Probiotics are frequently prescribed in clinical practice. Their efficacy in treating gastrointestinal disorders is supported by a significant number of clinical trials. However, the correct prescription of these agents is hampered due to a lack of knowledge of the scientific evidence and to the different presentations and microbial compositions of the probiotics that are currently available.
AimTo provide the clinician with a consensus review of probiotics and recommendations for their use in gastroenterology.
Materials and methodsControlled clinical trials, meta-analyses, and systematic reviews published up to 2015 were selected, using the MESH terms: probiotics, gastrointestinal diseases, humans, adults, AND children. The Delphi method was employed. Eighteen gastroenterologists treating adult patients and 14 pediatric gastroenterologists formulated statements that were voted on until agreement of >70% was reached. The level of evidence based on the GRADE system was evaluated for each statement.
Results and conclusionsEleven statements on the general concepts of probiotics and 27 statements on the use of probiotics in gastrointestinal diseases in both adults and children were formulated. The consensus group recommends the use of probiotics under the following clinical conditions: the prevention of diarrhea associated with antibiotics, the treatment of acute infectious diarrhea, the prevention of Clostridium difficile infection and necrotizing enterocolitis, the reduction of adverse events from Helicobacter pylori eradication therapy, relief from irritable bowel syndrome symptoms, the treatment of functional constipation in the adult, and the induction and maintenance of remission in patients with ulcerative colitis (UC) and pouchitis, and the treatment of covert and overt hepatic encephalopathy.
El uso de los probióticos es común en la práctica clínica. Existe un número significativo de estudios que apoyan la eficacia de los probióticos en algunos trastornos digestivos. Sin embargo, el desconocimiento de la evidencia científica y las diferentes presentaciones y composiciones microbianas de los probióticos disponibles dificultan su prescripción.
ObjetivoProveer al clínico de una revisión consensuada sobre los probióticos y recomendaciones de su uso en gastroenterología.
Material y métodosSe seleccionaron los ensayos clínicos controlados, metaanálisis y revisiones sistemáticas publicados hasta 2015, usando los términos MESH: probiotics, gastrointestinal diseases, humans, adults and children. Se utilizó la metodología Delphi. Diecisiete gastroenterólogos de adultos y 12 de niños elaboraron enunciados los cuales fueron votados hasta obtener acuerdo>70%. Para cada enunciado se evaluó el nivel de evidencia basado en el sistema GRADE.
Resultados y conclusionesSe generaron 11 enunciados sobre conceptos generales de probióticos y 27 enunciados sobre uso de probióticos en enfermedades gastrointestinales tanto en niños como en adultos. El grupo de consenso recomienda el uso de probióticos en las siguientes condiciones clínicas: prevención de la diarrea asociada a antibióticos, tratamiento de la diarrea aguda infecciosa, prevención de infección por Clostridium difficile y enterocolitis necrosante, para disminuir los eventos adversos de la terapia de erradicación del Helicobacter pylori, el alivio de los síntomas del síndrome de intestino irritable, en el estreñimiento funcional del adulto, para inducir y mantener la remisión en pacientes con colitis ulcerosa crónica idiopática y pouchitis, y en la encefalopatía hepática oculta y manifiesta.
Probiotics, regarded as live microorganisms that are beneficial to health, are frequently used in clinical practice. There is consistent evidence that probiotics can prevent different diseases or be useful in their treatment, especially in relation to gastrointestinal disorders, in both children and adults.1–3 The majority of gastroenterologists in Mexico and worldwide recommend probiotic use. However, in clinical practice, the physician is confronted with a wide variety of commercial products with different presentations (capsules, tablets, envelopes, vials, foods, supplements, milk formulas, etc.) with varying doses and microbial compositions, making the choice of a probiotic difficult. Even though different guidelines on the use of probiotics have been published,1,2,4 they are generally not well-known by specialists in Mexico.5
AimThe aim of the Mexican Consensus on Probiotics by the Asociación Mexicana de Gastroenterología is to provide a document on the general knowledge of probiotics and recommendations for their use in the treatment of gastrointestinal diseases in children and adults. These recommendations are based on an extensive review of the medical literature and the consensus opinion of specialists.
MethodsThe Delphi process6 was employed to develop the consensus. The following were the main steps of the process: a) selection of the consensus group; b) identification of the areas of clinical importance; c) a systematic review of the literature to identify the evidence supporting the statements; d) the formulation of the statements; e) the rounds of anonymous electronic voting with the discussion and analysis of the resulting statements, and their correction and modification.
Two groups of statements were formulated: those relative to the general concepts of probiotics and those that were specific statements on the use of probiotics in gastrointestinal diseases. The latter included statements relative to the use of probiotics in gastroenterology in both adult and pediatric patients.
A thorough search was carried out for the specific statements on the use of probiotics in gastroenterology in the following databases: The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search strategy included a validated filter for the identification of clinical trials, systematic reviews, and clinical practice guidelines using the following MeSH terms: (“probiotics” [MeSH Terms] OR “probiotics” [All Fields]) AND (“gastrointestinal diseases” [MeSH Terms] OR “gastrointestinal” [All Fields]) AND (“diseases” [All Fields]) OR “gastrointestinal diseases” [All Fields]) AND (Randomized Controlled Trial [ptyp] OR systematic [sb]) AND (“humans” [MeSH Terms] AND “adult” [MeSH Terms]) AND Clinical Trial [ptyp] OR Controlled Clinical Trial [ptyp] OR Randomized Controlled Trial [ptyp] OR systematic [sb] OR Meta-Analysis [ptyp] OR Practice Guideline [ptyp]”. All the clinical practice guidelines, systematic reviews, and clinical trials that analyzed the administration of probiotics compared with placebo or any other probiotic for the treatment of gastrointestinal diseases in adult and pediatric populations published up to the year 2015 were included. Studies that were open design, cross-over, quasi-experimental, observational, or narrative, and those that were case reports, were not eligible. Only articles in Spanish or English were evaluated.
Two independent reviewers carried out the risk for bias evaluation of the studies included utilizing the Cochrane Collaboration criteria for clinical trials, AMSTAR for systematic reviews, and AGREE II for clinical practice guidelines.7–9
The working group with MAV, EM, ATA, and SH formulated the general statements. Eighteen gastroenterologists treating adults and 14 gastroenterologists treating children formulated the statements specific for gastroenterology. Through an electronic voting system, all the members of the consensus anonymously voted upon each statement by selecting one of the following options: “complete agreement”, “partial agreement”, “uncertain”, “partial disagreement”, and “complete disagreement”. The results of each vote were presented to the members of the consensus group and were analyzed after discarding the indifferent responses through the chi-square test for trend to determine the degree of agreement. The rounds ended when there was agreement of more than 70%, with statistical significance (p<0.05). One of the voting sessions was carried out at a face-to-face meeting.
The level of evidence was evaluated (very high, high, moderate, or low) for each specific statement based on the GRADE system,10 which takes into account: A) limitations in the design and risk for bias of the studies, B) inconsistency in the results based on the heterogeneity of the studies, which was considered substantial when above 30% (I2>30%), C) a lack of direct evidence depending on the interventions and outcomes evaluated in the studies, D) result imprecision, analyzing the confidence intervals of the results reported, and E) publication bias utilizing funnel graphs. For the recommendation grades the words “we recommend” were used for a strong recommendation grade and “we suggest” for a weak recommendation grade.
Utilizing the responses of the Delphi panel, the recommendations were then formulated based on 4 criteria for determining their strength and direction: A) the balance between benefits and risks, B) confidence in the magnitude estimates, C) patient values and preferences, and D) resource use and costs.2
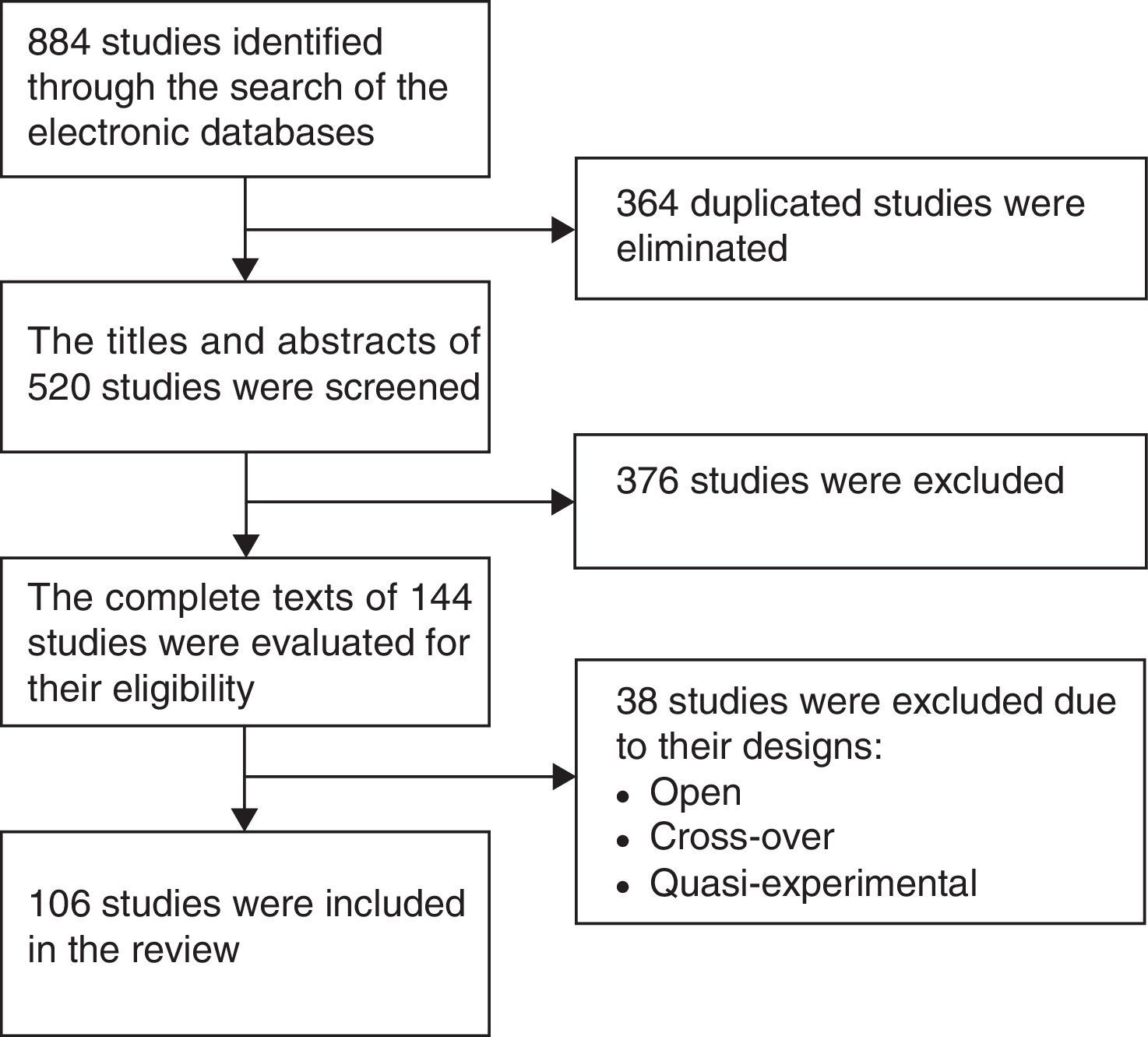
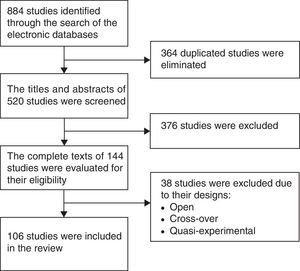
ResultsFigure 1 is a flow diagram with the medical literature search results and the selection process of the studies. The initial search identified 884 studies. A total of 364 duplicate studies were removed and 376 were excluded because their titles and abstracts did not meet the selection criteria. Thirty-eight studies were eliminated because they were cross-over, open, or quasi-experimental studies, leaving 106 studies in total for the final review.
The consensus group formulated 10 statements on general concepts of probiotics and 27 statements on the use of probiotics in gastrointestinal diseases.
In 8 of the 27 statements, the evidence is analyzed in both adults and children and the analyses are presented as subsections of the main statement.
General statements regarding probiotics1. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host
Complete agreement: 96.3%; partial agreement: 3.7%.
The original definition of probiotics was developed at a Joint Food and Agriculture Organization (FAO) of the United Nations and World Health Organization (WHO) Expert Consultation in 2011.11 Since then, it has been the most extensively used definition worldwide. The International Scientific Association for Prebiotics and Probiotics (ISAPP)12 recently published a consensus document on the appropriate use of the term probiotic. It maintains the definition proposed by the FAO/WHO, with a minor grammatical change. This is the definition used in the Mexican consensus on probiotics.
It emphasizes 3 characteristics of probiotics: the viability of the microorganisms, the number or quantity of the microorganisms, and the beneficial effects demonstrated in the health of the host.
2. Prebiotics are fermentable, nondigestible compounds that selectively stimulate the growth and activity of a number of bacterial genera/species of the microbiota that confer health benefits on the host
Complete agreement: 96.3%; partial agreement: 3.7%.
The definition of prebiotics has changed over time. Gibson and Roberfroid13 initially proposed the concept of “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon, and thus attempt to improve host health”. In 2004, the authors revised that definition and proposed that prebiotics are “selectively fermented ingredients that allow specific changes, both in the composition and/or activity of the gastrointestinal microbiome, that confer benefits upon host well-being and health”. The World Gastroenterology Organization (WGO)1 has recently defined prebiotics as “dietary substances (mostly consisting of polysaccharides and oligosaccharides poorly digested by human enzymes) that nurture a selected group of microorganisms living in the gut. They favor the growth of beneficial bacteria over that of harmful ones”. A prebiotic must have 3 characteristics: 1) be nondigestible, resistant to gastric acid and proteolytic enzymes, and not be absorbed in the proximal digestive tract; 2) be fermented by the gut microbiota and promote the growth of beneficial bacteria; and 3) be capable of producing beneficial effects on health.14 The most widely studied prebiotics that have a greater level of scientific evidence are inulin, fructo-oligosaccharides, galacto-oligosaccharides, lactulose, and oligosaccharides from breast milk.14
Prebiotics stimulate the increase in the number of bifidobacteria in the colon, the increase in calcium absorption and weight of fecal matter, and the reduction of gastrointestinal transit and possibly of blood lipid levels.15
3. Synbiotics are products that contain probiotics and prebiotics
Complete agreement: 96.3%; partial agreement: 3.7%.
Synbiotics, a combination of prebiotics and probiotics, can modulate the gut microbiota.16 As synbiotics, probiotics arrive at the intestine accompanied by the prebiotics that aid in their growth and colonization. Human milk is a synbiotic food, given that it contains lactic acid bacteria (lactobacilli and bifidobacteria), as well as fructo-oligosaccharides and nucleotides, which are nutrients that favor the development of the lactic acid bacteria.
4. The strain of a probiotic is identified by genus, species, and alphanumeric designation. For example: Lactobacillus casei (L. casei) DN-114 001
Complete agreement: 100%.
Probiotics should be named according to the International Code of Nomenclature of Prokaryotes.17 Probiotic identification should include:
- 1.
Genus: a group of species of microorganisms with similar qualities, such as physical characteristics, metabolic needs, and metabolic end products
- 2.
Species: a group of strains that share numerous stable properties
- 3.
Strain: a population of microorganisms that descend from a single organism or from a pure culture isolate
It is important to know the nomenclature of a probiotic strain, because the health benefits of probiotics are specific for each species.
4. Pharmaceutical products, foods, supplements, infant formulas, or bacterial consortia that define the microbial content of specific strains can be regarded as probiotics. Neither fermented foods that do not define microbial content nor the bacterial consortia of fecal microbiota transplantation are considered probiotics
Complete agreement: 96.3%; uncertain 3.7%.
In a published consensus on the use of the term “probiotic” the ISAPP12 expressed the following considerations:
If a food has a level of 1 x 109 colony forming units (CFU) per portion of bacteria known as probiotics, that food can be considered to “contain probiotics”. In contrast, foods and food supplements that contain potentially beneficial microbes, but do not meet that requirement, should be regarded as foods that “contain live and active bacterial cultures”. They should not be called probiotics.
Yoghurt is a product of milk fermentation by Streptococcus thermophilus (S. thermophilus) and Lactobacillus delbrueckii (L. delbrueckii) subspecies bulgaricus. There is sufficient evidence as to the beneficial effect of yoghurt on host health, thus it can be considered a probiotic.18
The bacterial consortia of fecal microbiota transplantation should not be considered a probiotic, given that it includes an unknown number of unclassified bacteria, yeasts, parasites, and viruses. Likewise, it is not known which microorganisms are responsible for therapeutic benefit, nor are the long-term effects of the consortia known.12
6. Probiotics have numerous mechanisms of action that have been described in relation to genus, species, and strain. The general and common mechanisms of action in probiotics are: increased resistance to colonization, normalization of the altered gut microbiota, promotion of the competitive exclusion of pathogens, increased short-chain fatty acid production, regulation of bowel transit, and increased enterocyte uptake
Complete agreement: 92.59%; partial agreement: 7.41%.
Probiotic research suggests that they provide a broad spectrum of possible health benefits. However, the effects described can only be attributed to the strain or strains studied, and not to the species or the entire group of bacteria.
In general, all probiotics affect the gut ecosystem, stimulating immune and non-immune mechanisms of the mucosa through antagonism and competition with potential pathogens.12
The relation that is maintained between the gut, the microbiota, and immunity is very complex and the effects of probiotics within this system may depend on the strain of probiotic or the individual's susceptibility.12
7. The beneficial effects of probiotics on host health should be demonstrated in humans in controlled clinical trials
Complete agreement: 96.3%; partial agreement: 3.7%.
A controlled clinical trial (CCT) is the criterion standard of the different types of research study for producing the best scientific evidence in relation to a medical intervention by a drug, food, or biologic product.19 The effects of any of these interventions should be studied in members of the same species. Therefore, the efficacy of probiotics in human health should be analyzed through CCTs in humans. Any effect of probiotics demonstrated in animals cannot be extrapolated to humans.
8. The demonstrated beneficial effects of probiotics are only applicable to the specific strain and clinical condition in the controlled clinical trials and systematic reviews and cannot be extrapolated to other strains of the same species or to different clinical situations
Complete agreement: 100%.
This statement emphasizes the fact that bacterial strains of the same species of probiotics can have significantly different actions, properties, characteristics, and therapeutic effects. These qualities are included in the concept of strain specificity. Likewise, the therapeutic outcomes with the use of one probiotic strain are specific for the clinical entity for which that particular probiotic was given. For example: Lactobacillus plantarum (L. plantarum) strain 299v20–21 has been shown to improve symptoms of irritable bowel syndrome (IBS), whereas L. plantarum MF1298 worsens symptoms in IBS patients.22 In the same manner, Lactobacillus rhamnosus (L. rhamnosus) GG (LGG) is effective in the prevention of antibiotic-associated diarrhea (AAD),23 but not in the prevention of urinary infection.24 The lack of awareness of the concept of strain specificity on the part of the physician or the probiotic consumer can cause incorrect interpretation or extrapolation of scientific research and result in an inadequate indication for a probiotic or in its inefficiency.
9. The beneficial effects of a probiotic strain observed in a specific population group (e.g., pediatric population) cannot be extrapolated to other population groups (adult population) or physiologic conditions (pregnancy)
Complete agreement: 100%.
The effects on health should be documented, taking into account the specific strain in the product that is administered. The different probiotic strains that are used should be supported by evidence of clinical effects in at least one well-designed clinical trial with sufficient statistical power, stating that the oral administration of the specific probiotic strain is efficacious and beneficial for health or as treatment for a given disease. Furthermore, the results of beneficial effects in the adult population cannot be extrapolated to the pediatric population or vice versa or to pregnant women.25
10. In general, probiotics are safe and are not a health risk
Complete agreement: 88.89%; partial agreement: 11.11%.
Probiotics that have been studied in CCTs have been well tolerated in humans and only minor adverse effects have been reported, such as abdominal colic and flatulence, among others. Microorganisms that have not been studied in CCTs should not be called probiotics and there is no evidence in relation to their safety.
There are reports in the medical literature of bacteremia and fungemia associated with the use of probiotics in neonates and in patients with immune deficiencies. Likewise, the presence of a central catheter is considered a risk factor for translocating the probiotic to the blood. Nevertheless, there are few such reports.26
Specific statements on gastrointestinal diseasesAntibiotic-associated diarrheaAAD is defined as the appearance of unexplained diarrhea transitorily associated with the use of antibiotics. It can occur as early as 2-7 days from the start of antibiotic therapy or as late as 2-8 weeks after therapy. It is a frequent entity in both the outpatient and hospitalized patient, with an estimated prevalence of 5-30% in the pediatric population27 and 5-70% in adults.28 The clinical spectrum of AAD can vary from mild diarrhea to severe colitis.
In adults11. The administration of specific strains of probiotics significantly reduces the risk for AAD
Complete agreement: 100%.
In the systematic review and meta-analysis by Hempel et al.,29 52 out of 82 CCTs were included, with a total of 11,811 patients. The main probiotics used were Lactobacillus alone or combined with other genera, such as Bifidobacteria, Saccharomyces boulardii (S. boulardii) CNCM I-745 (cerevisiae), or Saccharomyces Hansen CBS 5926 as the single probiotic, and strains of Streptococcus, Enterococcus, and/or Bacillus. The accumulated relative risk in the CCTs in which the number of patients was determined showed a statistically significant association in the administration of probiotics with a reduction in AAD (RR=0.58; 95% CI: 0.50-0.68; p=0.001; I2=54%) and a number needed to treat (NNT) of 13. The definition and outcomes of diarrhea were reported in 43 CCTs with results in favor of probiotic use (RR=0.56; 95% CI: 0.47-0.68; p=0.001; I2=57%) and a NNT of 10. The meta-regression of the CCTs on the effect of probiotics between children up to 17 years of age, adults up to 65 years of age, and older adults above 65 years of age showed no significant differences.
In the meta-analysis by Videlock and Cremonini,23 34 CCTs with 2,921 participants were evaluated in regard to probiotic use in AAD. Twenty-four of the CCTs were conducted on adults and 10 on the pediatric population. The accumulated results of the studies in children and adults showed an effect in favor of the use of probiotics (RR=0.53; 95% CI: 0.43-0.66; p=0.0001; I2=44%) and a NNT of 8. Lactobacilli were the most frequently used probiotic species in 24 studies, 8 of which evaluated the effect of Lactobacillus GG. S. boulardii was analyzed in 7 studies, and 10 studies used bifidobacteria (also as part of a combination). This meta-analysis in particular, showed the preventive effect of the probiotic added as a supplement, with respect to AAD incidence. It was relatively consistent in the different probiotic species and strains used in distinct antibiotic regimens.
The meta-analysis by Sazawal et al.30 included 34 CCTs that evaluated the efficacy of probiotics in acute diarrhea. Nineteen of the studies analyzed AAD, 13 of which were conducted on adults. The results showed a significant AAD reduction with the use of probiotics in 52% (RR=0.48; 95% CI: 0.35-0.65; I2=53%).
The 2011 WGO guidelines1 conferred a 1b level of evidence on different probiotic strains for the prevention of AAD that included Enterococcus faecium LAB SF 68, the S. boulardii cerevisiae strain (CNCM I-745), LGG, L. casei DN 114-001 in fermented milk, Bacillus clausii O/C, N/R, T, and SIN, and Lactobacillus acidophilus (L. acidophilus) CL 1285+L. casei LBC 80R.
In children11a. Probiotics are efficacious and safe in children for the prevention of AAD
Complete agreement: 100%.
The meta-analysis by Szajewska et al.31 evaluated 6 randomized controlled trials on 766 children. The authors concluded that treatment with probiotics, compared with placebo, reduced the risk for AAD in 28.5% to 11.9% (RR=0.44; 95% CI: 0.25-0.77; p=0.004; I2=69.9%). A subgroup analysis showed that the reduction in the risk for AAD was associated with the use of S. boulardii and LGG.
In a meta-analysis of 15 randomized controlled trials that included 2,874 children, Johnston et al.32 showed that the incidence of AAD in the group of children treated with probiotics was 9%, compared with 18% in the control group (RR=0.52; 95% CI: 0.38-0.72; I2=56%). Those studies included treatment with Bacillus spp., Bifidobacterium spp., Lactobacilli spp., Lactococcus spp., Leuconostoc cremoris, Saccharomyces spp., and Streptococcus spp., either alone or in combination.
In the meta-analysis by Videlock and Cremonini,23 the 10 CCTs conducted on pediatric patients showed a lower incidence of AAD in the group treated with probiotics with a RR of 0.48 (95% CI: 0.35-0.65; p<0.000; I2=36%), similar to that observed in studies on adults. The probiotics used were LGG, L. acidophilus, L. casei, S. boulardii, Lactobacillus reuteri (L. reuteri), Bifidobacterium lactis (B. lactis), and S. thermophilus, among others, either alone or in combination.
Acute infectious diarrheaThe WHO defines diarrhea as the presence of 3 or more loose or watery stools and an increase in the number of bowel movements in a 24-h period. Diarrhea is considered acute if duration is under 14 days.
Acute diarrhea continues to be a worldwide public health problem, especially in the developing countries. The most common cause is infection from different microorganisms. The majority of episodes are self-limited and do not require investigation as to the causal agent. The most frequent complication is dehydration and initial management is directed toward improving and maintaining hydration. Nevertheless, oral rehydration does not reduce the volume of the bowel movements or shorten the duration of the diarrheic episodes.33 Therefore, different treatment modalities have been employed that are associated with oral or intravenous rehydration, one of which includes probiotics.34,35
In adults12. Probiotics reduce the duration and number of bowel movements in acute infectious diarrhea
Complete agreement: 93.75%; partial agreement: 6.25%.
In the Cochrane systematic review by Allen et al.36 on probiotics in acute infectious diarrhea, 63 studies were evaluated, 56 in children and 7 in adults, with a total of 8,014 participants. The authors could not evaluate the effect of probiotics in adults with infectious diarrhea due to the heterogeneity in the definition of outcomes and in the duration of the diarrhea. The overall results, and those in the pediatric patient, are commented on further ahead.
In the meta-analysis by Sazawal et al.,30 21 studies on adults measuring the effect of probiotics on the prevention of acute diarrhea were evaluated. The authors concluded that probiotics reduced the risk for acute diarrhea in that population in 26% of the cases (RR=0.74; 95% CI: 0.59-0.93; p=0.011; I2=42%). Their results included studies on acute infectious diarrhea, traveller's diarrhea, and AAD. No significant difference was observed between the strains utilized, which included S. boulardii, Lactobacillus GG, L. acidophilus, and Lactobacillus bulgaricus (L. bulgaricus), either alone or in combination with 2 or more strains. Only 2 studies analyzed the effect of acute infectious diarrhea in adults. One evaluated diarrhea caused by Escherichia coli (E. coli) in 48 adults treated with L. acidophilus combined with L. bulgaricus in granules, with a reduction close to 30%, but no statistical significance (p=0.275), and the other was a community study conducted on 502 adults treated with L. casei in yoghurt, for which there are no data. The authors estimated a NNT of 4 in that meta-analysis.
In children12a. S. boulardii, as well as Lactobacillus GG, L. casei, L. acidophilus, L. bulgaricus, and Bifidobacterium longum (B. longum), alone or in combination, reduce the duration of acute diarrhea by approximately one day and reduce the frequency of the bowel movements on the second day of their consumption
Complete agreement: 83.33%; partial agreement: 8.33%; complete disagreement: 8.33%.
The meta-analysis by Szajewska et al.37 included 5 CCTs conducted on a total of 619 healthy children between the ages of 2 months and 12 years that received S. boulardii in doses of 250-750mg daily for 5-6 days. The results showed diarrhea was reduced by 1.1 days in 4 studies (standardized mean difference [SMD]=–1.1; 95% CI: –1.3 to –0.83, p=0.000; I2=0%) and another study reported a decrease in the risk for diarrhea lasting more than 7 days. The studies did not state whether the patients were outpatients or hospitalized patients or both.
The Cochrane systematic review by Allen et al.36 included 63 studies, 56 of which corresponded to a total of 6,489 pediatric patients. A single organism was evaluated in 46 of the studies and combinations of 2 to 8 organisms were evaluated in 17 studies. L. casei GG was the organism that was studied the most (13 studies), followed by S. boulardii (10 studies). The doses and treatment durations varied widely. Fifteen studies utilized high doses of the organisms (≥ 109 CFU/day) and 26 studies used low doses (≤ 1010 CFU/day). The results showed that probiotics reduced the mean duration of diarrhea by 24.76h (95% CI: –33.61 to –15.91, p<0.000; I2=97%), as well as the frequency of bowel movements on day 2 after the intervention (SMD: –0.80; 95%CI: –1.14 to –0.45, p<0.000; I2=71%).
L. casei GG and S. boulardii alone, as well as the combination of L. delbrueckii, L. acidophilus, S. thermophilus, Bacillus bifidum, and S. boulardii, or the combination of L. acidophilus, L. rhamnosus, B. longum, and S. boulardii, were the probiotics that significantly reduced the duration of diarrhea.
Eight CCTs were evaluated in the systematic review by Applegate et al.38 The studies utilized different combination of probiotics: a) L. bulgaricus with S. thermophilus; b) L. acidophilus, L. bulgaricus, S. thermophilus, and Bifidobacterium bifidum (B. bifidum); c) L. acidophilus and Bifidobacterium infantis (B. infantis); d) Lactobacillus GG, L. acidophilus, L. casei, L. plantarum, and B. infantis. The Lactobacillus GG, S. boulardii, L. acidophilus, Bacillus clausii, and Enterococcus faecium strains were used individually. The results showed a 14% reduction in the mean duration of diarrhea (95% CI: –24.2 to –3.8%, p=0.73; I2=0%) and a 13.1% reduction in the frequency of bowel movements on day 2 of treatment (95% CI: –25.3 to –0.8%, p=0.73; I2=0%) with Lactobacillus GG and with some of the probiotic combinations.
A review by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)39 was published in 2014 on the use of probiotics in acute gastroenteritis, reporting that they were able to reduce the duration of diarrhea by approximately one day. The efficacy and safety of probiotics depend on the strain employed and the dose at which they are administered. The probiotics with the higher level of evidence and greater recommendation grade are Lactobacillus GG and S. boulardii. L. reuteri DSM 17938 and L. acidophilus LB have a lower recommendation grade due to scant evidence. The rest of the probiotics cannot be recommended.
12b. The preventive administration of Lactobacillus GG, S. boulardii, L. acidophilus, L. bulgaricus, and Bifidobacterium, alone or in combination, to hospitalized children reduces the incidence of in-hospital acute diarrhea of different etiologies, including rotavirus
Complete agreement: 91.67%; uncertain: 8.33%.
Thirty-four randomized clinical trials were included in the meta-analysis by Sazawal et al.,30 12 of which were conducted on hospitalized children. The results showed that the administration of S. boulardii, LGG, L. acidophilus, L. bulgaricus, and Bifidobacterium, alone or combined, reduced the incidence of acute diarrhea of different etiologies, compared with the control group (RR=0.43; 95% CI: 0.29-0.65; p<0.001; I2=65%).
Wanke40 evaluated 6 CCTs on 1,343 hospitalized children. The authors demonstrated that the administration of LGG reduced the incidence of in-hospital acute diarrhea, including that caused by rotavirus, when compared with the control group (RR=0.37; 95% CI: 0.23-0.59; p<0.000; I2=21%).
Traveller's diarrheaAcute diarrhea is a health problem that affects travellers. The estimated incidence is from 10-40%, depending on the travel destination.41 The use of probiotics in the prevention of traveller's diarrhea is still a subject of debate.
In adults13. There is insufficient evidence for recommending the use of probiotics in the prevention of traveller's diarrhea
Complete agreement: 100%.
A systematic review42 that evaluated 12 randomized, controlled, and blinded trials showed that in 6 of them, probiotics prevented traveller's diarrhea. One demonstrated a trend favoring the use of probiotics, and 5 of the studies showed no significant difference between the groups treated with probiotics and the controls. When the 12 studies were analyzed together, a beneficial effect in favor of the use of probiotics was observed (RR=0.85; 95% CI: 0.79-0.91; p<0.001). S. boulardii and combinations, such as the mixture of L. acidophilus and B. bifidum, were the probiotic strains that were efficacious in the prevention of traveller's diarrhea. The contradictory results of the clinical trials may be due to the differences in populations and destinations studied, the type of probiotic, dose, and treatment duration. Further investigation controlling these variables is required to understand the role of probiotics in the prevention of traveller's diarrhea.
13a. There is limited evidence on the use of probiotics in the treatment of persistent diarrhea and traveller's diarrhea in children
Complete agreement: 100%.
The systematic review by Bernaola Aponte et al.43 that included 4 clinical trials with a total of 464 patients showed that there is insufficient data for reaching a conclusion in relation to the use of probiotics in persistent diarrhea. The current systematic reviews do not mention studies that can support the use of probiotics in traveller's diarrhea in children.36,38,39
Clostridium difficile infectionClostridium difficile infection (CDI) has been recognized as the most common cause of healthcare-associated diarrhea. Since 2000, the incidence of CDI has increased significantly, with greater morbidity and mortality. Advanced age, hospitalization, antibiotic use, immunosuppression, gastrointestinal surgery, and the use of proton pump inhibitors are the main risk factors for developing CDI.44 Probiotics have been evaluated for its treatment and prevention.
In adults14. There is no evidence that probiotics are useful in the treatment of CDI
Complete agreement: 81.25%, partial agreement: 18.75%.
A recent systematic review45 included 4 studies that examined the use of probiotics combined with antibiotics. Three of the studies were conducted using S. boulardii and one of the studies with L. plantarum for the treatment of an initial episode or recurrence of CDI in adults. The studies had small samples and certain methodological problems. Only one of the studies with S. boulardii showed significant difference in favor of that probiotic, which reduced the duration of diarrhea (RR=1.33; 95% CI: 1.02-1.74) and its recurrence after treatment suspension (RR=0.59; 95% CI: 0.35-0.98).
The other 3 studies did not show the combination of probiotics with antibiotic to be superior to antibiotic alone, regarding duration or intensity of diarrhea, recurrence, cure through fecal toxin analysis, or adverse events.
15. Some probiotic strains are useful in preventing the recurrence of CDI
Complete agreement: 87.50%; partial agreement: 12.50%.
Several systematic reviews and meta-analyses have been published in recent years that evaluate the role of probiotics in the primary23,32,45–47 and secondary 45,48,49 prevention of CDI. The first analysis included 11 studies on adults that received a probiotic or placebo and whose primary outcome measure was the primary prevention of CDI. The results showed that S. boulardii (4 studies) and the combination of L. acidophilus, L. casei, and L. rhamnosus (3 studies) had an effect in favor of probiotics (RR=0.39; 95% CI: 0.19-0.179; p<0.05; I2=44%). A sub-analysis showed that the combination of lactobacilli was associated with a significantly lower risk for infection than with placebo and that S. boulardii also had a protective effect, albeit not statistically significant.47 The meta-analysis by Johnston et al.,32 unlike that of Johnson et al.,47 included a pediatric population and a summary of some published works. They found significant reductions of risk for CDI with S. boulardii, as well as with the combination of L. acidophilus, L. casei, and L. rhamnosus, but not with LGG.
Three systematic reviews and meta-analyses have evaluated the use of probiotics in recurrent CDI.45,48,49 Pillai and Nelson45 demonstrated that the administration of S. boulardii was associated with lower CDI recurrence (RR=0.59; 95% CI: 0.35-0.98). O’Horo et al.49 included 8 clinical trials, 4 with S. boulardii, 3 with Lactobacillus spp., and one with a non-toxigenic strain of Clostridium difficile (C. difficile). The best evidence was obtained with S. boulardii, which showed a beneficial effect in 2 of the 4 studies. However, one of those studies did not control prior antibiotic use and the other reported a beneficial effect only when the probiotic was associated with vancomycin. Evidence with respect to Lactobacillus spp. and non-toxigenic C. difficile came from case series and case reports, respectively. In the review and meta-analysis by Evans and Johnson,48 4 randomized controlled trials were included: the first showed that the combination of antibiotics and S. boulardii was more effective for preventing a second CDI episode, compared with antibiotic use only (RR=0.43; 95% CI: 0.20-0.97). The second study controlled the type and dose of antibiotic and showed no beneficial effect by adding S. boulardii, but a sub-analysis reported a beneficial effect with the combination of high doses of vancomycin. The fourth work added Lactobacillus plantarum 299v or placebo for 38 days to the treatment with metronidazole for recurrent C. difficile, and recurrence was lower with the probiotic (36 vs 67%), although the difference was not statistically significant.
In children15a. The use of probiotics as a preventive measure reduces the incidence of C. difficile-associated diarrhea caused by antibiotics
Complete agreement: 100%.
The meta-analysis by Johnston et al.50 included 20 clinical trials conducted on a total of 3,810 patients treated with antibiotics, and 636 were children. L. acidophilus, L. casei, L. rhamnosus, S. boulardii, and other probiotics were administered, alone and in combination. The occurrence of CDI-associated diarrhea was analyzed, demonstrating that the incidence of this complication was lower in the group treated with probiotics (RR=0.4; 95% CI: 0.17-0.96) in relation to the control group.
The review by Goldenberg et al.46 included a total of 4,213 child and adult patients treated with antibiotics. L. acidophilus, L. casei, and L. rhamnosus were administered alone or combined. The presence of C. difficile-associated diarrhea was analyzed, demonstrating that its incidence was lower in the group treated with probiotics (RR=0.36; 95% CI: 0.26-0.51; I2=0%). The subgroup analysis showed that efficacy in relation to prevention was maintained, upon comparing children and adults.
Helicobacter pylori infectionIt has been estimated that Helicobacter pylori (H. pylori) infection affects approximately half of the world population.51 Different conditions have been associated with H. pylori infection, including peptic ulcer, MALToma, and gastric carcinoma.52 Thus, it is necessary to eradicate H. pylori in those conditions, requiring the use of antibiotics with triple, sequential, or quadruple regimens. However, due to antibiotic resistance, a lack of treatment adherence, and adverse events associated with treatment, eradication schemes often fail. Therefore, the use of probiotics as supplementary therapy to antibiotic use has been proposed in an effort to improve infection eradication rates and reduce the treatment side effects.
In adults16. There is limited evidence that supplementary treatment with specific probiotic strains can increase H. pylori eradication rates
Complete agreement: 93.75%; partial agreement: 6.25%
Six meta-analyses were analyzed that evaluated the effect of probiotics combined with antibiotics on the eradication rate of H. pylori infection. The results showed an increase in eradication rates in the intention-to-treat analysis (from RR=1.122; 95% CI: 1.086-1.159 to RR=2.066; 95% CI: 1.3-3). Nevertheless, those results applied to specific Lactobacilli,53–56S. boulardii,57 and Bifidobacteria53 strains. The majority of the studies included in meta-analyses on this subject are Asian, Italian, and Middle Eastern. Few are from other parts of Europe and there is only one Argentinian and one Brazilian study. There are no published studies on the Mexican population. Dang et al.53 make the interesting observation that their results suggest supplementation with probiotics is useful only when antibiotic therapy is less effective (eradication ranges<80%).
17. Probiotics are useful in the prevention and reduction of gastrointestinal adverse events associated with H. pylori eradication therapy and they improve treatment adherence
Complete agreement: 87.50%; partial agreement: 6.25%; uncertain: 6.25%.
One of the main disadvantages of H. pylori eradication therapy is the presence of adverse effects (diarrhea, nausea, vomiting, epigastric pain, and dysgeusia) that can present in 5-30% of patients57 and contribute to poor treatment adherence, and consequently, to lower eradication rates.
Six meta-analyses were identified that, in addition to eradication, analyzed the effect of probiotics on the prevention and reduction of adverse effects, some with apparently opposing conclusions. Zheng et al.55 and Zou et al.56 reported that they found no significant reduction of side effects in general. However, the reduction of side effects was 30.84% (95% CI: 24.82-36.86%) and 42.24% (95% CI: 35.89%-48.59%) with placebo and probiotics, respectively (OR=0.49; 95%CI: 0.24-1.02; p=0.06, I2=62%). The group treated with lactobacilli had less diarrhea, tympanites, and taste alterations. Zhu et al.58 and Szajewska et al.57 also found that diarrhea was the adverse effect that presented the least with probiotic use (OR=0.21, 95%CI: 0.06-0.74; p=0.000, I2=91%).6 Wang et al.54 reported that the incidence of side effects was significantly lower in the group treated with probiotics (OR=0.30; 95% CI: 0.12-0.79; p=0.000; I2=72%). Dang et al.53 included 20 studies in their meta-analysis that evaluated adverse effects of eradication treatment. Their results showed a trend for probiotics to reduce the general incidence of side effects (RR=0.735; 95% CI: 0.598-0.902). No meta-analyses or systematic reviews were found that provided evidence to support the idea that probiotics improve eradication treatment adherence.
In children17a. There is limited evidence on the use of probiotics for increasing H. pylori eradication rates
Complete agreement: 100%.
In a meta-analysis of 7 randomized clinical trials that included a total of 508 pediatric patients, Li et al.59 found that supplementing triple H. pylori eradication therapy with probiotics had beneficial effects, especially on the adverse effects of antibiotics, such as diarrhea (RR=1.96; 95% CI: 1.28-3.02).
In the systematic review and meta-analysis by Szajewska et al.60 that evaluated 11 clinical trials, they concluded that the addition of S. boulardii to H. pylori eradication therapy marginally increased the eradication rate, but it reduced the adverse effects secondary to the triple eradication regimen.
Functional dyspepsiaFunctional dyspepsia (FD) is a functional gastrointestinal disorder that affects 11-29% of the world population.61 According to the Rome III criteria, it is defined as postprandial fullness, early satiety, and epigastric pain and burning sensation in the absence of organic disease that could explain the symptoms. Treatment includes the use of proton pump inhibitors, prokinetics, visceral pain modulators, and alternative and complementary medicine.62 Probiotics have been used in the management of abdominal pain associated with this disorder in the pediatric population.
18. Probiotics have not been shown to be useful in children with FD or functional abdominal pain (FAP)
Complete agreement: 100%.
In the systematic review by Korterink et al.63 that evaluated 5 CCTs conducted on children and adolescents with abdominal pain associated with IBS, FD, and FAP, they found a beneficial effect of probiotics only on abdominal pain associated with IBS. In a meta-analysis, Horvath et al.64 evaluated 3 CCTs conducted on a total of 290 children with functional gastrointestinal disorders (IBS, FAP, and FD) according to the Rome II criteria. Two of the CCTs were conducted on 103 patients with FAP and one was carried out on 20 patients with FD. The results showed that supplementary treatment with LGG did not improve abdominal pain in children with FAP or FD (RR=1.08; 95% CI: 0.77-1.50; p<0.05; I2=57% and RR=0.83; 95% CI: 0.37-1.85; p<0.05; I2=35%, respectively).
Irritable bowel syndromeIBS is a frequent functional gastrointestinal disorder of complex pathophysiology, with difficult-to-control clinical manifestations. Different types of drugs, such as antispasmodics, antidiarrheals, dietary fiber, 5HT3 antagonists, 5HT4 agonists, guanylate cyclase 2C agonists, CIC-2 chloride channels, and antidepressants, among others, have been used in its management with variable responses.65 There is evidence that alterations in the microbial composition of the gut microbiota can play an important role in IBS pathophysiology.66 Therefore, the use of antibiotics and probiotics has been proposed for IBS management.
In adults19. The administration of specific probiotics improves overall symptom perception in patients with IBS
Complete agreement: 100%.
Six systematic reviews and meta-analyses were identified that evaluated the effects of probiotics on IBS. Ford et al.67 carried out the most complete review, including 35 CCTs in their analysis that evaluated the efficacy of probiotics vs placebo in a total of 3,452 patients with IBS. Symptom persistence or the absence of symptom improvement as primary outcome measures were studied in 23 CCTs. Their results favored the use of probiotics vs placebo (55.8 vs 73.1%, respectively, with RR=0.79; 95% CI: 0.70-0.89), with a NNT of 7. Combinations of probiotics maintained the beneficial effect over placebo (RR=0.82; 95% CI: 0.69-0.98), and the NNT was 8. Some species and specific strains that also showed a positive effect over placebo were L. plantarum (RR=0.67; 95% CI: 0.51-0.87), Escherichia (RR=0.86; 95% CI: 0.79-0.93), and S. faecium (RR=0.72; 95% CI: 0.53-0.99).
In a meta-analysis of 8 CCTs conducted on a total of 1,011 patients with IBS, Nikfar et al.68 reported that there was clinical improvement in favor of probiotics of 53 vs 44.9% with placebo, with a RR of 1.22 (95% CI: 1.07-1.4; p=0.0042).
In a systematic review of 19 CCTs on 1,650 patients with IBS, Moayyedi et al.69 showed that probiotics were significantly more efficacious than placebo, with a NNT of 4.
Hoveyda et al.70 evaluated 14 CCTs, finding that probiotics produced overall symptom improvement superior to placebo, with an OR of 1.6 (95% CI: 1.2-2.2; p=0.0007; I2=27%).
McFarland and Dublin71 evaluated 20 CCTs on 1,404 patients with IBS and their results showed that probiotics improved overall symptom perception, compared with placebo, with a RR of 0.77 (95% CI: 0.62-0.92) and a NNT of 7.3.
20. Some specific strains of probiotics improve abdominal pain, bloating, and flatulence in patients with IBS
Complete agreement: 100%.
The systematic reviews and meta-analyses of McFarland and Dublin,71 Hoveyda et al.,70 Moayyedi et al., 69 and Ortiz-Lucas et al.72 showed that probiotics were significantly better than placebo for alleviating abdominal pain, bloating, and flatulence, but they did not improve straining or the sensation of defecation urgency in IBS patients.
For example, probiotics in combination, or that contain Bifidobacterium breve (B. breve), B. longum, or L. acidophilus, improved abdominal pain scores. Bloating was improved with the B. infantis, L. casei, or L. plantarum strains. On the other hand, the B. breve, B. infantis, L. casei, L. plantarum, B. longum, L. acidophilus, L. bulgaricus, and Streptococcus salivarius (S. salivarius) ssp. thermophilus strains reduced flatulence scores.
In the systematic review and meta-analysis by Ford et al.,67 the authors evaluated the effect of probiotics on abdominal pain in 24 CCTs conducted on 2,011 patients with IBS. The results showed that probiotics significantly reduced abdominal pain (SMD=0.25; 95% CI: –0.36 to –0.14]. The Lactobacillus strains in 6 CCTs did not improve abdominal pain and 2 CCTs with Bifidobacterium showed a trend toward abdominal pain improvement with probiotics (SMD=–0.33; 95% CI: –0.90 to –0.24). In the evaluation of 15 CCTs conducted on 1,038 patients with IBS, the combination of probiotics significantly improved abdominal pain over placebo (SMD=–0.24; 95% CI: –0.37 to –0.12). In that same review, probiotics were significantly superior to placebo in reducing abdominal bloating in 17 CCTs (SMD=–0.15; 95% CI: –0.27 to –0.03). Likewise, probiotics significantly reduced flatulence in 10 CCTs conducted on 741 patients with IBS, compared with placebo (SMD=–0.23; 95% CI –0.38 to –0.07).
In children20a. The administration of probiotics is more effective than placebo in the treatment of abdominal pain associated with IBS
Complete agreement: 100%.
Korterink et al.63 evaluated 5 studies conducted on a total of 464 patients. Different strains of probiotics that included Lactobacillus GG, L. reuteri DSM 17 938, and VSL #3 were used in patients with IBS, FAP, and FD. Horvath et al.64 analyzed 3 studies on chronic abdominal pain with a total of 290 children and 3 studies on IBS conducted on 167 children. Both meta-analyses confirmed that probiotics were more effective than placebo in treating abdominal pain in children with IBS.
In adults21. The administration of probiotics does not improve stool consistency or quality of life in patients with IBS
Complete agreement: 100%.
The meta-analysis by Ortiz-Lucas72 included 16 studies that evaluated the effect of probiotics on stool consistency in patients with IBS. The results showed that the probiotics that contain B. breve, B. infantis, B. longum, L. acidophilus, L. bulgaricus, L. casei, L. plantarum, or species of S. salivarius ssp. thermophilus, did not improve stool consistency.
The parameter of quality of life improvement after probiotic administration in patients with IBS has not been consistently evaluated in studies. In fact, only 2 reviews70,72 carried out an evaluation of the effect of probiotics on quality of life. The analysis by Hoveyda et al. included 4 studies, and even though they found that patients that received probiotics had lower scores on the scales employed (IBS QoL, HAD, and FDD QoL questionnaires or the RAND questionnaire with 36 items), the difference was not statistically significant, when compared with those that received placebo. Ortiz-Lucas et al.72 reported that of the 12 studies evaluating the effect of probiotics on quality of life in IBS patients, no significant improvement in this variable was found in 7 of them.
Chronic constipationFunctional chronic constipation is a frequent gastrointestinal disorder affecting approximately 14% of the world population. Despite the use of dietary fiber, laxatives, prokinetics, and other agents, approximately 47% of constipated patients are not satisfied with those treatment modalities.73 Because of their effect on the gut microbiota and the motor function of the digestive tract, probiotics have been proposed for the management of functional chronic constipation.
In adults22. The administration of specific probiotics in patients with chronic constipation accelerates bowel transit and increases the frequency of bowel movements
Complete agreement: 94%; partial agreement: 6%.
Three meta-analyses were identified, the first of which included 14 randomized controlled trials with a total of 1,182 patients,74 the second included 11 studies and a total of 464 patients,75 and the third analyzed both IBS with constipation and chronic constipation, with a total of 245 patients,67 in addition to a systematic review that included 5 clinical trials conducted on 266 adults.76
The first meta-analysis included 10 studies that evaluated the frequency of bowel movements as the primary aim of the intervention with probiotics and it showed that the administration of specific probiotic strains increased stool frequency by 1.3 bowel movements per week (95% CI: 0.7-1.9; p<0.0001; I2=90%) (p=0.00001), compared with the control groups. The B. lactis strain was the one that favored the greatest increase, that of 1.5 (95% CI: 0.7-2.3; p=0.0003). Stool consistency based on the Bristol scale was evaluated in 11 studies and a change in consistency was observed in stool consistency from hard to soft (RR=0.55; 95% CI: 0.27-0.82; p=0.0001; I2=80%). In the subgroup analysis, B. lactis improved stool consistency the most. In the meta-analyses by Dimidi et al.74 and Miller and Ouwehand,75 bowel transit was objectively evaluated through radio-opaque markers, and the administration of probiotics was shown to reduce transit time in a mean of 12.4h (95% CI: –2.5 to –22.3; p=0.01; I2=23%). B. lactis was also the probiotic that favored a greater transit time reduction, especially at the level of the rectosigmoid colon, but not in the right or left colon.74,75
The specific strains that had favorable effects on bowel transit in subjects with constipation were B. lactis HN019 (2 trials) and B. lactis DN-173 010 (3 trials). However, the effect was only demonstrated in participants above 40 years of age.74
A limited number of clinical trials on subjects with chronic constipation were included in the meta-analysis by Ford et al.67 They concluded that the use of probiotics, prebiotics, and synbiotics has uncertain value in relation to chronic constipation. The systematic review by Chmielewska and Szajewska76 showed a favorable effect on constipated adults treated with B. lactis DN-173 010, L. casei Shirota, and E. coli Nissle 1917 in stool consistency and the frequency of bowel movements.
In children22a. There is no evidence about the efficacy of probiotics in the treatment of functional constipation
Complete agreement: 100%.
The systematic review by Korterink et al.63 included 4 CCTs conducted on 282 constipated pediatric patients. The probiotics used were LGG, L. casei DN 114 001, B. lactis DN 173 010, or B. longum for 3 to 12 weeks. The authors concluded that there was no evidence demonstrating that probiotics were more effective than placebo, with respect to treatment response or an increase in the frequency of bowel movements in constipated children. Chmielewska and Szajewska76 found no beneficial effect of probiotics on constipated children, either.
Infantile colic23. There is insufficient evidence for recommending probiotics in the management of infantile colic
Complete agreement: 50%; partial agreement: 30%; partial disagreement: 10%; total disagreement: 10%
The systematic review by Anabrees et al.77 evaluated 3 CCTs conducted on 209 breastfed infants. The probiotic utilized was L. reuteri DSM 17938 for 21-28 days. Two of the studies were financed by the industry. The results showed that supplementation with probiotics, when compared with simethicone or placebo, significantly and progressively reduced the periods of crying at 7 days, achieving a plateau at 3 weeks after treatment commencement (SMD: –56.03min, 95% CI: –59.92 to –52.15). The probiotics also increased therapeutic success in infantile colic, compared with placebo, with a RR of 0.06 (95% CI: 0.01-0.25) and a NNT of 2. In contrast, a recent CCT by Sung,78 with a significantly larger sample size than in the previous studies (n=167), showed that at one month of treatment, the infants fed with breast milk or formula, supplemented with L. reuteri DSM 17938, had a duration time of daily crying or continuous complaint (primary outcome measure) 49min longer (95% CI: 8-90min, p=0.02) than the group treated with placebo, especially in the infants fed with formula. There were no differences between the probiotic and placebo in relation to the secondary outcome measures of duration of crying or continuous complaint, number of episodes of crying or complaint, duration of sleep, maternal health, functioning of the family and infant, or in the microbiota of the patients treated.
Lactose intoleranceDeficient lactose absorption is a frequent disorder that occurs due to reduced intestinal lactase activity. Lactose intolerance is characterized by the presence of abdominal symptoms, such as bloating, pain, diarrhea, tympanites, and flatulence after the ingestion of dairy products. The development of those symptoms is related to the dose of lactose, lactase expression, gut microbiota, and digestive tract sensitivity. Management includes the restriction of dairy products and the use of lactase. Probiotics have been proposed as agents that improve the symptoms associated with lactose consumption.
24. Some specific probiotic strains improve symptoms in patients with lactose intolerance
Complete agreement: 93.75%; partial agreement: 6.25%.
Two systematic reviews and an expert panel were identified. In their systematic review, Levri et al.79 included 10 studies with probiotics analyzing whether the addition of probiotics to non-fermented dairy products reduced the intolerance to lactose. The majority of the studies employed L. acidophilus. The authors concluded that probiotics in general did not reduce lactose intolerance, but that some specific concentrations could be useful. In their systematic review on the treatment of lactose intolerant individuals, Shaukat et al.80 included 7 studies with a total of 105 patients treated with probiotics. The authors concluded that there was not enough evidence to determine the effectiveness of yoghurt or probiotics for improving the symptoms of lactose intolerance.
The Panel on Dietary Products, Nutrition, and Allergies of the European Food Safety Authority (EFSA)81 evaluated whether live yoghurt cultures containing L. delbrueckii subspecies bulgaricus and S. thermophilus aided lactose digestion in individuals with deficient lactose absorption. Based on 14 intervention studies in humans, the Panel concluded that there was a cause-effect relation between yoghurt consumption and improved lactose digestion in individuals with poor lactose digestion.
Inflammatory bowel diseaseInflammatory bowel disease includes Crohn's disease and ulcerative colitis (UC). It is an inflammatory disorder of the gastrointestinal tract of complex pathophysiology in which there is an abnormal immune response to the gut microbiota in a genetically susceptible subject.82 Standard treatment includes anti-inflammatory drugs, immunosuppressants, and biologic agents. Recent interest in dysbiosis associated with inflammatory bowel disease has fomented the use of probiotics in association with standard therapy in the management of this disease.
In adults25. The use of probiotics has not shown consistent beneficial effects on the induction or maintenance of remission or on recurrence prevention in Crohn's disease
Complete agreement: 100%.
In the Cochrane systematic review83 there were 7 studies that evaluated several probiotic strains in patients with Crohn's disease. The results showed that the administration of E. coli Nissle was not useful for inducing remission (RR=0.83; 95% CI: 0.25-2.80; p=0.77) or for preventing postoperative relapse (RR=0.43; 95% CI: 0.15-1.20). The administration of Lactobacillus GG did not induce remission (RR=1.58; 95% CI: 0.30-8.40) or prevent recurrence of Crohn's disease (RR=0.67; 95% CI: 0.13-3.30). In addition, the administration of S. boulardii was not effective in maintaining remission in patients with Crohn's disease (RR=0.17; 95% CI: 0.02-1.23).
In 2 other systematic reviews, the results showed that the use of probiotics was not efficacious in remission induction (OR=0.80; 95% CI: 0.04-17.20) or in remission maintenance (RR=0.43; 95% CI: 0.15-1.20) in patients with Crohn's disease.83,84
A double-blind placebo-controlled clinical trial was recently published that employed a mixture of 8 different probiotic species called VSL# 3 (4 Lactobacillus strains, 3 Bifidobacterium strains, and one S. salivarius subspecies thermophilus strain). There was only a trend toward significance in reducing postoperative recurrence of Crohn's disease in 20.5% of the group with VSL#3 started immediately after surgery, compared with 42.1% of the group with VSL#3 started one year after surgery (p<0.05).85
In adults26. Some specific strains of probiotics are useful for inducing and maintaining remission in patients with mild or moderate UC
Complete agreement: 93.7%; uncertain: 6.3%.
In a meta-analysis of 20 controlled clinical studies that used different probiotic strains, especially VSL#3, there was a favorable clinical response with a RR of 1.72 (95% CI: 1.35-2.20; p=0.43; I2=0%) and a favorable response in regard to the maintenance of remission (RR=1.56; 95% CI: 0.95-2.56; p=0.047; I2=58%) in patients with UC.86
In another meta-analysis that included 13 CCTs utilizing different probiotic strains in patients with UC, 7 of those studies had a remission induction rate in the probiotics group with a RR of 2 (95% CI: 1.35-2.96). In 8 studies that evaluated remission maintenance in UC patients that received treatment with probiotics for at least 12 months, there was a RR of 1.36 (95% CI: 1.07-1.73; I2=62%). In particular, the administration of B. bifidum reduced the recurrence rate with a RR of 0.25 (95% CI: 0.12-0.50).87
Finally, in the last published meta-analysis that analyzed 23 CCTs with a total of 1,763 patients with UC, a significant increase in remission induction rates was documented (RR=1.51; 95% CI: 1.10-2.06; p=0.01). Remission maintenance was significantly higher in the probiotics group (RR=1.80; 95% CI: 1.36-2.39; p=0.0001; I2=4%). In the probiotic strain subgroup analysis, only a significant increase in the remission rates was confirmed for VSL#3, compared with the control group (RR=1.74; 95% CI: 1.19 to 2.55; p=0.004; I2=60%), as well as in remission maintenance or relapse reduction (RR=0.18; 95% CI: 0.10-0.34; p<0.001).88
27. Some specific probiotic strains are effective for maintaining pouchitis remission after antibiotic therapy
Complete agreement: 93.75%; partial agreement: 6.25%.
In the Cochrane systematic review89 of 13 studies that included a total of 517 patients, VSL#3 administration was effective in 85% of the patients, compared with 3% in the placebo group, for maintaining chronic pouchitis remission 9-12 months in patients with proctocolectomy and ileal reservoir (RR=20.24; 95% CI: 4.28-95.8).
In addition, 2 double-blind, placebo-controlled studies showed the efficacy of VSL#3 at a dose of 450 billion bacteria for maintaining remission in patients with chronic pouchitis. In one of the studies, 40 patients that presented with clinical and endoscopic remission after one month of combined antibiotic treatment (rifaximin 2g/day+ciprofloxacin 1g/day) were randomized to receive VSL#3, 6g/day (18×1,011 bacteria/day) or placebo, for 9 months.90 All the patients that received placebo had some type of relapse. Only 85% (17/20) of the patients were treated with VSL#3 and they remained in clinical and endoscopic remission at the end of the study, compared with the placebo group (p<0.001). In the other study, 36 patients with refractory chronic pouchitis that achieved remission (pouchitis activity index=0) after one month of combined antibiotic treatment (metronidazole+ciprofloxacin) received 6g per day of VSL#3 or placebo for one year. The remission rate was 85% at one year in the group treated with VSL#3, compared with 6% in the placebo group (p<0.001).91
In children27a. There is insufficient evidence on the use of probiotics for the induction of remission or maintenance in Crohn's disease, UC, and pouchitis in pediatric patients
Complete agreement: 90%; partial agreement: 10%.
In the meta-analysis by Li et al.,59 they evaluated 13 studies conducted on adults and children with high-quality methodology and concluded that the use of probiotics did not produce additional benefits in induction therapy to remission of ulcerative colitis. However, they stated that probiotics were better in the maintenance treatment of ulcerative colitis, compared with placebo.
In their systematic review, Shen et al.,88 based on the evaluation of 23 randomized studies with a total of 1,763 participants, concluded that there was a higher remission rate in patients with pouchitis and ulcerative colitis, treated with a mixture of VSL #3 probiotics, as well as a lower recurrence rate of the disease.
Both meta-analyses had very heterogeneous populations, and therefore no recommendation can be made for the management of inflammatory bowel disease.
Diverticular disease of the colonThe spectrum of diverticular disease of the colon includes asymptomatic diverticulosis, acute diverticulitis, and symptomatic uncomplicated diverticular disease. Different therapeutic modalities have been used to prevent episodes of acute diverticulitis and improve the symptoms of symptomatic uncomplicated diverticular disease, and they include dietary fiber, anti-inflammatory agents, antibiotics, and probiotics.92
28. There is insufficient evidence for recommending the use of probiotics as monotherapy in symptomatic uncomplicated diverticular disease or in the prevention of acute diverticulitis
Complete agreement: 93.75%; partial agreement: 6.25%.
There are no quality CCTs that evaluate the effect of probiotics on diverticular disease. In a systematic review of 31 clinical trials, Maconi et al.93 found that probiotics were used for treatment or prophylaxis in patients with diverticular disease of the colon in only 4 of those studies. Said analyses were open studies, with no placebo group, that included a total of 235 patients, showing beneficial effects of the therapy that combined mesalazine or balsalazide with a probiotic (L. casei or VSL#3). There were no symptoms at 12 months in 90.5% of the patients, compared with 84.9% of the patients that received mesalazine or balsalazide, alone, and with 75% of the patients that received probiotics, alone.
Those same 4 studies reported no significant differences between the different treatments for preventing episodes of acute diverticulitis.
Radiation enteritis29. Current evidence does not support the use of probiotics in the prevention and treatment of radiation enteritis
Complete agreement: 93.75%; partial agreement 6.25%.
In the systematic review by Fuccio et al.,94 they evaluated 3 CCTs conducted on a total of 632 patients with post-radiotherapy enteritis. The results showed no significant differences in the management of diarrhea between the group treated with probiotics and the controls (OR=0.47; 95% CI: 0.13-1.67). Suadoni et al.95 evaluated 4 CCTs on 1,457 patients that were predominantly women. Three of those studies showed an effect in favor of probiotics in the management of diarrhea caused by radiation and one of them showed no benefit with the use of probiotics. The studies included in the review were greatly heterogeneous, with differences in the indication for radiation and the therapeutic outcomes.
The systematic review and meta-analysis by Hamad et al.96 evaluated 10 studies on 1,449 patients with radiation enteritis. Eight of those studies compared the use of probiotics vs placebo or no treatment and 2 of them compared 2 treatments with probiotics. The joint results of 6 of the 10 studies showed a benefit in favor of probiotic use in the management of diarrhea (OR=0.44; 95% CI: 0.21-0.92; p=0.03; I2=77%), albeit with significant study heterogeneity. There were no significant differences in decreasing loperamide use (OR=0.29; 95% CI: 0.01-6.80; p=0.44; I2=93%) or in the incidence of watery stools (OR=0.36; 95% CI: 0.05 to 2.81; p=0.33; I2=84%) with probiotic use.
Necrotizing enterocolitis30. Regardless of gestational age and the stage of necrotizing enterocolitis (NEC), the use of probiotics could significantly reduce the risk for NEC in premature neonates
Complete agreement: 100%.
In a meta-analysis, Wang et al.97 evaluated 20 clinical trials, with a total of 3,861 premature neonates. The results showed a reduction in the risk for presenting with NEC in the group supplemented with probiotics (RR=0.33; 95% CI: 0.24-0.46; p<0.00001; I2=0%). In a systematic review, Bernardo et al.98 concluded that probiotics could prevent NEC in premature infants. In the Cochrane review conducted by Alfaleh and Anabrees,99 they evaluated 19 clinical trials with a total of 1,650 premature neonates and concluded that supplementation with probiotics prevented the presentation of enterocolitis in premature infants. In a meta-analysis that included 6,655 pre-term neonates, Yang et al.100 concluded that, regardless of gestational age, supplementation with probiotics reduced the risk for necrotizing enterocolitis, without presenting differences in the presence of sepsis as an adverse effect. Dilli et al.101 carried out a study on patients that had very low birth weight and concluded that B. lactis could reduce the presence of NEC.
Food allergy31. There is insufficient evidence for recommending the preventive use of probiotics in children for allergic diseases or food hypersensitivity
Complete agreement: 100%.
The Cochrane review by Osborn and Sinn102 included 12 studies, 6 of which were evaluated for the outcome of food hypersensitivity, with a total of 2,080 infants and a progression report on 1,549 infants. The meta-analysis of 5 studies that reported the progression of 1,477 infants showed a significant reduction in infantile eczema (RR=0.82; 95% CI: 0.70-0.95), with important heterogeneity between the studies. Upon restricting the analysis to studies that evaluated atopic eczema (confirmed through cutaneous hypersensitivity tests or specific IgE test), the findings were no longer significant (RR=0.80; 95% CI: 0.21-1.02). A meta-analysis103 that included 29 studies on the supplementation of probiotics in pregnant women, breastfeeding mothers, and infants showed that probiotics reduced the risk for eczema, but there was no evidence as to effects on other allergies.
32. The prenatal or postnatal administration of probiotics does not prevent food allergies
Complete agreement: 100%.
In a meta-analysis of 10 CCTs that included 2,701 cases, Kong et al.104 showed that the preventive effect of prenatal or postnatal probiotic supplementation for the prevention of food allergies was not significant, with a RR of 0.88 (95% CI: 0.76-1.03; p=0.11; I2=33%).
In the meta-analysis by Zuccotti et al.105 a reduction in the frequency of eczema was found in infants treated with probiotics, but there were no differences in relation to the prevention of asthma (RR=0.99; 95% CI: 0.77-1.27; p=0.95), wheezing (RR=1.02; 95% CI: 0.89 to 1.17; p=0.76), or rhinoconjunctivitis (RR=0.91; 95% CI: 0.67-1.23; p=0.53).
Acute pancreatitisAcute pancreatitis causes a catabolic state and promotes the appearance of a systemic inflammatory response and nutritional deterioration, which is why nutrient support is an important therapeutic maneuver. It is known that severe cases benefit from receiving enteral nutrition support. It significantly reduces mortality, organ failure, infections, and the need for interventions, compared with parenteral support. Therefore, its use should be considered in all cases of severe acute pancreatitis that require nutritional support.106
There are different enteral formulas, some of which add immunomodulator components, including probiotics.
33. The use of enteral feeding enriched with probiotics has not been shown to be useful in acute pancreatitis
Complete agreement: 93.7%; partial agreement: 6.25%.
A Cochrane review107 that evaluated studies on different enteral nutrition formulas in acute pancreatitis had inconsistent and contradictory results, especially in relation to the occurrence of adverse events and complications, raising doubts as to its safety. The outcomes evaluated were: a) mortality (n=666; 6 studies: RR=1.13; 95% CI: 0.66-1.91), b) systemic inflammatory response (n=223; 3 studies: RR=1.07; 95% CI: 0.9-1.27), c) organ failure (n=644; 5 studies: RR=0.84; 95% CI: 0.67-1.04), and d) adverse events (n=133; 2 studies: RR=1.18; 95% CI: 0.33-4.2).
A subgroup of 9 patients that received probiotics died due to intestinal ischemia, compared with no deaths in the control group (n=298; a very poor-quality study).
These findings do not support the administration or use of enteral nutrition formulas enriched with probiotics in patients with acute pancreatitis that require nutritional support.
Liver diseasesFatty liver and nonalcoholic steatohepatitisThe development of nonalcoholic steatohepatitis is considered to require 2 events. First, the presence of insulin-resistance that produces liver steatosis, and second, an event that produces oxidative stress and that triggers and amplifies the hepatic inflammatory cascade. There is evidence in both animal models and in individuals with obesity and steatosis that they frequently present with intestinal bacterial overgrowth and that it is closely tied to nonalcoholic fatty liver.108,109 The mechanism involves the production of ethanol and an increase in the intestinal permeability to other bacteria and certain lipopolysaccharides that induce the hepatic inflammatory cascade. The use of antibiotics, such as metronidazole or tetracycline, has also been documented to reduce the liver damage induced by bacterial overgrowth in animals and in individuals that have undergone gastrojejunal deviation due to morbid obesity.110,111 The administration of probiotics in those individuals, for the purpose of achieving competitive inhibition of pathogenic strains, aims to reduce both the damage caused by those strains and the anomalous intestinal permeability.
34. The use of specific probiotics improves the inflammation of the liver in nonalcoholic steatohepatitis and tends to reduce cholesterol levels and insulin-resistance in patients with fatty liver and nonalcoholic steatohepatitis
Complete agreement: 100%.
A meta-analysis112 of 4 CCTs was identified that evaluated the use of probiotics in nonalcoholic fatty liver with different probiotic strains in 134 patients. There was a significant reduction in the indirect parameters of liver inflammation of ALT (95% CI: –33.46 to –13.95; p<0.00001) and AST (95% CI: –32.55 to –7.00; p=0.002). There was also a reduction in the tumor necrosis factor alpha levels (95% CI: –0.48 to –0.17; p<0.0001) and total cholesterol (95% CI: –0.55 to –0.01; p=0.04), as well as a reduction in the insulin-resistance index HOMA-IR (95% CI: –0.73 to –0.19; p=0.0008). It should be mentioned that 3 of the 4 studies had a documented histologic baseline, but only one study documented the histologic results after treatment. The probiotics utilized were Lactobacilli, Bifidobacterium, and Streptococcus (L. bulgaricus, Lactobacillus GG, Bifidobacterium longum, Streptococcus thermophilus) and fructo-oligosaccharides were added to 2 of them. Administration duration varied from a few weeks to a few months and one of the 4 studies was conducted on a pediatric population. The sample size of the patients was small.
35. Probiotics improve the covert forms of hepatic encephalopathy, quality of life, and they reduce ammonia concentrations
Complete agreement: 100%.
Eight studies with adequate methodologies were identified.113–120 Probiotics were associated with improvement in the covert forms of hepatic encephalopathy and prophylaxis for overt forms of encephalopathy (RR=1.52%; 95% CI: 1.00-2.33).
Studies with probiotics showed a reduction in levels of ammonia (SMD=–0.32; 95% CI: –0.54 to –0.11).
Probiotics improved the psychosocial profile, physical activity, and quality of life (SMD=–3.13; 95% CI: –4.1 to –2.17).
36. Some specific probiotic strains improve and prevent the development of overt hepatic encephalopathy, but do not influence mortality associated with liver disease
Complete agreement: 100%.
Five randomized clinical trials with different probiotic strains were identified. Dhiman et al.121 studied the use of probiotics in patients with a previous episode of overt hepatic encephalopathy: VSL#3 showed lower relapse to a new episode of hepatic encephalopathy (34.8% with probiotics vs 51.6% with placebo) (RR=0.65; 95% CI: 0.38-1.11; p=0.12), a lower number of hospitalizations due to hepatic encephalopathy (19.7 vs 42.2%, respectively) (RR=0.45; 95% CI: 0.23-0.87; p=0.02) or complications from cirrhosis (24.2 vs 45.3%) (RR=0.52; 95% CI: 0.28-0.95; p=0.034).10
Liu et al.113 studied 55 cirrhotic patients with CHE confirmed by neuropsychologic tests and auditory potentials. A synbiotic treatment was administered for 30 days that consisted of Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subspecies paracasei 19, and L. plantarum 2592, at a dose of 1010 CFU combined with 10g of fermentable fiber (beta-glucan, 2.5g; inulin, 2.5g; pectin, 2.5g; and fermentable fiber 2.5g). The cirrhotic patients with encephalopathy presented with substantial improvement in gut microbiota, manifested by a decrease in E. coli and Staphylococcus strains. The synbiotic treatment increased the fecal content of non-urease-producing Lactobacillus species. There was a reduction in ammonia levels and correction of the covert forms of hepatic encephalopathy in 50% of the cases. There was also a significant reduction in the circulating levels of endotoxins and improvement in the Child-Pugh stage in half of the cases treated with synbiotics. No significant changes were observed in those variables in the group treated with fermentable fiber or in the placebo group.
In a study by Bajaj et al.,114 25 patients were analyzed. Seventeen of the patients were assigned to treatment with yoghurt and 8 to treatment with placebo for 60 days. The patients had Child A compensated cirrhosis and CHE. CHE improved in the patients treated with yoghurt (71 vs 0%, p=0.003), as did the neuropsychologic tests. During follow-up, 25 vs 0% of the patients presented with clinical evidence of encephalopathy. Treatment adherence was 80%.11 Sharma et al.115 studied 105 cirrhotic patients with CHE, 37.4% Child A, 37.9% Child B, and 24.7% Child C. They randomly received lactulose (group A) vs probiotics (one capsule 3 times a day that contained 60 million Streptococcus faecalis, 4 million Clostridium butyricum, 2 million Bacillus mesentericus, 100 million lactic acid bacilli (group B), and lactulose plus probiotics (group C). Treatment was administered for 30 days. Normalization in the psychometric tests and the evoked potentials was evident in the group treated with probiotics.
Sharma et al.119 evaluated 206 cirrhotic patients, 124 (60.19%) of whom had CHE. Among the patients with CHE, 87 (70.16%) presented with flicker<39Hz, 112 (90.32%) presented with altered neuropsychologic tests, and 75 (60.48%) had alterations in both tests. Rifaximin treatment was administered to group A, probiotics to group B, L-ornithine-L-aspartate to group C, and placebo to group D.
Probiotic treatment was given in the form of capsules with L. acidophilus, L. rhamnosus, L. plantarum, L. casei, B. longum, B. infantis, B. breve, S. boulardii, and S. thermophilus. Treatment was administered for 2 months and there was improvement in 50% of the cases (16/32), with a p<0.05, when compared with the placebo group.9
Infant nutrition37. The available scientific data suggest that there are no safety concerns with the administration of probiotics in infant formulas with respect to adverse effects. However, the data are currently insufficient for recommending the routine use of infant formulas supplemented with probiotics
Complete agreement: 100%.
A systematic review by Mugambi et al.122 included 3 studies with synbiotics (n=475), 10 studies with probiotics (n=933), and 12 studies with prebiotics (n=1,563). Probiotics in infant formulas had no significant effect on growth, stool consistency and frequency of bowel movements, and did not reduce the incidence of diarrhea, colic, regurgitation, crying, restlessness, or vomiting. Thus the authors concluded that supplementation of infant formulas with probiotics for neonates and full-term infants was not associated with an increase in growth or with favorable clinical effects.
Another systematic review by Mugambi et al.123 on the use of infant formulas for pre-term infants or those with low birth weight, included 4 studies with probiotics (n=2,112) and 4 with prebiotics (n=126). No significant differences were found in the group supplemented with probiotics in relation to weight increase (SMD=1.96; 95% CI: –2.64 to 6.56) or maximum enteral feeding (SMD=35.20; 95% CI: –7.61 to 78.02), but there was a significant increase in the number of bowel movements per day (SMD=1.60; 95% CI: 1.20-2).
The ESPGHAN published a systematic review in 2011124 in which they concluded that the available scientific data suggest that: a) probiotic supplementation in infant formulas for healthy children gives no cause for safety concerns with respect to growth and adverse effects, b) the administration of probiotics in infant formulas in young children (≤ 4 months of age) has no clear clinical effects, and c) even though some probiotics added to infant formulas, either alone or in combination, can have beneficial clinical effects, the routine use of formulas supplemented with probiotics is not recommended for infants.
38. The use of probiotics in pregnant women that then breast-feed their babies can reduce the risk for eczema in the infants. Nevertheless, the evidence is insufficient
Complete agreement: 91%; partial agreement: 8.33%; uncertainty: 8.33%.
The meta-analysis by Cuello-Garcia et al.103 included 29 studies of probiotic supplements given to pregnant women, breast-feeding mothers, and infants. The probiotics reduced the risk for eczema when they were used in the third trimester of pregnancy (RR=0.71; 95% CI: 0.60-0.84), in mothers that breast-fed their babies (RR=0.57; 95% CI: 0.47-0.69), or when they were administered to the infants (RR=0.80; 95% CI: 0.47-0.69). No evidence was found of effects on other allergies, nutritional status, or incidence of adverse effects.
Another meta-analysis105 included 17 studies and reported the data of 4,755 infants (2,381 in the probiotic group and 2,374 in the control group). The infants treated with probiotics had a significantly lower RR for eczema, compared with the controls (RR=0.78; 95% CI: 0.69-0.8; p=0.0003). There were no differences in terms of asthma prevention (RR=0.99; 95% CI: 0.77-1.27; p=0.95), wheezing (RR=1.02; 95% CI; 0.89-1.17; p=0.76), or rhinoconjunctivitis (RR=0.9; 95% CI: 0.67-1.23; p=0.53). The results of the meta-analysis showed that probiotic supplementation prevented infantile eczema.
In 2015, the World Allergy Organization (WAO) published guidelines125 on the use of probiotics in the prevention of allergic disease, in which they suggested: a) to use probiotics in pregnant women with a high risk for having a child with allergies, b) to use probiotics in women that breast-fed infants at high risk for developing allergies, and c) to use probiotics in infants at high risk for developing allergies.
ConclusionsDue to their numerous mechanisms of action, probiotics have been shown to be effective in the prevention of infectious, inflammatory, and functional diseases of the digestive tract. The specificity of the strain and the indication in the precise clinical entity evaluated in the CCTs are determining factors for the adequate use of probiotics. The Mexican consensus group on probiotics recommends their use in the following gastrointestinal disorders: in the prevention of AAD, in the treatment of acute infectious diarrhea, in the prevention of CDI and NEC, to reduce the adverse events of H. pylori eradication therapy, to alleviate symptoms of IBS and functional constipation in the adult, to induce and maintain remission in patients with UC and pouchitis in the adult, and to treat CHE and overt hepatic encephalopathy.
FundingThe authors wish to thank the Instituto Danone for the financial support given in carrying out the first meeting of the members of the consensus group. Said organization did not influence the design or direction of the consensus, nor did it influence the collection, management, analysis, or interpretation of the results. It also had nothing to do with the preparation and writing of the manuscript to be published.
Conflict of interestMiguel A. Valdovinos is a Member of the Advisory Board of Biocodex, Mayoly-Spindler, Takeda, and Commonwealth. He is a Speaker for Takeda, Ferrer, Mayoly-Spindler, Danone, Columbia, and Biocodex. He has received financial support for academic development from Takeda, Alfa Wassermann, and Danone.
Ericka Montijo is a Speaker for Mead Johnson, Danone, and Biocodex.
Ana Teresa Abreu y Abreu is a Member of the Advisory Board for Sanofi, Takeda, Mayoly-Spindler, and Almirall. She is a Speaker for Takeda, Sanofi, Mayoly-Spindler, Alfa Wassermann, Almirall, Carnot, and Biocodex. She has received financial support for academic development from Takeda, Sanofi, Asofarma, Biocodex, and Danone.
Sollange Heller is a Member of the Advisory Board of Mayoly-Spindler and a Speaker for Mead Johnson Nutrition, Novamil (Bayer), Mayoly-Splinder, and Biocodex.
María Victoria Bielsa Fernández is a Speaker for Alfa-Wassermann and Almirall. She is a Member of the Advisory Board of Alfa-Wassermann.
Francisco Bosques-Padilla is a Member of the Advisory Board of Takeda and a Speaker for Abvie, Janssen, and Bristol-Myers Squibb México.
Ramón Carmona-Sánchez is a Member of the Advisory Board of Mayoly-Spindler and a Speaker for Mayoly-Spindler and Asofarma.
Alejandra Consuelo is a Member of the Advisory Board of Sanofi Laboratories and a Speaker for Sanofi and Mead Johnson Nutrition.
Enrique Coss-Adame is a Speaker for Takeda, Mexico. He has been a Consultant for and has collaborated with Asofarma Laboratories, Mexico.
José Antonio Chávez-Barrera is a Member of the Advisory Board of Mayoly-Splinder, Nestlé, Mead Johnson, Aspen, Sanfer, Carnot, Italmex, and Takeda. He is a Speaker for the Nestlé, Aspen, Mead Johnson, Bayer, Sanfer, Astra Zeneca, Carnot, Italmex, Mayoly-Splinder, and Takeda laboratories.
Mauricio de Ariño is a Speaker for Takeda.
Octavio Gómez-Escudero is a Member of the Advisory Board of Almirall Laboratories and a Speaker for Takeda, Astra-Zéneca, Almirall, Asofarma, and Alfa-Wassermann.
María Sarai González-Huezo is a Member of the Advisory Board of Bayer, Bristol, Roche, and Janssen Cilag. She is a Speaker for Bayer, Roche, Janssen, and Merck.
María Eugenia Icaza-Chávez is a Member of the Advisory Board of Mayoly-Spindler and a Speaker for Mayoly-Spindler and Asofarma.
Miguel Morales-Arámbula is a Speaker for Takeda and Almirall.
Jaime Ramírez-Mayans is a Speaker for Biocodex.
José María Remes-Troche is a Member of the Advisory Board of Takeda Pharmaceuticals, Alfa-Wassermann, and Almirall. He is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Almirall, and Astra-Zeneca.
Eric Toro is a Speaker for Sanofi and Mead Johnson Nutrition
Aldo Torre-Delgadillo is a Speaker for Merz Pharma and receives financial support for research from Medix.
Rodrigo Vázquez-Frías is a Speaker for and has received financial support for research from Alexión, Mayoly-Spindler, Nestlé, and Sanofi.
Jesús Kazuo Yamamoto-Furusho is a Member of the Advisory Board of Takeda, Abbvie, Schering, MSD, Ferring, Pfizer, Janssen, and Hospira. He is a Speaker for Takeda, Abbvie, Schering, MSD, Janssen, Danone, Farmasa, Ferring, Novartis, and Astra. He has been a Researcher and has received financial support for research from Bristol, Icon, and Pfizer.
Francisco Guarner is a Member of the Scientific Committee of the Instituto Danone in Spain and a Member of the Advisory Board of Biocodex and Clasado. He has participated in educational events financed by Danone, Biocodex, Sanofi, Stada, Casen-Recordati, Ferring, and Abbvie.
Alejandro González-Garay, Dante Bacarreza, Maria del Carmen Bojórquez-Ramos, Ana I. Burguete-García, Judith Flores-Calderón, Alfredo Larrosa-Haro, Chiharu Murata, Mario Peláez-Luna, and Martha Eugenia Urquidi-Rivera declare that they have no conflict of interest.
Please cite this article as: Valdovinos MA, Montijo E, Abreu AT, Heller S, González-Garay A, Bacarreza D, et al. Consenso mexicano sobre probióticos en gastroenterología. Revista de Gastroenterología de México. 2017;82:156–178.