Drug-induced liver injury (DILI) is one of the most challenging disorders faced by the gastroenterologist.1 The estimated annual incidence rate of DILI is 13.9-24 per 100,000 inhabitants.2 It is still a diagnosis of exclusion based primarily on a detailed history and the judicious use of blood tests, hepatobiliary imaging, and liver biopsy.1,3
Herbal and dietary supplements (HDSs) are currently consumed on a large scale in Mexico, due to the common misperception that they are innocuous because they are “natural”. However, HDS hepatotoxicity has received increasing attention over the past few years, in part because HDSs have been recognized in the United States as being the second most common cause of DILI cases.1 The Adverse Event Reporting Program of the Food and Drug Administration is responsible for the increased awareness of safety concerns and encourages individuals and healthcare professionals to report adverse effects.4 We present herein the case of a 25-year-old woman diagnosed with acute herbal-induced hepatotoxicity secondary to the consumption, in the form of an infusion, of Cochlospermum vitifolium (C. vitifolium). Commonly known as “yellow rose,” “panicua”, or “pongolote”, it is used as an herbal remedy for the “treatment” of jaundice and metabolic syndrome.5,6
Wild C. vitifolium belongs to the Cochlospermaceae family and is a shrub with yellow flowers that grows up to five meters in height. The patient had no concomitant diseases when she was admitted to the Instituto Nacional de Ciencias Médicas y Nutrición (a tertiary care hospital) with a 7-day history of jaundice. During the initial medical assessment, blood pressure and heart and respiratory rates were within normal values and her temperature was 36.7°C. Physical examination showed she was icteric, with conjunctival icterus and weakness, and her body mass index was 24.5kg/m2. She did not complain of fever, changes in level of consciousness, vomiting, abdominal pain, seizures, or gastrointestinal bleeding. The serum liver function tests were altered, reporting serum ALT and AST > 3 x the upper limit of normal (ULN), serum total bilirubin elevated to > 2 x the ULN and the calculated R-value (ALT/ULN divided by AP/ULN), suggesting a cholestatic injury pattern.1
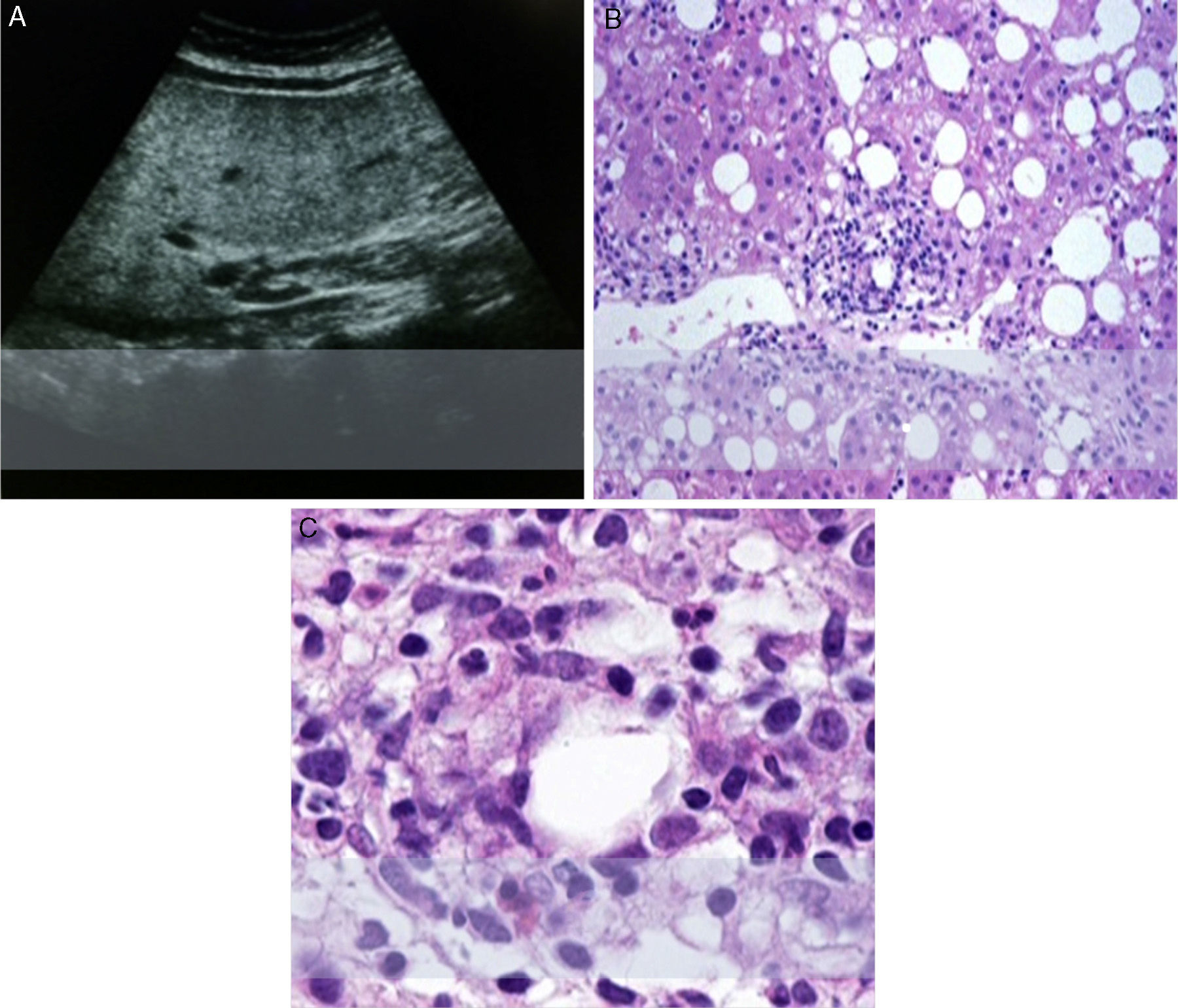
Serologic testing for hepatitis A, B, C and E viruses, and serologic tests for antinuclear antibodies, anti-mitochondrial antibodies, and anti-smooth-muscle antibodies were all negative (table 1). Hepatobiliary ultrasound reported hepatosplenomegaly and severe steatosis. In the hepatic biopsy the pathologist reported steatohepatitis with severe activity and mild fibrosis (Brunt-Kleiner 3) with mixed macro and microvesicular steatosis, and marked aggregates of inflammatory cells predominantly in the perivenular spaces with subtle necrosis. Moderate hepatocyte ballooning and satellitosis were found, characterized by the presence of neutrophils and eosinophils. The patient revealed that she had been taking a “panicua” infusion as a recommendation from a relative “just to stay healthy”, drinking up to 1-2 cups daily for 3-4 months before medical examination (fig. 1).
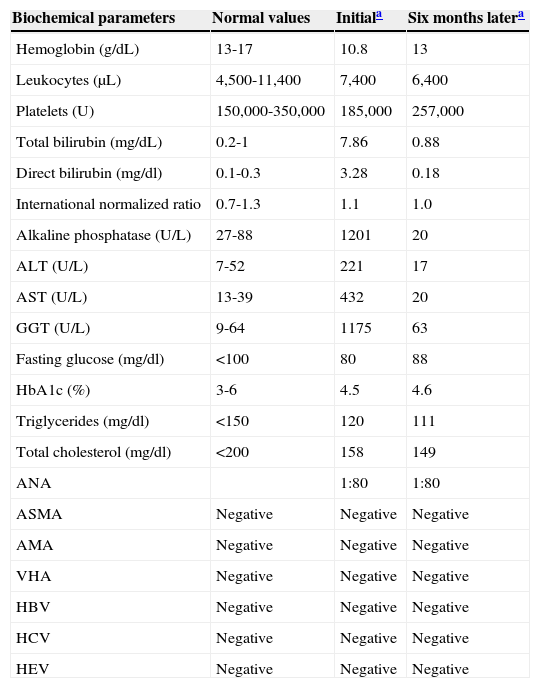
Biochemical parameters during patient progression: initial values and at 6 months after suspending “panicua” consumption).
| Biochemical parameters | Normal values | Initiala | Six months latera |
|---|---|---|---|
| Hemoglobin (g/dL) | 13-17 | 10.8 | 13 |
| Leukocytes (μL) | 4,500-11,400 | 7,400 | 6,400 |
| Platelets (U) | 150,000-350,000 | 185,000 | 257,000 |
| Total bilirubin (mg/dL) | 0.2-1 | 7.86 | 0.88 |
| Direct bilirubin (mg/dl) | 0.1-0.3 | 3.28 | 0.18 |
| International normalized ratio | 0.7-1.3 | 1.1 | 1.0 |
| Alkaline phosphatase (U/L) | 27-88 | 1201 | 20 |
| ALT (U/L) | 7-52 | 221 | 17 |
| AST (U/L) | 13-39 | 432 | 20 |
| GGT (U/L) | 9-64 | 1175 | 63 |
| Fasting glucose (mg/dl) | <100 | 80 | 88 |
| HbA1c (%) | 3-6 | 4.5 | 4.6 |
| Triglycerides (mg/dl) | <150 | 120 | 111 |
| Total cholesterol (mg/dl) | <200 | 158 | 149 |
| ANA | 1:80 | 1:80 | |
| ASMA | Negative | Negative | Negative |
| AMA | Negative | Negative | Negative |
| VHA | Negative | Negative | Negative |
| HBV | Negative | Negative | Negative |
| HCV | Negative | Negative | Negative |
| HEV | Negative | Negative | Negative |
AMA: anti-mitochondrial antibody; ANA: antinuclear antibody; ASMA: Anti-smooth muscle antibody; HBV: hepatitis B virus; HCV: hepatitis C virus; HEV: hepatitis E virus.
The patient was told to suspend the “panicua” infusion immediately. She had a medical follow-up once a month for the next 6 months at the steatohepatitis clinic in our hospital. During the first 4 weeks of surveillance there was a clinically significant decrease in hepatic enzymes and after the 6-month wash-out period the hepatic enzymes and bilirubin returned to normal values (table 1).7 A liver biopsy was not performed at the end of the follow-up, because the patient did not give her consent for the procedure. A key question in herbal hepatotoxicity is the appropriate attribution of causality method for suspected cases. Data acquisition, documentation, and presentation of these kinds of problems are a diagnostic challenge.
Some appropriate causality assessment methods for suspected herbal hepatotoxicity are the Council for International Organizations of Medical Sciences (CIOMS) scale and the Roussel Uclaf Causality Assessment Method (RUCAM).1 Both scales are structured, quantitative, liver-specific, and validated for hepatotoxicity. They are intended for bedside use in a clinic and yield a summed score indicating the likelihood of DILI.1,8
Points are given or taken away based on the timing of exposure, liver biochemistry dechallenge, risk factors for DILI, competing medications, competing diagnoses, and rechallenge information.1 In the present case, both the RUCAM and CIOMS scales showed a “probable” DILI due to C. vitifolium consumption as the primary candidate (+6 points and +9 points, respectively).1,8 In the case of HDS hepatotoxicity, important limitations to the causality assessment process must be considered:
None of the causality assessment processes currently used were created specifically for HDSs, dietary supplements are susceptible to ingredient or concentration variability over time, they are usually unlabeled, and in the specific case of herbal remedies, they could be contaminated with heavy metals or microbes. Although there is much information at the folk-knowledge level, there are hardly any health regulations regarding their collection, cultivation, local production, and processing.1,9
This case highlights the importance of encouraging patients to report the use of HDSs to their health-care providers. The same diagnostic approach for DILI is applicable to HDS hepatotoxicity suspicion and the exclusion of other causes and diagnosis can be made with confidence in the setting of recent HDS use.1,10 Patients with suspected HDS hepatotoxicity should suspend all HDSs and be monitored for resolution of their liver injury. This case is the first report of C. vitifolium (“panicua”) as a potential hepatotoxic plant. Experiments in rats showed that C. vitifolium extract plays an important role in hypertension regulation through nitric oxide synthesis and possibly prostaglandin I2 production and potassium channel activation under excessive endothelial dysfunction conditions.6,7
More studies on the potential toxic metabolites from “panicua” are needed.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Paris Ramos-Martínez, pathologist.
Please cite this article as: Martínez-Rodriguez L, Murguía-Hernández K, García-Juárez I, Uribe-Esquivel M, Gómez-Reyes E. La historia oscura de la rosa amarilla: un reporte de caso de toxicidad hepática asociado al consumo de Cochlospermum vitifolium como remedio herbolario. Revista de Gastroenterología de México. 2015;80:220–222.