Alcoholic hepatitis is a frequent condition in the Mexican population. It is characterized by acute-on-chronic liver failure, important systemic inflammatory response, and multiple organ failure. The severe variant of the disease implies elevated mortality. Therefore, the Asociación Mexicana de Gastroenterología and the Asociación Mexicana de Hepatología brought together a multidisciplinary team of health professionals to formulate the first Mexican consensus on alcoholic hepatitis, carried out utilizing the Delphi method and resulting in 37 recommendations. Alcohol-related liver disease covers a broad spectrum of pathologies that includes steatosis, steatohepatitis, different grades of fibrosis, and cirrhosis and its complications. Severe alcoholic hepatitis is defined by a modified Maddrey’s discriminant function score ≥ 32 or by a Model for End-Stage Liver Disease (MELD) score equal to or above 21. There is currently no specific biomarker for its diagnosis. Leukocytosis with neutrophilia, hyperbilirubinemia (>3 mg/dl), AST > 50 U/l (< 400 U/l), and an AST/ALT ratio > 1.5-2 can guide the diagnosis. Abstinence from alcohol, together with nutritional support, is the cornerstone of treatment. Steroids are indicated for severe disease and have been effective in reducing the 28-day mortality rate. At present, liver transplantation is the only life-saving option for patients that are nonresponders to steroids. Certain drugs, such as N-acetylcysteine, granulocyte-colony stimulating factor, and metadoxine, can be adjuvant therapies with a positive impact on patient survival.
La hepatitis alcohólica es una condición frecuente en población mexicana, se caracteriza por insuficiencia hepática aguda sobre crónica, importante reacción inflamatoria sistémica y fallo multiorgánico, que en la variante grave de la enfermedad implica una elevada mortalidad. Por lo anterior, la Asociación Mexicana de Gastroenterología y la Asociación Mexicana de Hepatología conjuntaron un equipo multidisciplinario de profesionales de la salud para elaborar el primer consenso mexicano de hepatitis alcohólica. El consenso fue elaborado con la metodología Delphi, emitiendo 37 recomendaciones. La enfermedad hepática relacionada al consumo de alcohol comprende un amplio espectro, que incluye esteatosis, esteatohepatitis, fibrosis en diferentes grados, cirrosis y sus complicaciones. La hepatitis alcohólica grave se define por una función modificada de Maddrey ≥32 o por un puntaje de MELD (Model for End-Stage Liver Disease) igual o mayor a 21. Actualmente no existe un biomarcador específico para el diagnóstico. La presencia de leucocitosis con neutrofilia, hiperbilirrubinemia (>3 mg/dL), AST > 50 U/L (<400 U/L), relación AST/ALT > 1.5-2 pueden orientar al diagnóstico. La piedra angular del tratamiento es la abstiencia junto con el soporte nutricional. Los esteroides estan indicados en la forma grave, en donde han resultado efectivos para reducir la mortalidad a 28 días. El trasplante hepático es en la actualidad la única opción con que se cuenta para salvar la vida de pacientes no respondedores a esteroides. Ciertos fármacos, como la N-acetilcisteína, el factor estimulante de colonias de granulocitos, y la metadoxina pueden ser una terapia adyuvante que puede impactar en la sobrevida de los pacientes.
Half of the deaths due to cirrhosis of the liver are related to alcohol consumption worldwide. In Mexico, alcohol-related cirrhosis of the liver is the cause of approximately 50% of the cases of cirrhosis and is a public health problem with a direct impact on an elevated mortality rate and the consequent high costs for the health system. Alcoholic hepatitis (AH) is a frequent condition in the Mexican population that is usually characterized by acute-on-chronic liver failure (ACLF), important systemic inflammatory response, and multiple organ failure. The severe variant of the disease implies an elevated mortality rate. Therefore, the Asociación Mexicana de Gastroenterología (AMG) and the Asociación Mexicana de Hepatología (AMH) brought together a multidisciplinary team of healthcare professionals made up of gastroenterologists, hepatologists, and clinical researchers in the field to formulate the first Mexican consensus on alcoholic hepatitis, to have a document with recommendations that can aid the entire medical community that provides clinical care to patients with AH.
The primary aim of the present consensus was to formulate a document containing descriptions and analyses of the current evidence on the basic concepts, epidemiology, diagnosis, and treatment of AH, with a focus on their application in daily clinical practice in Mexico.
MethodologyIn November of 2018, the AMH and AMG made a collaboration agreement to produce the first Mexican consensus on AH. The Delphi method was employed to develop the consensus.1 Two coordinators were designated, one from the AMH (JAVRV) and one from the AMG (MFHT), and 23 experts from the gastroenterology and hepatology specialties were invited to participate. In January of 2019, the coordinators (JAVRV and MFHT) and 3 other experts (RCO, JMAL, and ESGJ) carried out a thorough search of the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search encompassed the time frame of January 1, 1990, to February 2019, and in particular cases for the sections on basic concepts and treatment, covered the same period but beginning with the year 1971. All articles in English and Spanish were included. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses. Complementary electronic and manual searches were also carried out in the archives of the Revista de Gastroenterología de México and all the publications that the coordinators considered relevant up to February 2019. The search criteria included the term “alcoholic hepatitis” combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “mortality”, “diagnosis”, “differential diagnosis”, “treatment”, “antibiotics”, “infection”, “therapy”, “management”, “steroids”, “nutrition”, “review”, “guidelines”, “transplant”, “meta-analysis”, and their Spanish equivalents. The entire bibliography was made available to the members of the consensus through a virtual library.
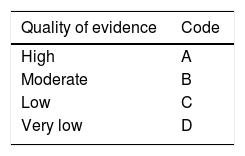
The coordinators then formulated 36 statements, which underwent a first round of anonymous electronic voting (February 21 to 27, 2019) to evaluate the drafting and content of the statements. The consensus participants emitted their votes, as follows: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement. After the first vote, the coordinators made the corresponding modifications. The statements that reached complete agreement in > 75% of the participants were kept and the ones that had complete disagreement in > 75% of the participants were eliminated. The statements that reached ≤ 75% complete agreement and ≤ 75% complete disagreement were reviewed and re-structured. The revised statements underwent a second round of anonymous, electronic voting (March 3 to 8, 2019). According to the comments from the second round of voting, the statements were revised and underwent a third round of voting (March 9 to 15, 2019). In that round, along with the drafting review, the strength of recommendation and quality of evidence for sustaining the recommendation were established, utilizing the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.2 In the GRADE system, the quality of evidence is not determined solely by the research design or methodology, but also by the function of a clearly posed question related to a clearly formulated outcome variable.3 Thus, evidence can be high, moderate, low, or very low. The GRADE system also establishes the strength of the recommendations as strong or weak and in favor of or against the intervention or statement. Table 1 shows the codes employed in the GRADE system: upper case letters classify the quality of evidence, followed by a number indicating the strength of recommendation in favor of or against the intervention or statement.
Grade system codes. GRADE system: Classification of the quality of evidence and the strength of recommendation.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | Code |
|---|---|
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
The results of the third round of voting were presented on March 15 and 16, 2019, at a face-to-face meeting held in Ensenada, Baja California. At that meeting, the statements that reached agreement in > 75% of the participants were ratified. Those that did not reach 75% agreement in the previous rounds of voting were discussed, in an effort to reach a consensus, and if none was attained, the statements were eliminated. The remaining statements under consideration were then voted on again.
Once all the consensus statements were established, the coordinators put together the present manuscript, which was reviewed and approved by all the members of the consensus.
Ethical considerationsThe authors of the present work declare that no experiments on humans or animals were performed for its formulation, and there were no confidentiality conflicts, given that no patient data appear. Because no data from persons or patients were utilized, no statements of informed consent were required.
ResultsThe coordinators initially proposed 36 statements. In the first voting round, one statement was eliminated due to lack of consensus. The second voting round was conducted on 35 statements, and according to the voting results, two new statements were proposed, resulting in a total of 37 statements for the third round of voting. In the face-to-face meeting, the 37 statements were presented, 31 (84%) to be ratified and 6 (16%) to be voted on again. At the end of the face-to-face meeting, once several statements were reviewed, eliminated, and fused together, the members of the consensus decided on 37 statements, classified into 6 sections:
- •
Basic concepts of alcohol-related liver disease
- •
Diagnostic tests for the detection of alcohol-related liver disease
- •
Diagnosis of alcoholic hepatitis
- •
Evaluation and prognosis of patients with alcoholic hepatitis
- •
Treatment of fibrosis due to alcohol-related liver disease
- •
Treatment of alcoholic hepatitis
The final statement recommendations and voting results are presented below.
- A
BASIC CONCEPTS OF ALCOHOL-RELATED LIVER DISEASE
- 1
Alcohol use disorder is defined as the hazardous and harmful consumption of alcohol that compromises social, family, and work environments. It causes clinically significant malaise or decline, with several grades of severity, depending on the number of criteria met. The term alcoholism should be avoided, given that, in addition to not being clinically useful, the social stigmatization it implies can be detrimental.
Agreement reached: 100% in complete agreement.
According to the World Health Organization (WHO), 50% of deaths by cirrhosis worldwide are related to alcohol consumption.4 In 2013, more than 23,000 persons died from cirrhosis in Mexico, and almost three-quarters of those deaths were in men. Cirrhosis of the liver is the fourth cause of loss of health in Mexico and accumulates 4.1% of the disease burden, according to the disability-adjusted life year (DALY) indicator. It is in fifth place, with respect to general mortality, and as a cause of premature death, it is in fourth place. Alcohol predominates in men as the cause of cirrhosis in the Mexican population, whereas hepatitis C is the most common cause in women. Nationally, 46% of the disease burden from cirrhosis is associated with alcohol consumption and 35% with hepatitis C.5
The fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) described two different alcohol-related disorders: alcohol abuse and alcohol dependence, each with specific criteria.6 The DMS-5 integrates those two disorders into only one, currently called alcohol use disorder (AUD), and it is classified as mild, moderate, or severe. According to the DMS-5, alcohol use disorder is diagnosed when two or more of the 11 criteria described in the manual are positive (Table 2). Alcohol use disorder severity is based on the number of positive criteria: mild (2-3 positive criteria), moderate (4-5 positive criteria), and severe (6 or more positive criteria).7
DSM-5 criteria for defining alcohol use disorder.
| 1 | Have you had times when you ended up drinking more or for a longer period of time than you intended? |
|---|---|
| 2 | Have you wanted more than once to cut down on your drinking or stop it altogether, or tried to, but couldn’t? |
| 3 | Do you spend a lot of time drinking or procuring alcohol, or are you sick much of the time due to drinking or to getting over the aftereffects? |
| 4 | Have you craved a drink so badly that you couldn’t think about anything else? |
| 5 | Have you found that drinking, or being sick due to drinking, often interferes with taking care of your home or family? Or caused problems at work? Or caused academic problems? |
| 6 | Have you continued to drink even though it was causing problems with your family or friends? |
| 7 | Have you given up or cut back on activities that were important or interesting for you or gave you pleasure, in order to drink? |
| 8 | Have you gotten yourself into situations more than once, while or after drinking, that increased your chances of getting hurt (such as driving, swimming, operating machinery, walking in a dangerous area, or having unsafe sex)? |
| 9 | Have you continued to drink even though it makes you feel depressed or anxious or has led to your having some other health problem, or becoming forgetful, or having memory loss, or blacking out? |
| 10 | Have you had to drink much more than before to feel the desired effect? Or found that the usual number of drinks has much less effect than before? |
| 11 | Have you found that when the effects of alcohol were wearing off, you had withdrawal symptoms, such as trouble sleeping, tremors, restlessness, nausea, sweating, tachycardia, or convulsions? Have you sensed things that actually were not there? |
All the criteria should be asked in relation to the past 12 months.
The terms alcoholism, alcoholic, and alcohol abuse have been eliminated and are no longer considered appropriate, given that they stigmatize the patient. The criterion of having had legal problems related to alcoholic beverage consumption for defining alcohol use disorder has also been eliminated.8
According to the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), when alcohol consumption causes physical or mental damage to health, it is considered harmful alcohol use.9
- 2
Alcohol-related liver disease covers a broad spectrum of pathologies that includes steatosis, steatohepatitis, different grades of fibrosis, and cirrhosis and its complications.
Agreement reached: 100% in complete agreement.
Alcoholic liver disease (ALD) encompasses different grades of lesions that range from simple steatosis to cirrhosis. They may not necessarily be progressive stages of the disease and all of them can coexist in the same patient.10 Simple steatosis, initially macrovesicular and later mixed (macrovesicular and microvesicular), is the earliest change and presents in 90% of the individuals that are hazardous drinkers11,12or binge drinkers 13 (see the definition of terms further ahead). However, that lesion is often reversible, with sustained alcohol abstinence.14 Although the prevalence of each histologic lesion in ALD is not precisely known,15 25% of patients with ALD are estimated to develop steatohepatitis and close to 15% progress to cirrhosis.16–18 The 5-year accumulated risk for developing hepatocellular carcinoma in patients with cirrhosis due to alcohol is an estimated 1%.19 The hepatic lesion (steatosis, steatohepatitis, and fibrosis) in ALD begins by affecting the perivenular hepatocytes, then progresses to the mid-lobule hepatocytes, and finally affects the periportal hepatocytes.13
- 3
Patients with alcohol-related liver disease require a comprehensive psychiatric evaluation and concomitant management by trained addiction personnel.
Agreement reached: 100% in complete agreement.
Alcohol abstinence is crucial in all patients with ALD and psychosocial intervention by trained personnel is an essential tool for achieving abstinence and preventing relapse in alcohol consumption.20
Different psychosocial interventions have been shown to be effective in favoring alcohol abstinence. Twelve-step facilitation (TSF) therapy,21 motivational enhancement therapy (MET),22 and cognitive-behavioral coping skills therapy23 are three interventions that have demonstrated equally significant and sustained improvement for achieving abstinence and preventing relapse up to one year of follow-up.24
In a systematic review, Khan A. et al. included 13 studies (five were randomized clinical trials and the others were observational studies) with a total of 1,945 patients. They evaluated the effect of motivational enhancement therapy, cognitive behavioral therapy (CBT), motivational interviewing, supportive therapy, and psychoeducation. Integrated therapy that combined CBT, motivational enhancement therapy, and comprehensive medical care were found to increase alcohol abstinence. Abstinence was not maintained with any of the psychosocial interventions, but relapse appeared to be reduced with the integrated therapy of CBT and medical care.25
According to the Mexican 2013-2018 Specific Action for Addiction Prevention and Comprehensive Care Program, the identification of risk groups is preponderant, so they can receive interventions in line with their necessities and conditions of risk. For those that present with use or abuse of tobacco, alcohol, and other drugs, it is critical to offer them brief interventions, specialized treatment, rehabilitation, and support for their social reintegration.26
- 4
The quantification of alcohol intake per gram/day can be calculated through the formula: gram/day of alcohol = (quantity ingested in milliliters) (alcohol content of the ingested beverage) (0.8)/100. As an alternative for standardizing the measurement of alcohol consumption, a proposed standard drink is equivalent on average to 10-14 grams of alcohol.
Agreement reached: 96% in complete agreement; 4% in partial agreement.
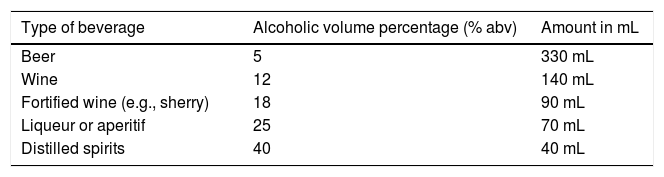
In their guidelines on brief intervention for preventing hazardous and harmful alcohol consumption, the WHO defines a standard drink as 10 g of pure ethanol and recommends not having more than two standard drinks per day, for both men and women.27 Importantly, that parameter suggested by the WHO appears to be the most appropriate, given that it is the simplest and easiest to replicate in clinical practice and for standardizing clinical trials.28 However, the quantity of alcohol in a standard drink varies, according to continental region, and differs even between countries. As found in the medical literature, amounts of alcohol in a standard drink vary from 8 g (Iceland) to 20 g (Austria) of pure ethanol.29 Regarding Mexico, the Norma Oficial Mexicana (NOM), NOM-142-SSA1/SCFI-2014 on “Alcoholic beverages. Health specifications. Health and commercial labeling”, published in the Diario Oficial de la Federación (DOF) in March 2015, specifically indicates that the approximate alcohol content in a “standard drink” is 13 g, considering that its specific gravity is 0.785 g/mL.30Table 3 shows the different types of standard alcoholic beverages and their composition, according to that established in the NOM-142-SSA1/SCFI-2014.
- 5
Alcohol consumption patterns are defined as: a) “hazardous drinking”, more than three standard drinks per day for men (> 30 g/day) and more than two standard drinks per day for women (> 20 g/day); b) “heavy episodic drinking”, six or more standard drinks (≥ 60 g of alcohol on at least one occasion); and c) “binge drinking”, four or more standard drinks for women (> 40 g of alcohol) and five or more standard drinks for men (> 50 g of alcohol) in fewer than two hours.
Types of standard alcoholic beverages according to the NOM-142-SSA1/SCFI-2014 on “Alcoholic beverages. Health specifications. Health and commercial labeling”.
| Type of beverage | Alcoholic volume percentage (% abv) | Amount in mL |
|---|---|---|
| Beer | 5 | 330 mL |
| Wine | 12 | 140 mL |
| Fortified wine (e.g., sherry) | 18 | 90 mL |
| Liqueur or aperitif | 25 | 70 mL |
| Distilled spirits | 40 | 40 mL |
abv: content of alcohol by volume.
Agreement reached: 100% in complete agreement.
The quantity of alcohol consumed, regardless of the consumption pattern, is the most important risk factor for developing ALD.31 Epidemiologic studies have shown a strong correlation between the quantity and duration of alcohol consumption and the presence of cirrhosis.32 In a cohort that included 6,970 adults from the general population, the frequency of cirrhosis was significantly higher in those that consumed ≥ 30 g/day of alcohol, compared with those that were abstinent or that consumed < 30 g/day (2.2% vs. 0.08%). The subjects that registered alcohol consumption > 120 g/day had the highest risk for presenting with cirrhosis (13.5%).33
Women are more susceptible to liver injury from alcohol than men.34,35 Some studies have even reported that women have a higher risk for presenting with ALD, consuming half the alcohol dose considered harmful in men. Likewise, women have a higher risk for accelerated disease progression and risk for developing cirrhosis than men. One study showed that women whose alcohol consumption was > 100 g/day developed cirrhosis in a mean length of time of 13.5 years, compared with a mean length of time of 20 years in men.34 Women with alcohol use disorder that ingested > 20 g/day of alcohol had a higher risk for developing ALD.36
Alcohol consumption pattern, especially “heavy episodic drinking” and “binge drinking”, is another factor that has been proposed in experimental models as a possible risk factor for developing ALD. Nevertheless, at present, a given consumption pattern has not been clearly related to a higher risk for ALD in humans,37 nor has an association been demonstrated in humans between the type of alcoholic beverage or the quality of alcohol consumed and the development of severe forms of the disease, such as alcoholic hepatitis (AH).38
In patients with comorbidities, such as metabolic syndrome, or chronic hepatitis B or hepatitis C virus infection, and alcohol consumption, even in quantities below those considered “hazardous drinking”, can favor and accelerate progression to liver injury.39,40 Those patients should avoid alcohol consumption.
Smoking is a condition that is frequently associated with alcohol use. There is a three-times higher risk for developing alcohol-related cirrhosis in persons that smoke one or more packs per day, compared with nonsmokers.41
- 6
“Alcoholic hepatitis” is a severe condition that frequently behaves as “acute-on-chronic liver failure”. It is characterized by systemic inflammation and a predisposition to the development of infections, kidney failure, encephalopathy, and multiple organ dysfunction, with an elevated mortality rate of 20-50% in the following three months, albeit possibly higher in the Mexican population.
Agreement reached: 100% in complete agreement.
Hepatitis or steatohepatitis due to alcohol has a wide clinical spectrum that ranges from an asymptomatic or minimal symptom status or mild clinical signs of disease to the presentation of severe acute-on-chronic liver failure.42–44 Severe AH is defined by a modified Maddrey’s discriminant function (MDF) score ≥ 32 or a Model for End-Stage Liver Disease (MELD) score equal to or greater than 21.42 Mortality at three months is high, but variable, depending on the population studied. Sidhu SS et al. calculated three-month mortality in AH at between 30-70%.45 In some populations, especially European ones, the general mortality rate for AH is low. In the STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH) study, mortality at 28 days varied between 13.5% and 19.4%.46
In a recent systematic review that included 77 studies published between 1971 and 2016, a total of 8,184 patients were analyzed, finding a general mortality rate due to AH at 28 days of 26%, at 90 days of 29%, and at 180 days of 44%. Comparing the mortality frequency between the different decades, there were no significant changes over time; not at 28 days or at 90 days (Pearson correlation coefficient r -0.216, p = 0.098; and r 0.121, p = 0.503, respectively). A small but significant increase was observed in relation to mortality at 180 days (r 0.461, p = 0.036).47
In Mexico, a multicenter study that included 175 patients at four different hospitals, found that 121 (69%) patients had underlying cirrhosis and 125 (71%) patients developed at least one complication during hospitalization: acute kidney injury (AKI) in 43%, infections in 48%, hepatic encephalopathy (HE) in 49%, and gastrointestinal bleeding in 17%. Overall intrahospital mortality and 90-day mortality were 36% and 51%, respectively. The main causes of death at 90 days were: sepsis (20%), liver failure (24%), and multiple organ failure (46%). In that same study, the quantity of alcohol consumed was shown to have a negative impact on patient survival. Seventy-six percent of the patients with an alcohol intake > 120 g/day died, compared with 46% of the patients that consumed lower quantities of alcohol (p < 0.0001). The mortality rate in Mexican patients classified as age-bilirubin-INR-creatinine (ABIC) B and C was as high as 50% and 81%, respectively.48
Another poor outcome factor related to higher mortality in Mexican patients is malnutrition. A study that included 76 patients with AH, of which 76.3% had underlying cirrhosis, and whose nutritional status was evaluated through the subjective global assessment (SGA), reported that 38 (50%) of the patients presented with severe malnutrition, 22 (28.9%) were at risk for malnutrition, and only 16 (21.1%) were well-nourished. Overall 30-day mortality was 60.5% and the multivariate analysis through logistic regression showed that the presence of severe malnutrition was associated with early death (30 days): odds ratio (OR) = 6.4; 95% confidence interval (95% CI) = 1.9-22.1; p = 0.003.49
- 7
Alcoholic hepatitis is classified as: a) “definitive” when there is histologic confirmation; b) “probable” when the clinical diagnosis is based on hazardous drinking, active drinking of up to eight previous weeks, the development of jaundice, an AST/ALT ratio > 1.5, elevation of aminotransferase levels usually < 400 IU/L, and the absence of other causes of liver injury; and c) “possible” when the clinical diagnosis is uncertain.
Agreement reached: 95% in complete agreement; 5% in partial agreement.
AH is classified as “probable” when the following diagnostic criteria are clinically met: serum total bilirubin (TB) > 3 mg/dL, elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) > 50 IU/L but < 400 IU/L, and an AST/ALT ratio > 1.5. They should all be present in both the clinical and hazardous drinking history contexts described above.42,50 In addition, there should be no confounding factors, i.e., the autoimmune profile should be negative (antinuclear antibodies [ANAs] < 1:160 or anti-smooth muscle antibodies [ASMAs] < 1:80), and there should be no metabolic liver diseases, sepsis, shock, cocaine use, or use of drugs or herbal medicine with hepatotoxic potential within the last 30 days.42
AH is considered “definitive” when the clinical diagnosis has been confirmed by typical biopsy findings: macrovesicular steatosis, lobe inflammation with mononuclear cell infiltration and a predominance of neutrophils, satellitosis, hepatocellular lesion identified by the presence of Mallory-Denk bodies or ballooning degeneration of hepatocytes, necrosis, canalicular or ductular bilirubinostasis, and fibrosis that is typically described as pericellular and perisinusoidal.51 Characteristic changes of liver injury due to alcohol are venous fibro-obliterative lesions and hyaline sclerosis, which are not observed in nonalcoholic liver injury.32
AH is classified as “possible” when it is clinically suspected but there are also confounding factors, such as the possibility of ischemic hepatitis in the face of severe gastrointestinal bleeding, hypotension, or recent cocaine use; the possibility of idiosyncratic damage from drugs or herbal medicine; uncertainty, with respect to alcohol use disorder (e.g., when the patient denies alcohol use); atypical laboratory test findings (e.g., AST < 50 IU/L or > 400 IU/L, an AST/ALT ratio < 1.5), ANAs > 1:160 or ASMAs > 1:80). In those cases, liver biopsy is recommended to confirm or diagnose AH.42
- B
DIAGNOSTIC TESTS FOR THE DETECTION OF ALCOHOL-RELATED LIVER DISEASE
- 8
Liver biopsy can be performed to make the definitive diagnosis of alcohol-related liver disease, to evaluate the exact stage and hepatopathy prognosis, and to rule out additional or alternative causes of liver injury. However, because it is an invasive procedure, it is not recommended in all cases and its risk-benefit must be individually assessed.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 95% in complete agreement; 5% in partial agreement.
Routine liver biopsy is not recommended for diagnosing ALD.52–54 Steatosis due to alcohol is apparent through noninvasive imaging studies, such as ultrasound, tomography, and magnetic resonance. Ultrasound is the most cost-effective54 and magnetic resonance is the most accurate for detecting, and even quantifying, steatosis but is expensive and not widely available.55 Biochemically, elevated AST and gamma-glutamyl transferase (GGT) are indicators of excessive alcohol consumption and their testing is widely available in clinical practice. On the other hand, liver biopsy is not considered necessary for diagnosing alcohol-induced cirrhosis, given that a history of hazardous drinking, biochemical alterations with elevated AST and GGT levels, an AST/ALT ratio > 1.5-2 .0, and elevated mean corpuscular volume (MCV), generally in the context of macrocytic, hyperchromic, or megaloblastic anemia, as well as ruling out chronic viral diseases, such as hepatitis B or C and autoimmune diseases, are usually sufficient for arriving at the diagnosis of cirrhosis due to ALD.52,53
In the context of “probable” AH, the clinical criteria are considered sufficient for making the diagnosis in clinical practice, generally reserving transjugular liver biopsy for patients with “possible” AH, as described above.42,56
Biopsy findings in ALD are practically indistinguishable from findings in nonalcoholic fatty liver disease (NAFLD), and therefore, histopathologic study for differentiating between the two diseases is not recommended.13,57
- 9
Liver stiffness measurement by elastography can be useful for evaluating liver fibrosis in alcohol-related liver disease.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 95% in complete agreement; 5% in partial agreement.
However, it should not be used in patients with alcoholic hepatitis because it overestimates fibrosis grade.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 85% in complete agreement; 10% in partial agreement.
Noninvasive methods for evaluating liver fibrosis are less validated in ALD, compared with other etiologies (e.g., chronic viral hepatitis or nonalcoholic steatohepatitis [NASH]).32
Among the radiologic techniques available for evaluating fibrosis, elastography is well validated for detecting advanced fibrosis and performs better in ruling out the presence of cirrhosis than confirming it.58 There is no agreement as to the cutoff values for defining cirrhosis in the context of ALD and there is a risk for false positives in patients with active alcohol consumption.32 In a recent systematic review with a meta-analysis, the diagnostic accuracy of transitory elastography for establishing fibrosis grade in patients with ALD was evaluated. Those authors suggested utilizing a cutoff value < 9.5 kPa to rule out advanced fibrosis (F3) and a cutoff value < 12.5 kPa to rule out cirrhosis (F4). However, they also recommended caution with respect to their results, given that the majority of data from the review came from retrospective studies and the overall risk for bias was high for most of the studies analyzed. In addition, prospective studies are required that evaluate the accuracy of the cutoff values proposed.59
Elastography is not recommended for estimating liver fibrosis grade in patients with AH because the presence of hepatic inflammation, as well as systemic inflammation, cholestasis, hyperbilirubinemia, steatosis, and hepatic vein congestion, among others, overestimate the grade of fibrosis.60
- 10
Ultrasound, tomography, and magnetic resonance can quantify steatosis and help rule out other causes of chronic liver injury and can also recognize advanced disease (cirrhosis) and its complications. However, they cannot distinguish whether injury is secondary to alcohol or to other etiologies.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 95% in complete agreement; 5% in partial agreement.
Ultrasound, tomography, and magnetic resonance are useful for detecting steatosis,61 but ultrasound is the imaging method with lower sensitivity and specificity, especially when the steatosis affects at least 20-30% of the liver parenchyma. The controlled attenuation parameter (CAP) has been shown to be useful in quantifying hepatic steatosis but it is not specific for ALD and therefore does not distinguish alcoholic steatosis from steatosis secondary to any other etiology. When a patient suffers from other diseases (e.g., hepatitis B or C, metabolic syndrome, obesity, etc.), in addition to hazardous drinking, the result should be interpreted according to the appropriate clinical context and in an individualized manner.62,63 Magnetic resonance can detect steatosis if 5-10% of the parenchyma is affected. Magnetic resonance elastography is more expensive, and generally less available, but it is more accurate for estimating steatosis and fibrosis grades, compared with other types of elastography.64
Imaging studies do not distinguish between ALD and other etiologies as causes of hepatopathy, but they are useful for anatomically evaluating the liver and bile ducts and for ruling out primary and secondary obstructive processes. In the context of the cirrhotic patient, regardless of etiology, they are useful for evaluating the presence and magnitude of complications derived from portal hypertension: ascites, collateral vessels, and focal lesions.61
- 11
In patients suspected of presenting with alcoholic hepatitis and jaundice, ultrasound is recommended as a screening tool for differentiating obstructive processes of the bile ducts.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Ultrasound is the first-line noninvasive imaging technique that enables intrahepatic cholestasis to be differentiated from extrahepatic cholestasis. Ultrasound is extremely accurate for identifying the site and cause of a biliary obstructive process.65 It is also cost-effective for identifying an obstructive process and distinguishing it from nonobstructive jaundice.66
- 12
When there is doubt, or a history of hazardous drinking cannot be readily established in the patient, an alcoholic liver disease/nonalcoholic fatty liver disease index (ANI) above 0 indicates the likelihood of alcoholic liver disease.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
The alcoholic liver disease/nonalcoholic fatty liver disease index (ANI) is a highly accurate tool for differentiating ALD from NAFLD. Its application is recommendable when there is doubt with respect to hazardous drinking as a trigger for liver injury in the presence of steatohepatitis. Short-term alcoholic abstinence does not affect the ANI result. Other liver diseases (e.g., viral or autoimmune) should be ruled out before applying the ANI. That index is more accurate when the MELD score is under 20. The ANI can be calculated free of charge on the website of the Mayo Clinic.67
The most relevant variables identified through the logistic regression analysis that are able to distinguish between ALD and NAFLD are: MCV, the AST/ALT ratio, body mass index (BMI), and sex. An ANI value above zero favors alcohol as the etiology and an ANI value below zero favors the diagnosis of NAFLD. The ANI had a c-statistic of 0.989 in the derivation sample and 0.974, 0.989, and 0.767 in the three validation samples of the model. The ANI is superior for differences between ALD and NAFLD, compared with other biomarkers, such as protein tyrosine phosphatase 1b, the AST/ALT ratio, GGT, and carbohydrate-deficient transferrin.68
- C
DIAGNOSIS OF ALCOHOLIC HEPATITIS
- 13
Alcoholic hepatitis is a clinical entity characterized by the sudden onset of jaundice and aminotransferase elevation (particularly AST) that occur in patients with continuous drinking in the hazardous drinking range. When severe, other signs of liver decompensation can also be found, such as bacterial infection, ascites, variceal bleeding and/or encephalopathy.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Severe AH is a clinical entity characterized by the sudden onset of jaundice and elevated aminotransferases, particularly AST, that occur after having engaged in hazardous drinking for more than six months, with fewer than 60 days (eight weeks) of abstinence before the presentation of jaundice.50 The cutoff point with respect to the duration of drinking and quantity of alcohol related to the development of AH are not completely established, but in general, an average alcohol intake ≥ 40 g/day for women and ≥ 50-60 g/day for men is a reasonable parameter for AH diagnosis. Regarding duration, patients with AH generally have a history of intense alcohol consumption for more than five years, possibly having had intermittent periods of abstinence.42 Jaundice is frequently accompanied by fatigue, hepatomegaly, and decompensation (ascites, HE, bacterial infection, variceal bleeding). Liver biopsy reveals steatohepatitis with Mallory-Denk bodies, ballooning degeneration of hepatocytes, bilirubinostasis, and advanced fibrosis/cirrhosis. 43
- 14
A detailed clinical history can identify alcohol as a liver aggressor. It can be supported by the AUDIT, AUDIT-C, and CAGE scales.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Different questionnaires are available for detecting alcohol use disorder and have generally shown greater sensitivity than any of the biochemical tests at hand.69,70
The CAGE (acronym for cut down, annoyed, guilty, eye-opener) is among the more widely used questionnaires and consists of four simple questions. If the answer is “yes” to two or more of the questions, severe alcohol dependence (the term used in the DSM-IV) is positively correlated. The CAGE questionnaire has been widely validated, with a reported sensitivity between 91% and 95% and specificity between 76% and 77% for identifying patients with excessive alcohol consumption and alcohol dependency or alcoholism, respectively (DSM-IV). However, it has a much lower sensitivity than that of the Alcohol Use Disorders Identification Test (AUDIT): 40% vs. 93%, respectively.71
The AUDIT questionnaire is considered the gold standard for identifying hazardous drinking.71 Designed by the WHO, it has been widely validated for detecting alcohol use disorder, even when mild. Its simple format is made up of 10 multiple choice questions whose answers are marked on a scale from 0 to 4. A result ≥ 8 indicates alcohol use disorder (92% sensitivity and 94% specificity) and a result ≥ 20 indicates severe alcohol use disorder.72
The AUDIT-C is a simplified version of the AUDIT. It has a similar sensitivity and specificity to the AUDIT and consists of 4 multiple choice questions. Practical online calculators are also available. As with the AUDIT, and the answer to each question is marked on a scale from 0 to 4. A score ≥ 3 for women and ≥ 4 for men indicates alcohol use disorder.73
- 15
There is no specific biomarker for the diagnosis of alcoholic hepatitis. Alterations that aid in diagnosing alcoholic hepatitis are leukocytosis with neutrophilia, hyperbilirubinemia (generally > 3 mg/dL), AST > 50 U/L (usually < 400 U/L), and an AST/ALT ratio > 1.5-2 .0.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
To make the clinical diagnosis of AH (“possible” AH), the following values are indispensable: TB > 3 mg/dL, elevation of AST and ALT > 50 IU/L but < 400 IU/L, and an AST/ALT ratio > 1.5 (previously, it was an AST/ALT > 2).42,50,56 AH diagnosis is supported by neutrophilic leukocytosis, coagulopathy, and thrombocytopenia.43
- 16
Liver biopsy is utilized to confirm diagnosis, but it is not indispensable and should be reserved for cases in which there is diagnostic uncertainty.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
In clinical practice, percutaneous ultrasound-guided liver biopsy or transjugular liver biopsy can be performed due to the frequent presence of thrombocytopenia and coagulopathy in patients with AH. The transjugular approach appears to be the route of choice.54–56 Nevertheless, in patients with “probable” AH, liver biopsy is not essential, given that the possibility of finding a diagnosis different from AH in the histopathologic study is less than 10%. On the other hand, liver biopsy is indeed recommended in patients with “possible” AH to either confirm or rule out the diagnosis of AH.42
- 17
Histopathologic findings, such as macrovesicular steatosis, steatohepatitis, ballooning degeneration of hepatocytes, infiltration of polymorphonuclear neutrophils, and Mallory-Denk bodies, can aid in making the diagnosis, but they are not pathognomonic.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
The definitive diagnosis of AH is made through liver biopsy.42,48,49 It is also useful for estimating the short-term outcome of AH50 and alcohol-related acute-on-chronic liver failure.51 Fibrosis grade (bridging, advanced, or the presence of cirrhosis), a severe grade of neutrophilic infiltration, and the type of hepatocellular bilirubinostasis, added to the presence of ductular or canalicular bilirubinostasis and the presence of megamitochondria, are histologic factors independently associated with 90-day mortality. In addition, the type of bilirubinostasis with hepatocellular plus ductular or canalicular involvement, is also a predictive factor for the development of bacterial infections.52 Those findings are not pathognomonic. NASH can histologically present with similar findings, thus the clinical criterion is fundamental for diagnosing AH.51,57
D. EVALUATION AND PROGNOSIS OF PATIENTS WITH ALCOHOLIC HEPATITIS
- 18
Complete nutritional, psychologic, psychiatric, and social work evaluations should be carried out in all patients with alcoholic hepatitis.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
For several years, patients with AH and malnutrition have been reported to have higher rates of morbidity and mortality.74 That has been corroborated in the Mexican population, with the report that malnutrition, as an independent risk factor, increases mortality in Mexican AH patients. Higuera-de la Tijera et al. found that severe malnutrition was associated with a higher 30-day mortality rate (OR = 6.4; 95% CI = 1.9-22.1; p = 0.003).49
The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends offering nutritional therapy to all patients with severe AH that do not meet the caloric requirements for spontaneous dietary intake to improve survival, reduce infection rates, improve liver function, and resolve HE, if present. However, survival improvement has not been demonstrated in different meta-analyses.75
A daily caloric intake of 35-40 kcal/kg and a daily protein intake of 1.2-1.5 g/kg are recommended. Nevertheless, they are difficult-to-achieve objectives in clinical practice. Therefore, patients that do not meet those requirements should be given enteral nutritional support through a nasoenteral tube.75
In the most recent multicenter study that combined steroids and enteral diet, no improvement in survival was demonstrated with their combination but there was a significantly higher mortality rate in the patients that had a hypocaloric diet (21.5 Kcal x kg-1 x d-1).76
Likewise, micronutrient supplementation, such as the B complex (especially thiamine), vitamin D, and zinc, is recommended by the ESPEN.74
For the abovementioned reasons, the present consensus recommends that all patients with AH have an adequate nutritional evaluation, a daily energy intake of 35-40 kcal/kg, a daily protein intake of 1.2-1.5 g/kg, and that they receive micronutrient supplementation.
The oral route should be the first feeding option but nasoenteral administration can be used if there is an impediment, such as HE, cough syncope, or swallowing alterations. Parenteral feeding should be used as a last resort and only in patients contraindicated for enteral feeding.
Patients with alcohol use disorder have a high prevalence of psychiatric comorbidity, especially anxiety disorders, mood disorders, psychosis, post-traumatic stress disorder, and schizophrenia.77 They can also have a history of sexual abuse, physical abuse, and social isolation.50 Such factors can increase the risk for alcohol relapse. Therefore, the present consensus recommends evaluations by psychologists and psychiatrists that should be in charge of substance abuse management, including alcohol.
There is also a high risk for developing other addictions to substances such as opioids, benzodiazepines, and nicotine. The synergy of smoking and drinking is an important risk factor for cardiovascular diseases and cancer, including hepatocellular carcinoma. Due to the fact that patients with alcohol use disorder are heavy smokers, psychologic and psychiatric evaluation and referral to addiction clinics are recommended.28,78
In addition, evaluation by a social work team is recommended because any addiction, including that of alcohol, adversely affects the family system and all its members, including children, thus contributing to family separation. The financial and emotional burden caused by the disease results in the suffering of the individual family members. Children that have a parent with an addition are at an increased risk for low academic performance, behavior disorders, psychiatric disorders, and presenting with substance abuse, themselves.79
- 19
The comprehensive evaluation of the patient with confirmed alcoholic hepatitis should include:
- a)
the search for infectious foci.
- b)
the ruling out of gastrointestinal bleeding.
- c)
the ruling out of encephalopathy.
- d)
the ruling out of acute kidney injury.
- e)
the search for chronic complications of hepatopathy
- a)
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
It is vitally important to search for primary infection sites because they can be poor outcome factors. Undetected and untreated infections can lead to the development of acute kidney injury (AKI) and multiple organ failure, increasing mortality. Louvet et al. reported a 25% infection rate in patients with severe AH upon hospital admission and the mortality rate at two months increased by 30% in the infected patients.80 In the STOPAH study, 24% of the deaths were in patients with infections.81
A high level of suspicion is needed to identify bacterial or fungal infections, given that cardinal signs, such as fever, can be absent. Other signs, such as tachycardia and leukocytosis may not be very specific for infection in those patients. Systemic inflammatory response syndrome (SIRS) can be present, with or without infection upon hospital admission, and is a risk factor for the development of multiple organ failure. Serum procalcitonin is a useful marker for detecting bacterial infections.82 Similar to the European guidelines, we recommend herein the performance of chest x-ray, urinalysis, diagnostic paracentesis in the patient with ascites, and the ruling out of soft tissue infections. Pan-culturing is also strongly recommended in all patients.28
Another recommendation is ruling out the presence of AKI, given that it has been reported as one of the most important predictors of 90-day mortality. The presence of SIRS, increased bilirubin level, and coagulopathy upon hospital admission are useful predictors for the development of AKI.83
In an observational study of 71 Mexican patients, Higuera-de la Tijera et al. found that the quantity of alcohol consumed was related to AKI, reporting intakes of 219 g/day vs. 101 g/day; p = 0.001. They also found that the presence of AKI was an isolated associated factor for greater risk of death (RR = 6.7, p = 0.02). Strikingly, the presence of other complications that should be monitored, such as upper gastrointestinal bleeding (UGIB) or HE, did not, separately, increase mortality, but their combination or the added presence of AKI, resulted in an increase in mortality. The following combinations behaved as predictive factors of death: AKI plus HE (RR = 8.9, p = 0.001) and HE plus UGIB (RR = 6.7, p = 0.01). The presence of AKI, UGIB, and HE showed the highest risk (RR = 10.0, p = 0.001).84
- 20
The severity of alcoholic hepatitis should be established, as it defines the type of treatment to administer.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
- 21
The application of the different scales (MDF, MELD, Glasgow, ABIC) is useful for establishing disease severity, predicting mortality, and indicating corticosteroid treatment.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Determining the severity of disease in patients with AH is essential because it establishes the conduct to be followed, such as whether the patient should be hospitalized or managed as an outpatient, the treatment to be employed, and the prognosis for survival.
There are different scales for determining disease severity. MDF was the first scale that differentiated individuals with higher short-term mortality, and it continues to be one of the most widely used.85 A cutoff point ≥ 32 identifies patients with severe AH and high short-term mortality ( 20-50% ). Thirty-day mortality is under 10% in patients with mild AH (MDF < 32).28,50,81,85–87
Other severity evaluation scales include:
- •
MELD
- •
ABIC
- •
Glasgow
The MELD is already a well-validated prognostic score for advanced liver disease. It has also been shown to be useful for evaluating mortality in AH, in which a MELD score ≥ 20 suggests elevated 30-day and 90-day mortality.28,50,86
The acronym ABIC signifies A for age, B for serum bilirubin, I for INR, and C for serum creatinine. The calculated 90-day mortality risk on the ABIC scale is 6.71 (low risk), 6.71-9 (intermediate risk), and ≥ 9 (high risk), with reported survival of 100%, 70%, and 25%, respectively.28,50,86,87
The Glasgow scale is more recent and utilizes the following variables: age, serum bilirubin from day one, urea from day one, bilirubin from days 6-9, prothrombin time, and peripheral leukocyte count. The score ranges from 5-12, the cutoff point for defining severity is ≥ 9, and 90-day mortality is 52%.28,50,87
Forrest et al. recently conducted a study on patients from the STOPAH trial, in which they compared the effectiveness of the different scales (MDF, MELD, ABIC, Glasgow) for predicting mortality at 28 days and at 90 days. They reported that the MDF had poorer performance and concluded that the MELD, ABIC, and Glasgow scales were superior for predicting mortality (AUROC: 0.670, 0.704, 0.726, and 0.713, respectively).88
In Mexico, Altamirano et al. reported that the ABIC and MELD scales had better performance and prognostic accuracy for predicting severity and mortality in a Mexican population, with an AUROC of 82 and 83, respectively.48
The present consensus recommends establishing the severity and prognosis of AH by utilizing any of the scales described above to define the starting of steroid management.
- 22
The Lille model is useful for evaluating treatment response and is calculated seven days after starting corticosteroid treatment. If it is > 0.45, the patient is considered a nonresponder.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
The scarceness of effective therapies for severe AH and the need for early identification of the patients that do not respond to steroids led to the development of the Lille model.89 It includes six variables: age, creatinine, albumin, prothrombin time, TB, and TB evolution at day seven. Patients with a Lille model score ≥ 0.45 have a six-month survival rate of 25%, compared with an 85% rate in others, suggesting they are nonresponders and should suspend steroid treatment. The main advantage of the Lille model is its dynamic component in relation to TB evolution, which is the most accurate and precise variable for predicting six-month mortality.87
In a meta-analysis by Mathurin et al. that newly evaluated the Lille model, they identified three patient groups according to steroid response: 1) complete responders (Lille model score ≤ 0.16), 2) partial responders (Lille model score of 0.16-0.56), and 3) nonresponders (Lille model score ≥ 0.56), providing a window for suspending steroids in the nonresponders.90
Applying the Lille model on day four of steroid treatment was recently shown to be similar to applying it on day seven, in relation to accuracy for predicting mortality, but that finding needs to be validated.91 On the other hand, Louvet et al. demonstrated the usefulness of combining the different scales and reported that the best combination was that of the Lille model + the MELD score for predicting mortality in severe AH patients more accurately.92
- 23
Alcohol abstinence is the most important prognostic factor for long-term survival in patients with alcoholic hepatitis. In the short term, mortality is determined by causes related to inflammatory response, liver injury (variceal bleeding, portal hypertension), infections, and organ failure.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Long-term survival after a severe episode of AH is strongly related to successful alcohol abstinence. Louvet et al. conducted a study that described factors for predicting short-term and long-term survival, reporting that alcohol intake, defined as ≥ 30 g/day, was not associated with short-term mortality (p = 0.24) but was strongly associated with long-term mortality (> 6 months), with a hazard ratio (HR) of 3.9 (p < 0.001). They also reported the association between the quantity of alcohol consumed and long-term mortality: HR = 2.36 (p = 0.052) for intake of 1-29 g/day; HR = 3.2 (p = 0.003) for intake of 30-49 g/day; HR = 3.51 (p = 0.0001) for intake of 50-99 g/day; and HR = 5.61 (p = 0.0001) for intake > 100 g/day,93,94 implying that the cornerstone of survival improvement is the effective management of alcohol use disorder.28
Short-term survival is determined by the presence of SIRS, regardless of the presence of infection, and decreases if it presents together with variceal bleeding, AKI, or HE.28,82
Higuera-de la Tijera et al. have demonstrated an increase in short-term mortality in Mexican patients with severe AH + AKI (RR of 6.7, p = 0.02). They also showed that the combination of AKI + UGIB + HE elevated the risk of death to a RR of 10, p = 0.001.84
E. TREATMENT OF FIBROSIS DUE TO ALCOHOL-RELATED LIVER DISEASE
- 24
The time at which fibrosis becomes irreversible is not precisely known in patients with alcoholic hepatitis. However, there is evidence that eliminating alcohol consumption can reverse or stop fibrosis.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Some of the complications observed in ALD are not due to the inherent toxicity of alcohol but are a result of the different stages of liver fibrosis. Despite the fact that the prevention and detention of fibrosis is an adequate aim in that context, the exact time at which it begins, the individual phenomena that determine the pattern of progression, and the point at which fibrosis becomes irreversible are not known. The main factors for the development and progression of ALD are quantity of alcohol consumed, consumption duration, consumption pattern, hepatitis viruses, interactions with the environment of the host, sex, genetic factors, and nutritional factors.95 For reasons not yet understood, most heavy drinkers do not develop severe liver disease with fibrosis. The majority develop alcoholic fatty liver but only 10-35% develop alcoholic steatohepatitis and 8-20% develop cirrhosis of the liver, even with an alcohol intake of 12-24 g/day.96
At any rate, sustained alcohol consumption is related to episodes of AH, which leads to more serious cases of decompensated ALD, and in turn, greater morbidity and mortality. It is the main risk factor for liver injury and its complications.95 In addition, the coexistence of sustained alcohol consumption with other hepatic comorbidities (NAFLD, chronic hepatitis C, chronic hepatitis B, metabolic liver disease, or autoimmune liver, etc.) increases the risk for fibrosis.96,97 Therefore, total and sustained alcohol abstinence is the cornerstone for stopping, and even reversing, alcohol-related liver fibrosis, as long as the threshold of irreversible injury has not been crossed.28,50,98
- 25
There is no evidence that any medication (ursodeoxycholic acid, chenodeoxycholic acid, obeticholic acid, or others) is beneficial for the regression of fibrosis due to alcohol-related liver disease, especially alcoholic hepatitis.
Quality of evidence: B1
Strength of recommendation: strong, in favor of.
Agreement reached: 95.5% in complete agreement; 4.5% in complete disagreement.
Even though there is evidence, as well as pathophysiologic principles, that demonstrate the usefulness of ursodeoxycholic acid, chenodeoxycholic acid, and obeticholic acid for stopping fibrosis progression in experimental cholestatic liver disease and NAFLD models, at present there are no clinical trials that show safety and effectiveness in relation to histologic improvement of the inflammatory process or fibrosis regression in ALD at any of its different stages or degrees of injury, including AH.98–101
A phase 2 study is currently being conducted that is analyzing the changes in the MELD score after six weeks of obeticholic acid administration, and the results are pending.101
- 26
There is no evidence that antioxidants (silymarin, vitamin E) reduce hepatic inflammatory damage or lead to the regression of fibrosis in patients with alcoholic hepatitis.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 95.5% in complete agreement; 4.5% in complete disagreement.
Despite the fact that silymarin is a product with great commercial success that is popularly perceived as apparently harmless, there is no evidence of its usefulness in AH.102 According to two reviews in the medical literature (a meta-analysis and an evidence report), there are no conclusive benefits that support the prescription of that antioxidant and the studies analyzed were greatly heterogeneous, with conflicting results.103–105 With respect to vitamin supplements, including vitamin E, there is no scientific evidence supporting their use as monotherapy or in combination with traditional treatment for AH.106
F. TREATMENT OF ALCOHOLIC HEPATITIS
- 27
The therapeutic strategy that remains a cornerstone of treatment for acute alcoholic hepatitis is alcohol abstinence.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Given that AH, by definition, requires significant alcohol consumption that is sustained and recent, and added to the individual and environmental risks involved, alcohol suspension is the imperative measure recommended for those suffering with the disease. Maintaining abstinence beyond 90 days after the onset of AH is especially important because that is the expected threshold of time for a process of liver function recovery to take place.107 Patients that begin to drink again are known to have a worse outcome, in general. In the STOPAH study, sustained alcohol abstinence was the single factor associated with one-year survival. That benefit was lost with as little as one to two alcoholic beverages per day, increasing mortality.81,108 The present consensus group firmly believes that multidisciplinary management is necessary for the treatment of alcohol use disorder that must include evaluations in the areas of social work, psychology, and psychiatry, as well as guaranteeing an adequate support network.
- 28
Nutritional support is essential in the treatment of alcoholic hepatitis. The recommended caloric intake is 35-40 cal/kg/day, with protein intake of 1.2-1.5 g/kg/day. It is also important to treat other nutrient deficiencies, such as those of vitamins and minerals.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Albeit controversial, nutritional support has acquired great importance in the context of AH because it has been shown to be beneficial in relation to the mortality rate and the development of HE and infections.109 A supplemented diet has not been more advantageous than an unsupplemented one, therefore, the priority is to focus on the caloric content, which, when under 21.5 kcal/kg/day, impacts mortality. Particularly for severe AH, the current recommendation is a protein intake of 1.2-1.5 g/kg, with a caloric intake of 35-40 kcal/kg.28,50 Enteral nutrition as monotherapy has even been compared with prednisolone, describing statistical similarities in the clinical outcomes.110 Evaluation by nutrition personnel should be implemented at the time of diagnosis because the effects of malnutrition and catabolism can influence the response to pharmacologic treatment with steroids, increase the risk for infection, and increase morbidity and mortality.111
- 29
Treatment with corticosteroids in alcoholic hepatitis is indicated in cases of severe acute alcoholic hepatitis (MDF score above 32 or MELD score above 20).
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Pharmacologic treatment with corticosteroids in AH is justified only in severe cases defined by an MDF score ≥ 32 or a MELD score > 20, as long as there are no contraindications for their administration. The drug of choice is prednisolone, which has been substituted by prednisone in Mexican studies, for reasons of availability.112 The benefit of 40 mg daily of prednisolone is limited, given that in the STOPAH study only short-term survival, i.e., 28 days, was improved, compared with monotherapy with pentoxifylline or placebo and with the concomitant use of pentoxifylline-prednisolone. Unfortunately, corticosteroids have no effect on mortality beyond 28 days. Two meta-analyses that include results from the STOPAH study, confirmed the 28-day mortality benefit, with no six-month extension, suggesting the need to establish new clinical objectives and new therapeutic strategies.113,114 There is a risk for decompensating certain conditions after starting corticosteroid therapy, thus the contraindications for their administration must be taken into account (Table 4). Infections, which are a frequent cause of death in AH, are an extremely important contraindication. Classically, infection control before beginning treatment with a steroid has been suggested. However, there is evidence of similar mortality results in patients with AH plus active infection that began concomitant antibiotic and steroid therapy vs. AH patients with no infection.28,115
- 30
Intravenous infusion of N-acetylcysteine has shown an increase in survival in the short term, but not in the long term (three to six months), only when used together with prednisolone. Its routine use is not recommended.
Contraindications for corticosteroid use in patients with acute alcoholic hepatitis 28,115,116.
| Absolute |
|
| Relative |
|
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
N-acetylcysteine has been suggested as a therapeutic option because its mechanism of action consists of increasing glutathione reserves to reduce the oxidative stress that is a cardinal pathophysiologic factor in patients with AH. In a controlled clinical trial, the administration of prednisolone was compared with its concomitant administration with intravenous N-acetylcysteine in the management of severe AH. Several doses and infusion speeds were utilized for N-acetylcysteine on the first day of administration and the dose of 100 mg kg of body weight from days two to five. There was a significant decrease in mortality at one month in the prednisolone + N-acetylcysteine group (8%), compared with prednisolone alone (24%) but there were no differences in relation to mortality at three or six months. In a sub-analysis, reduced hepatorenal syndrome-specific mortality at six months and infection frequency were found.116,117 Despite those results, more evidence is needed to recommend its routine use, given that there is still no improvement in the overall mortality threshold after one month. Prednisolone is not available in Mexico, but the replication of those results with available formulations (prednisone) could be viable.
- 31
There is no current scientific evidence that pentoxifylline is useful, but some studies have shown it to be beneficial for reducing the risk for kidney injury, hepatorenal syndrome, and death.
Quality of evidence: C1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Pentoxifylline is a phosphodiesterase inhibitor that suppresses the actions of tumor necrosis factor-alpha (TNF-alpha), a key cytokine in the pathophysiology of AH.118 One of the first promising studies was conducted by Akriviadis et al. The results of that double-blind, randomized controlled trial on patients with severe AH (MDF score > 32) showed that treatment with pentoxifylline improved short-term survival and that the benefit appeared to be related to a significant decrease in the risk for the development of hepatorenal syndrome.119 Nevertheless, later studies have not shown any benefit for survival. A systematic Cochrane review that analyzed five clinical trials concluded that pentoxifylline use for the treatment of severe AH could not be supported or rejected, according to the available evidence.120 Further studies explored the possibility of evaluating whether pentoxifylline was an effective additive to the use of steroids in AH. However, a randomized, double-blind, multicenter study that included 23 hospitals in France found no improvement in six-month survival with the combination of 40 mg daily of prednisolone plus 400 mg of pentoxifylline three times a day for four weeks.121 The results of a systematic review and meta-analysis that included a total of 2,639 patients and 25 studies confirmed that pentoxifylline as monotherapy had no effect on decreasing mortality.122
- 32
Opportune treatment of infections is essential in alcoholic hepatitis.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
In patients with AH, the frequency of infections is reported at up to 25% of cases, increasing the rate of organ failure and death.123 In the STOPAH study, 24% of deaths were secondary to infectious events, regardless of the treatment given.81 Mortality in patients with infections and severe AH increases, according to the Lille model, even in responders to corticosteroid therapy.124 Considering that one of the contraindications for corticosteroid therapy is the presence of uncontrolled infection and the consequent increase in mortality due to infections in patients with AH, it is imperative to systematically look for infections, identify them opportunely, and treat them in those patients.
- 33
Anti-TNF-alpha biologic agents are not recommended for the treatment of alcoholic hepatitis. Those agents are associated with a high risk for infections, sepsis, and death.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Despite the pathophysiologic support due to the role of certain cytokines in AH, such as TNF-alpha, interleukin-1, and interleukin-8, current evidence is not consistent regarding the administration of anti-TNF agents in patients with severe AH. A systematic review conducted in 2019 that included the analysis of five studies concluded that infliximab could be a treatment alternative for patients in whom corticosteroids were contraindicated. However, the review was based on case series and two clinical trials. From the latter, the use of three doses of infliximab was shown to increase the risk for infection, reaching 89%, and consequently, death.125 The effect of the combination with a steroid was also studied, resulting in an increased risk for infection and complications. Even though apparent benefits were observed in the reduction of biochemical markers for inflammation and a comparable infection rate to that of therapy with corticosteroids alone (10-20%), the routine use of a single dose of infliximab is not recommended because of its greater adverse effects and higher treatment cost.126
- 34
There are new pharmacologic agents that are potentially beneficial for alcoholic hepatitis. The gut-liver axis, hepatic regeneration, apoptosis, oxidative stress, and inflammatory signaling are among the therapeutic targets.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Due to the economic burden and associated mortality of AH, clinical trials have been conducted in recent years to evaluate new objectives and develop viable treatment options for patients with severe alcoholic disease. AH is associated with dysbiosis of the gut microbiota due to alcohol consumption, resulting in the gut-liver axis becoming a potential target for therapy. AH-related dysbiosis has been shown to be associated with an increase in Bifidobacteria, Streptococci, and Enterobacteria, with a decrease in Clostridium leptum or Faecalibacterium prausnitzii.127 There is evidence that supplementation with zinc can preserve intestinal integrity, reduce hepatocytic cell death by restricting the pathway mediated by Fas/FasL, and reduce oxidative stress, proinflammatory cytokine production, and endotoxemia. Zinc is generally administered at a dose of 220 mg (50 mg of elemental zinc sulphate) per day with food.128 Fecal microbiota transplant has also been proposed as a treatment option in AH. A pilot study on patients with severe AH that were not candidates for steroid treatment underwent fecal microbiota transplant, improving the outcome scores and liver disease survival at one year.129 In an effort to increase the possibility of therapeutic success, studies on various drug combinations have been conducted. There are studies that have utilized the combination of an interleukin-1 (IL-1) receptor antagonist, called anakinra, with pentoxifylline and zinc, and other studies have evaluated the action of probiotics and bovine colostrum on the gut microbiota to reduce bacterial translocation. Despite promising preliminary results, there is not sufficient solid evidence for their recommendation in AH. Other drugs that have been evaluated as anti-inflammatory agents that are different from anakinra, are obeticholic acid, cenicriviroc, and alopurinol with probenecid but there are still no satisfactory results, or they have not yet been published.101
- 35
The use of granulocyte-colony stimulating factor has shown improvement in liver function and survival in patients with severe alcoholic hepatitis. Its routine use is not yet recommended.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
Granulocyte-colony stimulating factor (G-CSF) is a cytokine that normally acts on the bone marrow microenvironment to stimulate the formation of blood cells and there are several commercial presentations of the factor on the drug market.130 A randomized study on a small sample of patients with alcoholic steatohepatitis that included histologic studies evaluated the short-term effects of G-CSF. The results showed that a five-day course of G-CSF stimulated hepatic progenitor cells in alcoholic steatohepatitis patients with cirrhosis and moderate-to-severe liver failure. Those effects were observed seven days after treatment was begun, due to the fact that G-CSF promoted the mobilization of CD34+ cells and increased the hepatocyte growth factor.131 Those findings have also been documented in patients treated with G-CSF that have acute-on-chronic liver failure of alcoholic etiology.132 A randomized study published a few years ago reported favorable mortality results utilizing treatment with G-CSF in acute alcoholic hepatitis. In that clinical trial, subcutaneous G-CSF at a dose of 5 μg/kg every 12 hours for five days increased survival to 90 days, compared with standard treatment (p = 0.001).133 G-CSF has also been used for patients that are nonresponders to steroids. In a study that began in 2015 and is projected to be finished in 2020 (ClinicalTrials.gov, NCT02442180), the efficacy of G-CSF is being evaluated in patients with severe AH that have had a partial response to steroids or none at all. The randomized study is named GRACIAH and its hypothesis is that said therapy can aid in prolonging survival.134 Based on the evidence at hand, the routine use of G-CSF is not presently considered in all patients with AH.
- 36
Metadoxine is an antioxidant agent that can be used as adjuvant therapy, and when combined with corticosteroids, has shown improvement in survival.
Quality of evidence: B1.
Strength of recommendation: strong, in favor of.
Agreement reached: 95.5% in complete agreement; 4.5% in complete disagreement.
Metadoxine is a compound formed by in vitro crystallization of two molecules: vitamin B6 and pyroglutamic acid.135 It is an antioxidant drug that has been shown in experimental models to prevent glutathione depletion and the increase in lipid peroxidation damage caused by ethanol and acetaldehyde. In hepatic stellate cells, it prevents the increase of collagen and the attenuated secretion of tumor necrosis factor-alpha induced by acetaldehyde.136 In addition to improving glutathione availability, metadoxine inhibits hepatic steatosis in patients with AH.127
There are several clinical studies on the use of metadoxine in liver disease associated with alcohol consumption. In a randomized study by Mao et al., they used 1,500 mg of metadoxine daily for 42 days and improvement in liver function tests and the liver-spleen ratio evaluated through computed tomography were among the main results. Another non-randomized study reported improvement in liver function and metabolism with a dose of only 500 mg daily for 28 days. In both of those studies, the adverse effects were minor.136
In two Mexican studies conducted by Higuera-de la Tijera et al, they evaluated the effectiveness of metadoxine in patients with AH. The first study was open and randomized, and its aim was to evaluate the effectiveness of metadoxine added to steroids in the treatment of severe AH. One group received prednisone alone at 40 mg daily (n = 35) and another group received prednisone plus 1,500 mg of oral metadoxine daily (n = 35), for 30 days. Survival was evaluated at 30 and 90 days. The main results were better survival at 30 days (74.3% vs. 45.7%, p = 0.02) and at 90 days (68.6% vs. 20%, p = 0.0001) and less development or progression of encephalopathy and hepatorenal syndrome in the group that received metadoxine. There was also greater response to steroids in the metadoxine group.137 The second study was open and randomized, and unlike the first, it was conducted on four groups. Group 1 (n = 35) received 40 mg/day of prednisone, group 2 (n = 35) received 500 mg three times a day of prednisone + metadoxine, group 3 (n = 33) received 400 mg three times a day of pentoxifylline, and group 4 (n = 32) received 500 mg three times a day of pentoxifylline + metadoxine. Treatment duration was 30 days in all the groups. The results showed that metadoxine improved the three and six-month survival rates in patients with severe AH. Alcohol abstinence is a key factor for survival in those patients, and the patients that received the therapy combined with metadoxine were more likely to maintain abstinence than those that received monotherapy with prednisone or pentoxifylline.138
Based on the results of the abovementioned studies on Mexican patients, oral metadoxine at a dose of 1,500 mg daily is an antioxidant agent that can be considered additional treatment in patients with AH.
- 37
Liver transplantation can be considered a therapeutic option in selected cases of severe alcoholic hepatitis.
Quality of evidence: A1.
Strength of recommendation: strong, in favor of.
Agreement reached: 100% in complete agreement.
The selection of candidates for liver transplantation is always a great responsibility for the professionals that perform it. One of the main points against the use of liver transplantation for selected cases of AH is outcome, given the severity of the disease. Nevertheless, psychosocial aspects have always played an essential role in decision-making related to liver transplantation in ALD due to the risk for alcohol relapse that would go against the basic principal of any treatment of AH. ALD is currently the most common reason for liver transplantation, with similar outcomes at one and five years to those of other indications. The use of tools for predicting the risk for alcohol relapse is inaccurate, including the six-month alcohol abstinence rule. In addition, there is the virtual risk that if intense liver transplantation screening is eliminated, extending its indiscriminate use for ALD, it would result in a smaller number of donors than patients requiring liver transplant.139,140 Nevertheless, there are other considerations with respect to abstinence duration. Regarding the addition of patients with AH to the liver transplantation waiting list, three months of alcohol abstinence might be better than six months. Patients that lack social support, are active smokers, have psychotic or personality disorders, or a pattern of noncompliance should be added to the waiting list with reservations. Patients diagnosed with alcohol abuse rather than alcohol dependency can be better candidates. Patients that have regular addiction treatment appointments with a psychiatrist or psychologist also appear to have more favorable behavior.140
Thus, liver transplantation is currently a treatment indication for severe AH (MDF score > 32), in patients that are nonresponders to (Lille model > 0.45) or not eligible for corticosteroids, patients in their first hepatic decompensation event, and patients with a favorable psychosocial profile and social support. Transplantation exclusion criteria are: uncontrolled infection, comorbid systemic disease that impedes recovery, poor prognostic profile in relation to alcohol use, lack of social support, previous liver decompensation events, and uncontrolled psychiatric comorbidity.139
With respect to survival and the alcohol relapse rate in patients transplanted due to acute AH that did not comply with six months of alcohol abstinence, a 2018 meta-analysis concluded that early liver transplantation is a life-saving treatment in patients with medical treatment-refractory AH and with no increased possibility of alcohol relapse after transplantation, in well selected patients.141 In another recent meta-analysis that analyzed 11 studies to review the available evidence on liver transplantation in patients with AH and evaluate alcohol relapse and survival at six months, utilizing strict selection criteria, those authors found that 14% of the patients with severe AH had alcohol relapse after liver transplantation. The percentage of alcohol relapse in the transplanted patients with AH was similar to that of the patients with alcoholic cirrhosis that underwent elective liver transplantation.142 Other encouraging survival data are described in the study by Lee et al. According to a retrospective analysis of 147 patients that underwent early liver transplantation (before six months of abstinence) due to severe AH, they found that the majority of patients had one-year (94%) and three-year (84%) survival, which was similar to that of patients that received liver transplantations for other indications. Those authors also emphasized the fact that sustained alcohol consumption after transplantation was infrequent, but when it occurred, it was associated with a higher mortality rate.143
In the context of decision delay or in candidates on the transplantation waiting list, the MARS therapy has shown sufficient improvement in the biochemical profile as bridging therapy. However, improvement in short-term or long-term mortality has not been demonstrated.144
ConclusionsSevere AH is an entity with a high mortality rate. This is the first Mexican consensus on AH that addresses definitions of alcohol use disorders and the diagnosis and treatment of AH formulated in 37 recommendations. Importantly, AH is a condition that ranges from an asymptomatic status to the maximum expression of liver failure. Severe AH is defined by a Maddrey’s discriminant function score ≥ 32 or a MELD score equal to or above 21. There is no specific biomarker for its diagnosis and so it is important to be supported by laboratory tests. With respect to treatment, alcohol abstinence continues to be essential and nutritional support is important. Steroids are a therapeutic option in severe AH. The use of antioxidants, such as metadoxine combined with steroids, has been shown to increase survival. Transplantation is a treatment option in selected patients. There are new potentially beneficial drugs for AH but there is still no evidence for their use in daily clinical practice.
Financial disclosureThe present consensus was carried out in part with the logistics support of the Asociación Mexicana de Hepatología and the Asociación Mexicana de Gastroenterología. Laboratorio Sanfer provided funds for the face-to-face meeting. The authors received no fees for their participation.
Conflicts of interestDr. José Antonio Velarde-Ruiz Velasco: is or has been a speaker for Takeda, Asofarma, Alfa-Wassermann, Abbvie, Abbott, Gilead, and MSD.
Dr. María Fátima Higuera-de la Tijera: is or has been a speaker for Gilead, MSD, Medix, Takeda, Liomont, and Merz.
Dr. Graciela Elia Castro-Narro: is or has been a speaker for Gilead, Abbvie, and Abbott.
Dr. Felipe Zamarripa-Dorsey: declares that he has no conflict of interest.
Dr. Juan Miguel Abdo-Francis: is or has been a speaker for Medix, Takeda, Merz, Abbott, and Liomont.
Dr. Ignacio Aiza-Haddad: is or has been a speaker for Abbvie and Takeda.
Dr. Juan Manuel Aldana-Ledesma: declares that he has no conflict of interest.
Dr. María Victoria Bielsa-Fernández: is or has been a speaker for Chinoin, Takeda, and Sanfer.
Dr. Eira Cerda Reyes: is or has been a speaker for Gilead, Abbvie, MSD, Bayer, and Sanfer.
Dr. Laura Esthela Cisneros-Garza: is or has been a speaker for Abbvie, Bayer, Bristol, and Gilead.
Dr. Raúl Contreras-Omaña: is or has been a speaker for MSD, Bayer, and Schwabe.
Dr. Ángel Reyes Dorantes: declares that he has no conflict of interest.
Dr. Nicolás Joaquín Fernández-Pérez: is or has been a speaker for Takeda, Gilead, MSD, Medix, Mayoli, and Grunenthal.
Dr. Edgar Santino García-Jiménez: declares that he has no conflict of interest.
Dr. María Eugenia Icaza Chávez: declares that she has no conflict of interest.
Dr. David Kershenobich-Stalnikowitz: declares that he has no conflict of interest.
Dr. Marco Antonio Lira-Pedrin: declares that he has no conflict of interest.
Dr. Rosalba Moreno-Alcántar: declares that she has no conflict of interest.
Dr. José Luis Pérez-Hernández: declares that he has no conflict of interest
Dr. Mayra Virginia Ramos-Gómez: declares that she has no conflict of interest.
Dr. María Teresa Rizo-Robles: is or has been a speaker for Abbvie, Gilead, Merz, and MSD.
Dr. Sergio Solana-Sentíes: is or has been a speaker for Asofarma.
Dr. Aldo Torre-Delgadillo: declares that he has no conflict of interest.
Please cite this article as: Velarde-Ruiz Velasco JA, Higuera-de la Tijera MF, Castro-Narro GE, et al. Consenso Mexicano de hepatitis alcohólica. Revista de Gastroenterología de México. 2020;85:332–353.