Endoscopic submucosal dissection (ESD) is a well-established treatment for superficial gastrointestinal tumors and enables en bloc resection. Adequate tissue tension is important for safe and effective dissection. Simplified magnetic anchor-guided ESD (MAG-ESD) with a neodymium magnet has potential benefits, compared with other current traction methods. We evaluated the feasibility of simplified MAG-ESD in an ex vivo porcine model.
Materials and methodsAn experimental study was conducted, utilizing the standard ESD technique. An external magnet and an internal magnet, both neodymium magnets, were used for the magnetic anchoring. The internal magnet was attached to an arm of a hemoclip with a 2-0 silk suture. After the incision, the clip with the internal magnet was placed at the edge of the lesion. The external magnet was maneuvered around the surface to apply adequate tension.
ResultsA total of 15 en bloc ESDs (5 with no magnetic anchoring and 10 with magnetic anchoring) were carried out. Traction and dissection were feasible in all cases and the procedures were completed in fewer than 90min. Lesion size ranged from 15 to 50mm (mean 30mm). Two cases in the group with magnetic anchoring presented with punctate perforation (13.3%).
ConclusionsOur study demonstrated the feasibility of simplified MAG-ESD and en bloc resection in an ex vivo porcine model.
La disección endoscópica de la submucosa (DES) es un tratamiento bien establecido para las neoplasias superficiales del tracto gastrointestinal y permite la resección en bloque. La tracción adecuada del tejido es importante para una disección efectiva y segura. La DES guiada por anclaje magnético (DES-AM) con imán de neodimio (simplificada) tiene beneficios potenciales en comparación con otros métodos actuales de tracción. Evaluamos la factibilidad de DES-AM simplificada en modelo porcino ex vivo.
Materiales y métodosDiseño experimental, se empleó la técnica estándar de DES. Para el anclaje magnético (AM), utilizamos un imán externo y un imán interno de neodimio. El imán interno de neodimio se fijó a una rama de un hemoclip con sutura seda 2-0. Después de la incisión, el clip con el imán interno se colocó al borde de la lesión y luego se maniobró un imán externo alrededor de la superficie para aplicar una tracción adecuada.
ResultadosEn total se realizaron 15 DES (5 sin AM y 10 con AM), de las cuales el 100% se completaron en bloque. En todos los casos la tracción y la disección fueron factibles. Todos los procedimientos se completaron en menos de 90minutos. El tamaño de las lesiones fue de 15-50mm (promedio 30mm). Dos casos presentaron perforación puntiforme en el grupo con AM (13.3%).
ConclusionesNuestro estudio mostró la factibilidad de la DES-AM simplificada en modelo porcino ex vivo y resección en bloque.
Endoscopic submucosal dissection (ESD) has been developed for treating early gastrointestinal lesions by enabling en bloc resection, to make a safe histopathologic diagnosis and reduce local recurrence.1–3 However, ESD is technically difficult and the limited field of vision can produce severe complications, such as bleeding and perforation.4,5 Various devices and traction methods have been developed to facilitate ESD.6–16 Magnetic anchor-guided ESD (MAG-ESD), utilizing a large external magnet, provides several degrees of traction.17,18 A simplified MAG-ESD method utilizing small neodymium magnets, useful for daily practice, has been described in resected porcine stomachs and the stomachs of live dogs.19,20 A prospective case series by Matsuzaki et al. was recently published on 50 patients with stomach lesions, utilizing the simplified MAG-ESD method, in which successful resection was achieved in all cases.21 Similar results were described in 48 cases with colorectal lesions.22 To the best of our knowledge, there are no Mexican reports on MAG-ESD. In contrast, training on animal models (particularly utilizing the stomach) to master the learning curve for ESD has been described and incorporated at several centers for ESD training courses.23 The aim of the present study was to evaluate the feasibility of performing simplified MAG-ESD in ex vivo porcine models.
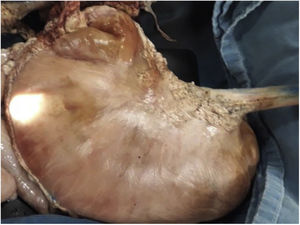
Materials and methodsEx vivo porcine modelResected porcine stomachs (Fig. 1), and in one case, the colon, were utilized. They were prepared according to the recommendations established by Ramírez et al.24 The procedures were performed by the same endoscopist (Miguel Ángel Ramírez Ramírez), who was at the beginning of his learning curve in ESD training. An expert in other advanced endoscopic procedures, he had 7 years of experience in endoscopic procedure training in ex vivo models.
Neodymium magnetInternal and external permanent neodymium magnets (Nd2Fe12B) for experimental use (Innova Endoscopy S.A. de C.V. Mexico City) were employed. Neodymium magnets are the strongest magnets available and are highly resistant to demagnetization, due to their atomic structure. The external magnet (attraction force, 845.8N; magnetic flow density, 534Mt) is shaped like a coin (3cm in diameter and 8mm in height) and the internal magnet (5.3N, 296Mt) is ring-shaped (external diameter of 5mm, internal diameter of 1mm, height of 3mm).
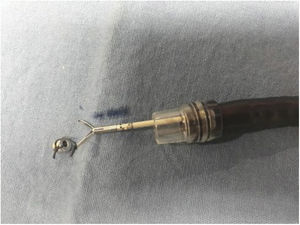
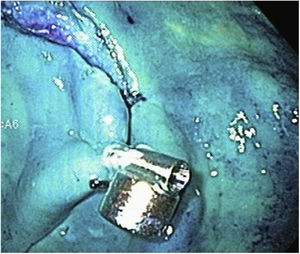
Magnetic anchoringMagnetic anchoring (MA) consists of attaching the internal neodymium magnet to an arm of the hemoclip. For MA preparation, a hemoclip was initially inserted into the working channel of the endoscope that had a plastic transparent cap attached to the tip. Once the hemoclip came out at the distal part of the endoscope, the magnet was attached to one of its arms, with 2-0 silk (Fig. 2). It was then moved a few centimeters until it was completely covered by the plastic cap (Fig. 3), ready to be introduced into the stomach or colon, without injuring the mucosa.
In the first training stage, five ESD procedures were carried out with no MA. In the second stage, 10 ESD procedures were performed, introducing the MA method. From this point forward in the text we will refer to two groups: the group with MA and the group without MA. The ESD technique utilized was made up of 4 stages: marking, submucosal injection, circumferential incision, and submucosal dissection.
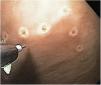
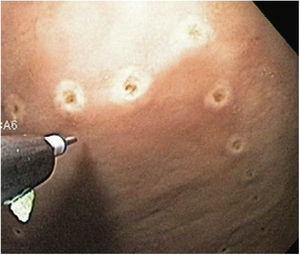
ESD technique with no MAA single-channel endoscope (GIF Q160; Olympus Medical System Corp, Tokyo, Japan) with a plastic transparent cap at the tip was employed, together with an electrosurgical unit (ERBE ICC, T200 Tübingen, Germany) and the following accessories: a rotatable cylindrical-type instrument, a cylindrical-type square knife, and a ball-type IT knife (Alton Medical Instruments CO., LTD, Shanghai, China). As the first step, a simulated lesion larger than 15mm was marked (Fig. 4), followed by the submucosal injection of saline solution and methylene blue. A circumferential incision was then made (Fig. 5), and finally, dissection was performed, utilizing the cap for countertraction.
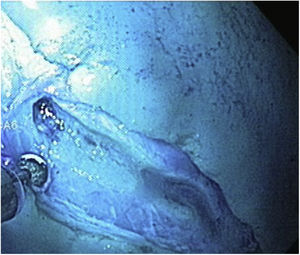
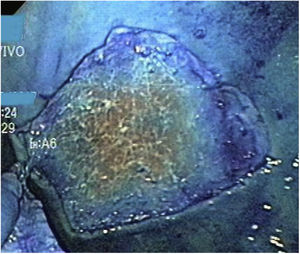
The stages of marking, injection, and circumferential incision were performed in the same manner. Before proceeding to the dissection stage, the endoscope was withdrawn so the magnetic anchor could be placed with the clip. The endoscope was then reintroduced, and the magnetic anchor was attached to the gastric mucosa at the edge of the lesion (Fig. 6). In the first case of the group with MA, the cap remained at the tip of the endoscope during dissection. However, in the rest of the cases of that group (9/10), once the clip with the magnet was deployed, the endoscope was withdrawn, and the cap was removed because we realized that it was no longer technically necessary, and its removal improved the field of vision. The external magnet was maneuvered around the surface of the ex vivo model to achieve adequate internal magnet traction and expose the correct dissection plane (Fig. 7). When the dissection was completed, the resected specimen and the in situ magnetic anchor were retrieved (Fig. 8).
A descriptive analysis was carried out. The categorical variables were expressed as percentage and the quantitative variables were expressed as mean and standard deviation.
Variables- 1
En bloc resection (categorical variable)
- 2
Adequate traction: achieving adequate traction and countertraction at all angles through MA (categorical variable)
- 3
Maximum duration (90min) of the dissection procedure, evaluated from the start of the circumferential cut to the end of the dissection (quantitative continuous variable)
- 4
Size (mm) of the resected tissue (discrete quantitative variable)
- 5
Perforation: defined as the loss of gastrointestinal wall continuity (categorical variable)
Ninety minutes was considered the maximum time limit for successfully performing ESD. We did not measure the time of each procedure because the feasibility of ESD performance, not the comparison of the procedure duration variable, was the aim of the study.
Ethical considerationsThe good clinical practice and animal experimentation norms were met. The study was conducted on ex vivo porcine models. No animals in vivo or patients were involved, and the equipment and accessories employed were exclusively those for use in animals.
ResultsA total of 15 ESD procedures were carried out (Table 1). The first five cases were performed with no MA (four in the stomach and one in the colon) and 10 were performed with MA (all in the stomach). En bloc resection was achieved in 100% of the cases. MA enabled adequate traction and countertraction at all angles and adequate dissection in 100% of the cases. All the procedures were carried out in fewer than 90min (100%). Lesion size ranged from 15mm to 50mm (mean 30mm). Two cases in the group with MA (13.3%) presented with punctate perforation. They were repaired and en bloc dissection was completed.
General population characteristics and the study variables.
| General population characteristics | Magnet anchoring | No magnetic anchoring | |
|---|---|---|---|
| n=10 | n=5 | ||
| Dissection tissue | Gastric 14 (93%) | 10 (100%) | 4 (80%) |
| Colonic 1 (7%) | 1 (20%) | ||
| Mean dissection size in mm (range) | 30±10.18 (15-50) | ||
| Use of cap | 1 (10%) | 5(100%) | |
| En bloc resection | 10 (100%) | 5 (100%) | |
| Perforation | Yes 2 (20%) | 0 | |
| No 8 (80%) |
In the present study we found that simplified MAG-ESD was a feasible procedure in ex vivo models. ESD has been developed for the treatment of early gastrointestinal lesions and its goal is en bloc resection for making a safe histopathologic diagnosis and reducing local recurrence.1–3 Training on animal models is the best way to master the learning curve for ESD.24 A formal sequential training program that includes ex vivo, in vivo, and human models can be useful in countries with a low volume of cases. In the present study on ex vivo models, MAG-ESD was successfully performed, with en bloc resection in all cases, even at the beginning of the learning curve of the operator, as mentioned above.25 The fact that the operator is an expert in advanced endoscopic techniques could be an important factor in the present results, which would not necessarily be the expected results for endoscopists with lower or different levels of experience. In the present study, we achieved traction and countertraction with the MA system at several angles during the ESD, which was the equivalent of having a first assistant during surgery. The MA technique could be incorporated into a future sequential training program, reproducing our results in in vivo models.
The transparent cap at the tip of the endoscope is useful and indispensable for ESD because it enables dissection through countertraction, but it can be a disadvantage regarding visualization when the diameter of the cap is small.26 In our study we found that leaving the cap at the tip of the endoscope in the MA procedures was unnecessary during the dissection stage, and not having the cap enabled better visualization. We used the cap in all five cases with no MA and in only one case in the group with MA. The cap was removed in the remaining nine cases in that group. Although the endoscope had to be withdrawn for its removal, it resulted in a better field of vision. In contrast, Matsuzaki et al.21,22 did not specify that potential advantage. Thus, more studies are needed to determine whether cap removal is advantageous or not. Importantly, the cap was used in all the cases when the magnetic anchor was placed, protecting both the mucosa and the anchor.
There are considerable technical risks involved in the performance of ESD, such as perforation. Two meta-analyses reported perforation in an average of approximately 4.5% of cases during gastric ESD27,28 and 4.8% during colonic ESD. Likewise, in a large case series on perforation, Minami et al.29 found that 98% of the cases had successful closure with the clip and did not require surgery. In our study, we had 2 cases (13%) of punctate perforation that were identified during the procedure and sutured, and en bloc MAG-ESD was completed in 100% of the cases.
Because we utilized an ex vivo model, we could not evaluate the risk for bleeding, and technically, we were not faced with the problem of peristalsis. Nevertheless, sequential training should gradually shift towards live models and humans. Future studies could be conducted on in vivo porcine models and then on humans (performed by experts) to measure the variables of bleeding and evaluate the factor of peristalsis and the physical barrier of the body to magnetic attraction, utilizing the present study as a base and prior reference.
A methodological limitation of the present study was the fact that there was lesion variability. We included 14 gastric lesions and only one colonic lesion (ESD without MA), and so, strictly speaking, the feasibility of MAG-ESD was established only for gastric lesions. However, we consider that the results could be extrapolated to lesions of the colon, but that was not evaluated. Another limitation was that even though it was not a study aim, the precise time of each ESD was not measured, thus we could not evaluate whether procedure duration was related to lesion size or location.
ConclusionOur study demonstrated the feasibility of simplified MAG-ESD and en bloc resection, in an ex vivo porcine model. The use of a cap at the tip of the endoscope was not needed during MAG-ESD, improving visualization.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Ramírez-Ramírez MÁ, Zamorano-Orozco Y, Beltrán-Campos EG. Disección endoscópica de la submucosa asistida con anclaje magnético simplificado: modelo porcino ex vivo. Revista de Gastroenterología de México. 2022;87:13–19.