermectin, albendazole, and piperacillin/tazobactam. Thiabendazole was not considered for use due to its poor safety profile. Treatment was extended up to 14 days, because even though the patient showed signs of intestinal transit at day 10, the exit of larvae persisted up to treatment day 13.
In conclusion, the early diagnosis of Ss hyperinfection requires a high level of clinical suspicion and the disease should be included in the differential diagnosis of patients with intestinal obstruction that live in endemic areas and/or are immunocompromised.
Ethical considerationsA written statement of informed consent was requested from the patient’s mother to publish patient data and images. We declare that this article contains no personal information that could identify the patient.
Data confidentiality. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this article.
AuthorshipAll the authors contributed to the conception and design of the work, data acquisition, the critical review of the intellectual content, and the definitive approval of the version presented herein.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Cuadros-Mendoza CA, Lozano-Agudelo K, Otoya-Castrillon JP, Serrato-Roa F, Navarro-Mejia YA. Gastroduodenitis severa por Strongyloides stercoralis: una causa rara de obstrucción intestinal. Rev Gastroenterol Méx. 2023;88:188–190.