Cadaveric donor liver graft retrieval is complex in Mexico. The aim of the present article was to present the experience in liver graft use during the first year of work of a local evaluation and procurement team.
Materials and methodsWe reviewed the organ donation report forms and allocation offer records covering the time frame of December 15, 2017 to December 15, 2018, and registered the donor characteristics, causes of organ discard, causes of declined offers, transport time, and graft and recipient survival at 30 days.
ResultsThere were 17 donations and we completed the evaluation of 14. Two donors were considered ideal (14.2%) and 12 were expanded criteria donors (ECDs) (85.7%). Two grafts with steatosis were not offered (14.2%). Twelve liver grafts were offered 88 times (mean 7.6 offers per graft). The acceptance rate was 6% for public hospitals and 23.6% for private hospitals (p = 0.016). One graft was discarded during the procurement process due to steatosis. The rate of use after evaluation was 78.5% (11/14). All the grafts were procured by the local team and 9 (81.8%) were transported by commercial airline (median 240 min, range 85 min). Graft and recipient survival at 30 days was 100%.
ConclusionsThe participation of a local evaluation and procurement team notably increased liver graft use with excellent results. Commercial airline transportation of the grafts to all active transplantation centers of the country resulted in cold ischemia times <6 h.
La recuperación de injertos hepáticos de donante cadavérico en México es compleja. El objetivo de este manuscrito es presentar la experiencia en la utilización de hígado durante el primer año de un equipo local de valoración y procuración.
Material y métodosRevisamos los formatos de reporte de donación y las bitácoras de oferta del 15 de diciembre de 2017 al 15 de diciembre de 2018. Registramos las características de los donantes, causas de descarte, causas de declinación, tiempo de transporte y supervivencia del injerto y receptor a 30 días.
ResultadosHubo 17 donaciones y completamos la valoración de 14; dos donantes fueron considerados ideales (14.2%) y 12 donantes por criterios extendidos (DCE 85.7%). Dos injertos no fueron ofertados por esteatosis (14.2%). Doce injertos hepáticos se ofertaron 88 veces (media 7.6 ofertas por injerto). La tasa de aceptación de hospitales públicos fue de 6% y privados 23.6% (p = 0.016). Un injerto se descartó durante la procuración por esteatosis. La tasa de utilización tras valoración fue 78.5% (11/14) Todos los injertos fueron procurados por el equipo local y nueve (81.8%) fueron transportados en vuelos comerciales (mediana 240 min, rango 85 min). La supervivencia del injerto y el receptor a 30 días fue 100%.
ConclusionesLa participación de un equipo local de valoración y procuración incrementó notablemente la utilización de hígado con excelentes resultados. El uso de vuelos comerciales permitió transportar los injertos con tiempos de isquemia fría <6 horas a todos los centros de trasplante activos del país.
Geographically, Chihuahua is the largest state in Mexico. The distances it encompasses limit the capacity to transport liver grafts by land within an appropriate cold ischemia time frame. Therefore, all liver donation processes in organs that are not utilized locally require air transport, which is not routinely available for the majority of transplantation centers of the country.
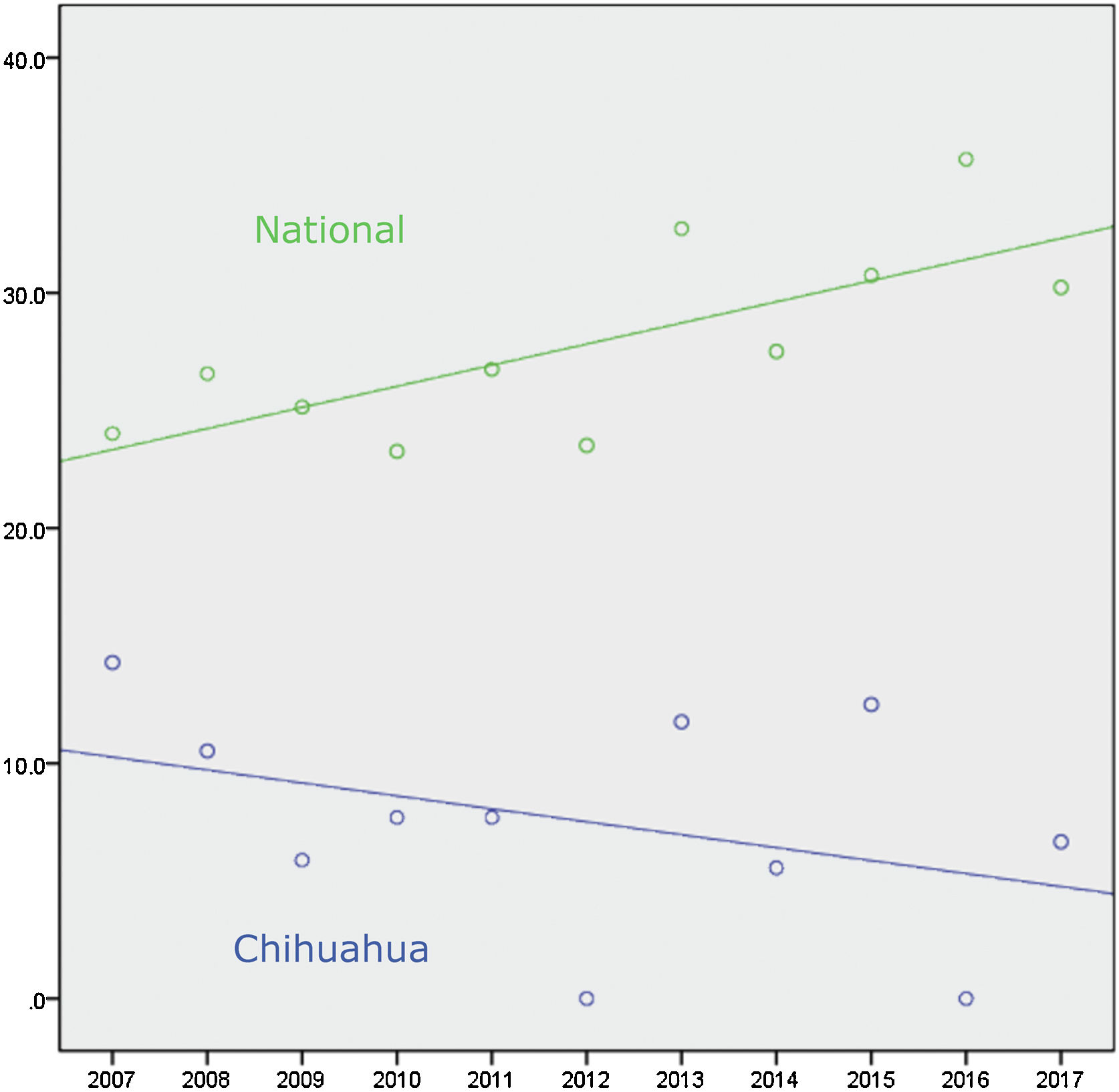
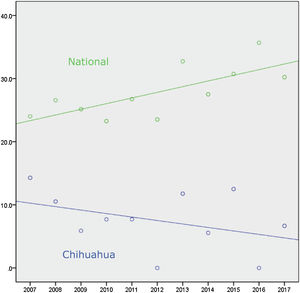
Connected to the absence of liver transplantation activity up to 2018 in the State of Chihuahua, the lack of offer, distance, air travel time, and cost of transport significantly limited the use of liver grafts from brain-dead donors. Figure 1 and Table 1 show there was a national positive trend toward and a statistically significant use of liver grafts from 2007 to 2017, whereas their use in Chihuahua did not significantly change.1 In December 2017, we established a systematized strategy of evaluation, offer, and procurement, to increase the number of liver grafts utilized in the state.
Number of brain-dead donors and cadaveric donor liver transplantations in Mexico from 2007-2017.
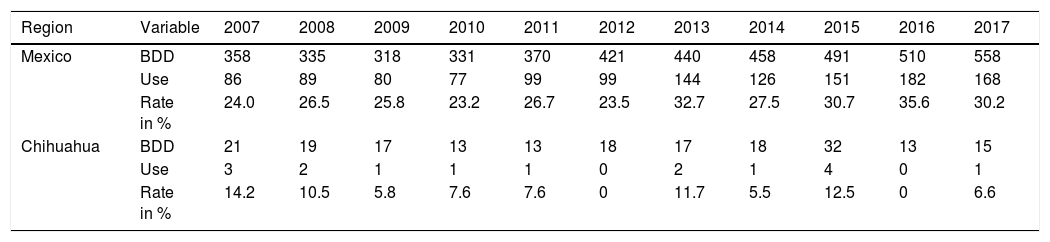
| Region | Variable | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mexico | BDD | 358 | 335 | 318 | 331 | 370 | 421 | 440 | 458 | 491 | 510 | 558 |
| Use | 86 | 89 | 80 | 77 | 99 | 99 | 144 | 126 | 151 | 182 | 168 | |
| Rate in % | 24.0 | 26.5 | 25.8 | 23.2 | 26.7 | 23.5 | 32.7 | 27.5 | 30.7 | 35.6 | 30.2 | |
| Chihuahua | BDD | 21 | 19 | 17 | 13 | 13 | 18 | 17 | 18 | 32 | 13 | 15 |
| Use | 3 | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 4 | 0 | 1 | |
| Rate in % | 14.2 | 10.5 | 5.8 | 7.6 | 7.6 | 0 | 11.7 | 5.5 | 12.5 | 0 | 6.6 |
BDD: brain-dead donors; Use: number of liver transplants from cadaveric donors during the same period; Rate: percentage of liver grafts used in relation to the number of donors per year.
The delay at the beginning of the procurement process has often been cited as a factor that can place organ retrieval at risk due to donor instability or the family’s withdrawal of consent. However, there is no database in Mexico, with which the time of offer, the number of offers per donor, or the number of donations lost in the attempt to use the liver can be known. We are also not aware of any report on the causes of why liver transplant offers are being declined by transplantation centers in Mexico.
The aims of the present article were to present our first-year experience and analyze donor characteristics, causes of discard of the unoffered grafts, causes of decline of the offers by the transplantation centers, length of time of graft transport, and results 30 days after graft use.
Materials and methodsOn December 15, 2017, we established a systematized strategy for evaluating all brain-dead cadaveric donors. All cases are assessed by a liver transplant surgeon and each donation is considered as soon as the family signs the consent form for cadaveric organ and tissue transplantation.
There are no limits regarding age, weight, height, or length of intrahospital stay for evaluating the candidate as a potential liver donor.2 Alcohol consumption is registered, no matter the grade. All causes of brain death are taken into consideration. In the case of primary tumors of the central nervous system, patients with anaplastic tumors, open surgery of the tumor, or ventriculoperitoneal diversion are ruled out.3 Donors with a personal history of cancer are evaluated case by case, and there are no impediments for those with a history of cancer limited to the skin (except melanoma), thyroid (except anaplastic disease), and the prostate.4,5 The presence and grade of hypernatremia is not a contraindication for evaluation, but if present, we request immediate medical management by the intensive care team.6 A history of cardiorespiratory arrest is not a contraindication for evaluation.7 Elevated liver enzymes is not a contraindication for evaluation, regardless of the level. Elevated total bilirubin level < 4 mg/dL is not a contraindication for evaluation, unless there is conjugated hyperbilirubinemia (> 20% of total bilirubin) upon hospital admission of the donor. The presence of thrombocytopenia upon admission is an alarm factor, discarding the donor if there are risk factors for liver diseases or abnormal laboratory test results. Donors at increased risk for parenteral transmission of infections (defined by the presence of one of the following within the last 12 months: intravenous drug use, tattoos, incarceration > 72 h, homosexual intercourse, sex worker, infection due to sexual transmission or hemodialysis, or massive transfusion with ≥ 10 units of blood derivatives within the last 72 h) undergo a nucleic acid test and the organ is offered if the test is negative.8 Donors serologically positive for hepatitis B or C are evaluated through biopsy and the livers are offered only to patients with known active infection. All donors with risk factors for fatty liver (BMI ≥ 28 kg/m2, a history of alcohol/drug use, or diabetes mellitus) undergo ultrasound. If the study result is normal, we perform intraoperative wedge liver biopsy at the beginning of procurement, only if necessary. If the ultrasound result identifies mild or moderate hepatic steatosis, we perform percutaneous liver biopsy with frozen tissue study before procurement; if the result of the ultrasound is severe steatosis (hyperechogenicity of the liver parenchyma in relation to the renal cortex, reduced visualization of the segments posterior to the right hemiliver, and loss of visibility of the portal pedicles), the graft is discarded.9 If the biopsy result is macrovesicular hepatic steatosis >30%, bridging fibrosis, or cirrhosis, the graft is discarded, and the rest of the findings are evaluated case by case. Grafts are not discarded due to the presence of microvesicular steatosis, regardless of the grade.10
If the transplant team receiving the graft has their own air transport means, they establish the logistics and time of procurement and clamping. If the team receiving the graft does not have their own air transportation, the local team performs the organ retrieval and the exact time that procurement begins is established at 210 min before take-off of the available commercial airline flight, whereas the time of clamping is programmed for 90 min before take-off. The organ is transported by hospital ambulance to the airport terminal, which is located 5.7 km from the hospital with the highest donation activity.
Our team performs the procurement of liver grafts, utilizing warm vascular dissection, which enables cold ischemia and warm ischemia times to be shortened by reducing bench surgery duration. If the arterial anatomy is normal, the only intervention needed on the graft during bench surgery is suprahepatic inferior vena cava preparation. If there is an aberrant right hepatic artery arising from the superior mesenteric artery, the pertinent reconstruction is required. During organ procurement, we perform sternotomy and an abdominal crossed incision, freeing the round and falciform ligaments, as well as the peritoneal attachments of the liver. The liver is photographed and shown to the recipient’s transplant team. If necessary, we take a wedge biopsy from the left lateral section, which is processed in an intraoperative study. We divide the right adrenal vein between sutures and caudally dissect the infrahepatic and retrohepatic inferior vena cava up to the renal veins. We dissect the common hepatic artery, the celiac trunk, and the supraceliac aorta, dividing the gastroduodenal, splenic, and left gastric arteries between sutures. We divide the common bile duct at its most distal part, without ligating the stump of the graft. We wash the gallbladder and the bile duct with 0.9% saline solution and dissect the portal vein and ligate its tributaries. If an aberrant left hepatic artery is identified, it is kept in continuity with the left gastric artery. If an aberrant right (or common) hepatic artery is identified, it is kept in continuity and retrieved with a short segment of the superior mesenteric artery. The aorta is cannulated just above its bifurcation and the portal vein is cannulated at its origin. Both are perfused with cold HTK solution and the liver is photographed and shown to the recipient’s transplant team.
We reviewed the formats of the donation reports of the CENATRA and the allocation offer records for liver grafts from brain-dead cadaveric donors, within the time frame of December 15, 2017 to December 15, 2018. We registered the demographic characteristics of the hospitals and donors, the laboratory, imaging, and pathology studies, the causes of discard of the unused grafts, the causes of declined offers by the transplant centers, and the final results in the transplanted grafts.
Donors were considered “ideal” when they met the following criteria: below 40 years of age, trauma as the cause of brain death, hemodynamic stability at the time of procurement, absence of steatosis or chronic liver disease, and absence of transmittable diseases. Any departure from those criteria defines the individuals as expanded criteria donors (ECDs).11 For the present study, we considered age > 65 as the expanded criterion, if it was the only departure.
Grafts were considered “ideal” if there were no abnormal intraoperative findings (e.g., biopsy with steatosis), no disease in the celiac trunk or hepatic artery (e.g., atheromatous plaque, calcifications), modifications in the graft (e.g., reduced liver), and prolonged cold ischemia time (>6 h). Grafts with the presence of any of those conditions was considered an expanded criteria graft (ECG).11
We registered the causes of liver graft offer decline by the transplant centers in record books. The causes were grouped into 8 categories: donor quality or age, discrepancy in donor/recipient size, specific problem with the graft, problems with the recipient, incomplete transplant team, inactive transplant program, lack of transfer capacity, and other causes (including all that could not be grouped into one of the previous categories described). We analyzed the number of offers made per donor until the organ was placed, hospital characteristics, the time interval from the first offer to graft acceptance, and the time interval from graft acceptance to the start of procurement.
We contacted the transplantation centers that received the liver grafts and the recipients to find out the result 30 days after graft transplant, without requesting any information that could violate recipient confidentiality. We defined “graft non-function” according to the criteria of the United Network for Organ Sharing (UNOS) by at least 2 of the following results up to 7 post-transplantation days: INR ≥ 2.5 with no response to vitamin K, AST ≥ 3,000 IU/L or ALT ≥ 2,000 IU/L, total bilirubin ≥ 10 mg/dL, metabolic acidosis (arterial pH ≤ 7.30 or venous pH ≤ 7.25), and lactate ≥ 4 mmol/L.12 We defined “early graft dysfunction” by at least one of the following: total bilirubin ≥ 10 mg/dL on post-transplantation day 7, INR ≥ 1.6 on post-transplantation day 7, ALT or AST ≥ 2,000 IU/L within the first 7 post-transplantation days.13
Statistical analysisThe dichotomous variables were presented as percentages and the quantitative variables as medians (minimum and maximum, when so marked). We compared the acceptance rate of public and private hospitals through the chi-square test. The correlation between livers obtained at the national level and in the state of Chihuahua over time was performed using the Spearman test. Statistical significance was set at a p < 0.05.
Ethical considerationsWe presented only the most relevant and necessary information, thus respecting donor and recipient confidentiality. Procurement dates were omitted from the manuscript and age was presented in ranges (<18, 18-40, 40-65, and >65 years). If a donor had an increased risk for parenteral transmission of infections, the cause was left out. The evaluation protocol and the present manuscript were authorized by the ethics committees of the participating hospitals. Patient anonymity has been maintained. Informed consent was not required for the present study, given that it contains no data through which patients can be identified. No funding was received in relation to this study.
ResultsWithin the time frame of the study, there were 17 donations from brain-dead donors in the State of Chihuahua. Mean donor age was 47.7 ± 14.8 years and 23.5% were women. One donor was < 18 years of age (5.8%) and the rest were adults (16 cases, 94.2%). Regarding ABO group, 11 (64.7%) donors were type O, 3 (17.6%) were type A, and one (5.8%) was type B. ABO group was not available in 2 cases and was not included in the evaluation (cases #2 and #6). The logistics of donor evaluation, offer, and graft procurement could not be completed in case #16, before the kidney procurement team began the retrieval. Within our study period, at least one kidney graft was obtained from those donors (94.1%) (16/17).
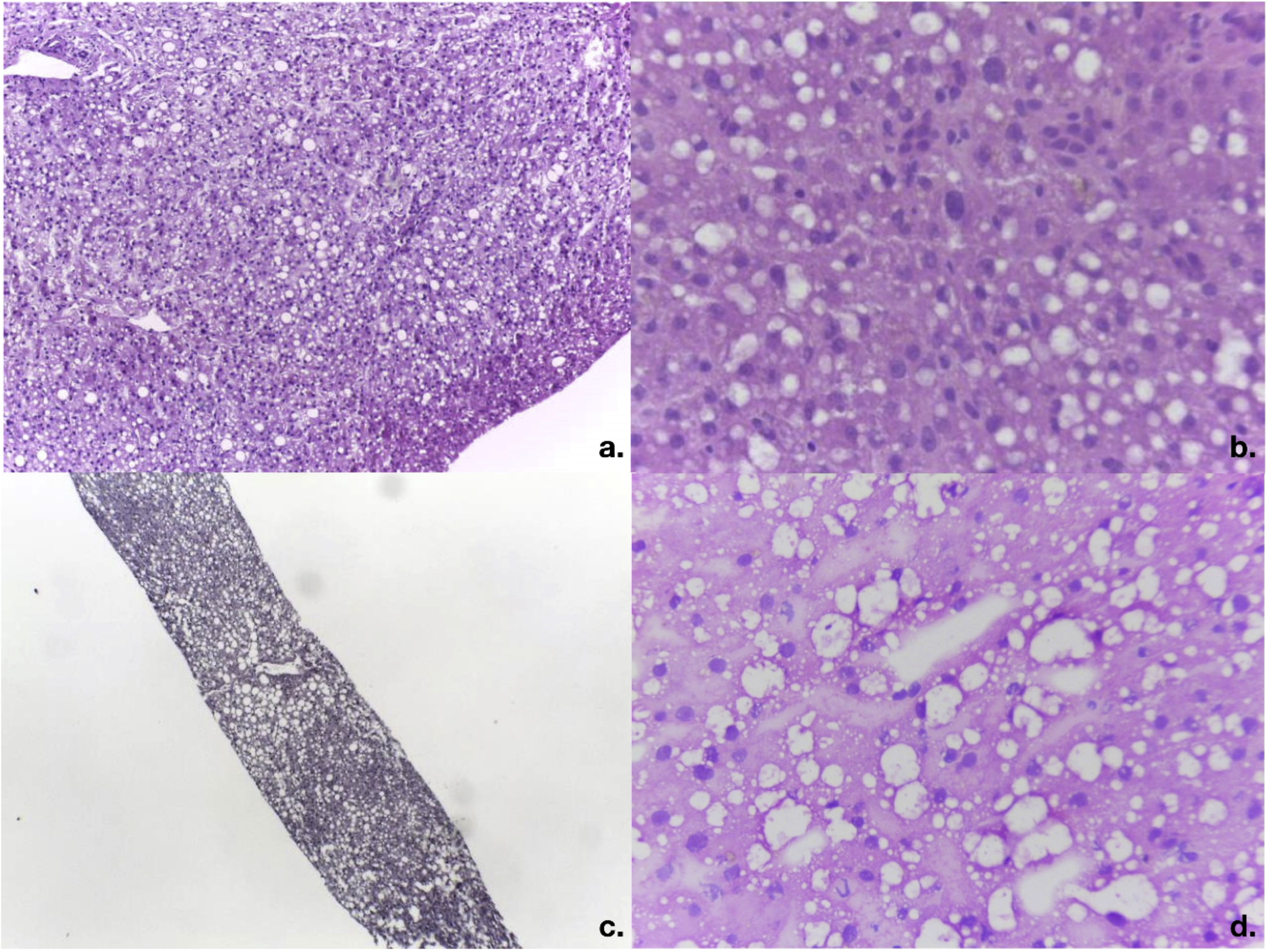
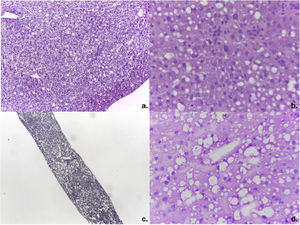
Of the 14 donors with complete evaluations, 5 had increased risk factors for parenteral transmission of infections (35.7%) and 12 were considered ECDs (85.7%). The causes for considering donors ECDs were: harmful alcohol use in 6 cases (41.6%), multiple causes in 4 cases (33.3%), and cerebrovascular disease as the cause of death in one case (7.1%). Three of the 6 cases (50%) with a history of alcohol use had a history of treatment in rehabilitation centers. The cases with multiple causes included: intracranial bleeding as the cause of death in 4 cases and age >65 years in 2 cases. Table 2 presents more information on donor characteristics. Two cases were discarded due to finding macrovesicular steatosis >30% in the intraoperative biopsy of one case (Fig. 2A, case #6) and the preoperative biopsy in another (Fig. 2B, case #8), with body mass indexes of 24.2 kg/m2 and 25.1 kg/m2, respectively. Case #9 was discarded because of the ultrasound finding of severe steatosis in a patient with a body mass index of 46.1 kg/m2.
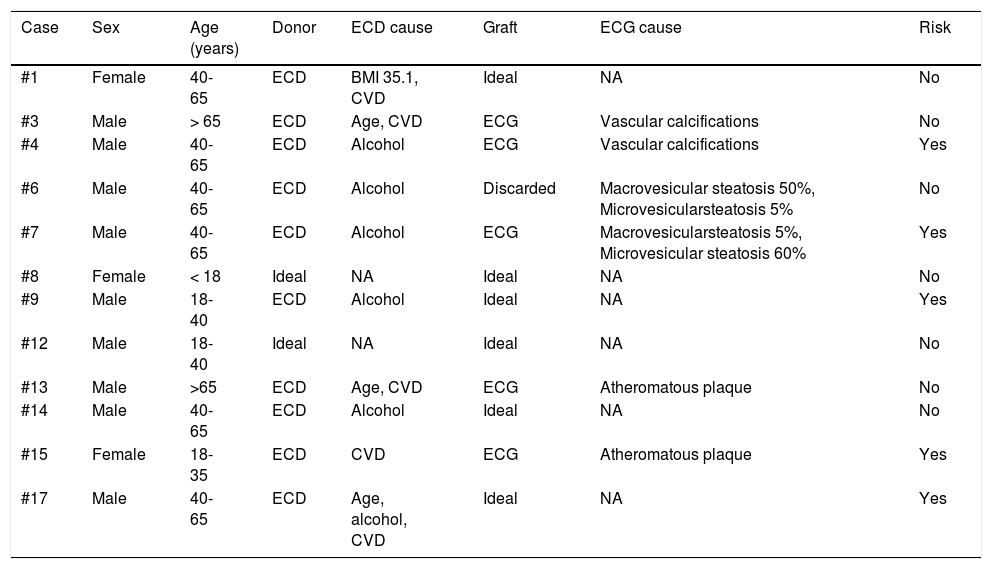
Characteristics of the 12 donors and liver grafts offered during the time frame of the study.
| Case | Sex | Age (years) | Donor | ECD cause | Graft | ECG cause | Risk |
|---|---|---|---|---|---|---|---|
| #1 | Female | 40-65 | ECD | BMI 35.1, CVD | Ideal | NA | No |
| #3 | Male | > 65 | ECD | Age, CVD | ECG | Vascular calcifications | No |
| #4 | Male | 40-65 | ECD | Alcohol | ECG | Vascular calcifications | Yes |
| #6 | Male | 40-65 | ECD | Alcohol | Discarded | Macrovesicular steatosis 50%, Microvesicularsteatosis 5% | No |
| #7 | Male | 40-65 | ECD | Alcohol | ECG | Macrovesicularsteatosis 5%, Microvesicular steatosis 60% | Yes |
| #8 | Female | < 18 | Ideal | NA | Ideal | NA | No |
| #9 | Male | 18-40 | ECD | Alcohol | Ideal | NA | Yes |
| #12 | Male | 18-40 | Ideal | NA | Ideal | NA | No |
| #13 | Male | >65 | ECD | Age, CVD | ECG | Atheromatous plaque | No |
| #14 | Male | 40-65 | ECD | Alcohol | Ideal | NA | No |
| #15 | Female | 18-35 | ECD | CVD | ECG | Atheromatous plaque | Yes |
| #17 | Male | 40-65 | ECD | Age, alcohol, CVD | Ideal | NA | Yes |
BMI: body mass index; CVD: cerebrovascular disease as cause of brain death; DM2: type 2 diabetes mellitus; ECD: expanded criteria donor; ECG: expanded criteria graft; NA: not applicable; Risk: increased risk for parenteral transmission infection.
Intraoperative study of frozen liver biopsies of the discarded cases. a) Photomicrograph of the intraoperative wedge biopsy of case #6 with 50% macrovesicular steatosis and 5% microvesicular steatosis (magnification x10). b) Detail of steatosis in case #6 (magnification x40). c) Photomicrograph of percutaneous liver biopsy of case #8 with 40% macrovesicular steatosis and 20% microvesicular steatosis (magnification x10). d) Magnification x40 of case #8.
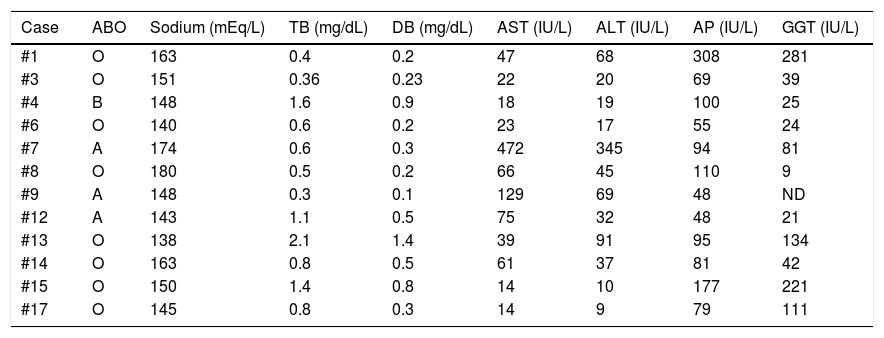
The laboratory test results at the time of the offer are shown in Table 3. Hypernatremia was present in 66.6% of the donors (upper limit of normal 145 mEq/L, minimum 148, maximum 180), conjugated hyperbilirubinemia in 25% (upper limit of normal 1.2 mg/dL, minimum 1.4, maximum 2.1), elevated AST in 50% (upper limit of normal 40 IU/L, minimum 47, maximum 472), elevated ALT in 33.3% (upper limit of normal 55 IU/L, minimum 68, maximum 345), elevated alkaline phosphatase in 16.6% (upper limit of normal 147 IU/L, minimum 177, maximum 308), and elevated GGT in 33.3% (upper limit of normal 50 IU/L, minimum 81, maximum 281).
Laboratory results of the 12 donors whose liver grafts were offered during the study period.
| Case | ABO | Sodium (mEq/L) | TB (mg/dL) | DB (mg/dL) | AST (IU/L) | ALT (IU/L) | AP (IU/L) | GGT (IU/L) |
|---|---|---|---|---|---|---|---|---|
| #1 | O | 163 | 0.4 | 0.2 | 47 | 68 | 308 | 281 |
| #3 | O | 151 | 0.36 | 0.23 | 22 | 20 | 69 | 39 |
| #4 | B | 148 | 1.6 | 0.9 | 18 | 19 | 100 | 25 |
| #6 | O | 140 | 0.6 | 0.2 | 23 | 17 | 55 | 24 |
| #7 | A | 174 | 0.6 | 0.3 | 472 | 345 | 94 | 81 |
| #8 | O | 180 | 0.5 | 0.2 | 66 | 45 | 110 | 9 |
| #9 | A | 148 | 0.3 | 0.1 | 129 | 69 | 48 | ND |
| #12 | A | 143 | 1.1 | 0.5 | 75 | 32 | 48 | 21 |
| #13 | O | 138 | 2.1 | 1.4 | 39 | 91 | 95 | 134 |
| #14 | O | 163 | 0.8 | 0.5 | 61 | 37 | 81 | 42 |
| #15 | O | 150 | 1.4 | 0.8 | 14 | 10 | 177 | 221 |
| #17 | O | 145 | 0.8 | 0.3 | 14 | 9 | 79 | 111 |
ABO: blood group system; AP: alkaline phosphatase; DB: direct bilirubin; TB: total bilirubin.
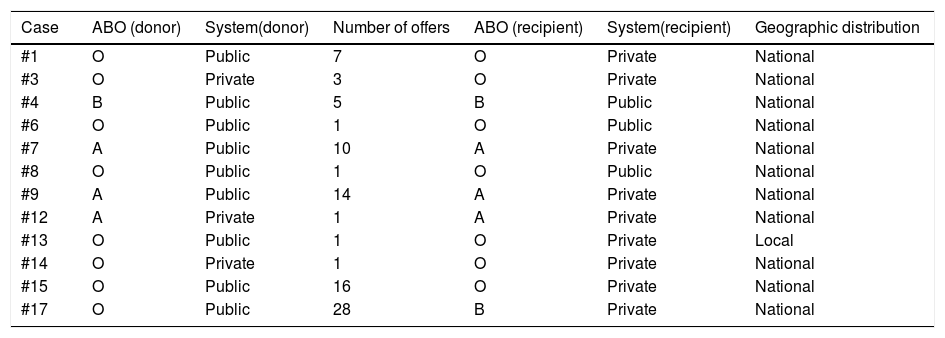
Table 4 shows the number of offers per case. We carried out a total of 88 offers for the distribution of 12 liver grafts. Case #6 was offered to a pediatric national emergency patient and accepted. The donor had an abdominal computed tomography scan that showed no steatosis, but during the procurement we encountered an orange-colored liver with smooth edges, and the intraoperative biopsy revealed macrovesicular steatosis at 50% and microvesicular steatosis at 5%: the liver was discarded due to specific problems with the graft (1/88, 1.3%). Eleven liver grafts were utilized for liver transplantation. Excluding the accepted offers, the rest of the 76 offers were discarded for the following reasons: donor quality or age (24 offers, 31.1%), problems with the potential recipient (22 offers, 28.5%), incomplete transplantation team (11 offers, 14.3%), donor/recipient size discrepancy (8 offers, 10.3%), lack of transfer capacity (7 offers, 9.1%), inactive program (3 offers, 3.9%), and other reasons (one offer, 1.3%), in which a patient had been registered with an incorrect ABO group that was corrected due to the offer.
Relation between the characteristics of the donor’s hospital, the number of offers, and the recipient’s hospital.
| Case | ABO (donor) | System(donor) | Number of offers | ABO (recipient) | System(recipient) | Geographic distribution |
|---|---|---|---|---|---|---|
| #1 | O | Public | 7 | O | Private | National |
| #3 | O | Private | 3 | O | Private | National |
| #4 | B | Public | 5 | B | Public | National |
| #6 | O | Public | 1 | O | Public | National |
| #7 | A | Public | 10 | A | Private | National |
| #8 | O | Public | 1 | O | Public | National |
| #9 | A | Public | 14 | A | Private | National |
| #12 | A | Private | 1 | A | Private | National |
| #13 | O | Public | 1 | O | Private | Local |
| #14 | O | Private | 1 | O | Private | National |
| #15 | O | Public | 16 | O | Private | National |
| #17 | O | Public | 28 | B | Private | National |
ABO: blood group system.
In the category of donor quality or age, the reasons the offers were declined were that the donor did not meet the criteria of the transplantation center (20 offers, 83.3%) and the donor was not apt for a pediatric recipient (4 offers, 16.6%). Donors were declined in relation to quality for numerous reasons, including obesity, a history of alcohol or drug use, tattoos, hypernatremia, and elevated transaminases. Of the cases with donor/recipient size discrepancy, 5 cases involved adult recipients (62.5%). The lack of transfer capacity was due to the fact that the transplantation center did not operate outside of its city (3 cases, 42.8%), the transplant team did not authorize the local team to perform the procurement and did not have the logistical means for transferring their team (3 cases, 42.8%), and transportation time was greater than 6 h, due to the need for a commercial airline connecting flight (1 case, 14.2%).
Problems with the potential recipients were due to medical conditions in potential recipients in 16 cases (16/20, 80%), potential recipient with overly stable status for transplant in 2 cases (2/20, 10%), potential recipient with no financial authorization for transplant in one case (1/20, 5%), and potential recipient unable to be located for the transplantation in one case (1/20, 5%).
Liver graft procurement was performed at public hospitals in 72.7% of the cases, whereas transplantations were performed at private hospitals in 81.8% of the cases. We carried out 50 liver graft offers at public hospitals (3 offers were accepted, 6% acceptance rate) and 38 offers at private hospitals (9 were accepted, 23.6% acceptance rate) (public vs. private: p = 0.016). All offers declined because of “incomplete team” of “lack of a bed in the intensive care unit” corresponded to public hospitals. Of the 20 offers declined because the donor did not meet the transplantation center criteria, 75% came from public hospitals, as did 87.5% of the declined offers due to donor/recipient discrepancy.
Liver grafts from ideal donors were accepted by the hospital in first place on the distribution list in all cases (2 offers from 2 ideal donors, 100%). Liver grafts from ECDs (n = 10) were accepted by the first hospital on the list in 2 cases (20%), whereas in 4 cases, the graft was accepted after ≥10 offers (40%). A mean of 8.6 offers were made for each ECD (maximum 28). The liver grafts considered ideal (n = 5) were accepted by the hospital in first place on the distribution list in 40% (2/5), whereas the ECGs (n = 6) were accepted by the hospital in first place on the distribution list in 33.3% (2/6).
The liver utilization rate in the total of brain-dead donors in Chihuahua was 64.7% during the study period (11/17). The liver utilization rate from the donors evaluated was 78.5% (11/14), given that 21.4% of the donors (3/14) had medical contraindications for liver graft use (hepatic steatosis in all cases) (Fig. 2).
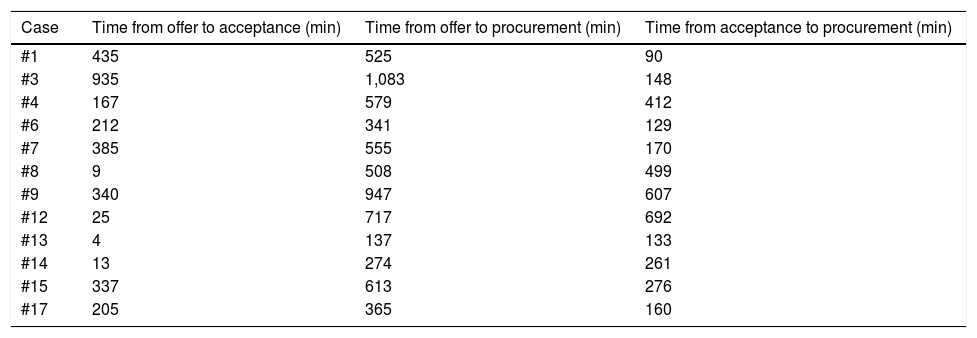
Table 5 shows the time intervals analyzed. The median time from the first offer to liver graft acceptance was 199 min (3 h and 19 min), with a minimum of 4 min and a maximum of 815 min (13 h and 35 min). The median time from liver graft acceptance to the start of procurement was 272 min (4 h and 32 min), with a minimum of 129 min (2 h and 9 min) and a maximum of 692 min (11 h and 32 min). No family withdrew their consent for the donation and no donor had circulatory arrest, within the interval from the signing of consent to procurement.
Time interval in minutes from the first offer to the acceptance of the graft, from the first offer to the beginning of procurement, and from the acceptance of the graft to the beginning of procurement.
| Case | Time from offer to acceptance (min) | Time from offer to procurement (min) | Time from acceptance to procurement (min) |
|---|---|---|---|
| #1 | 435 | 525 | 90 |
| #3 | 935 | 1,083 | 148 |
| #4 | 167 | 579 | 412 |
| #6 | 212 | 341 | 129 |
| #7 | 385 | 555 | 170 |
| #8 | 9 | 508 | 499 |
| #9 | 340 | 947 | 607 |
| #12 | 25 | 717 | 692 |
| #13 | 4 | 137 | 133 |
| #14 | 13 | 274 | 261 |
| #15 | 337 | 613 | 276 |
| #17 | 205 | 365 | 160 |
The grafts retrieved by our team were accepted and used by transplantation centers in Mexico City (4 cases, 36.3%), Hermosillo (2 cases, 18.1%), Guadalajara (2 cases, 18.1%), Monterrey (2 cases, 18.1%), and Chihuahua (one case, 9%). Procurement was performed by our team in all the cases. In one case, the graft was transported in a private airplane flight. The graft was retrieved at one hospital and used at another hospital in the same city. The rest of the grafts (9/11, 81.8%) were transported by commercial airlines. The median cold ischemia time due to transport was 240 min (4 h), with a minimum of 30 min and a maximum of 305 min (5 h and 5 min). The graft that was utilized locally had the shortest transport time and the graft transported in the private plane had the longest. The median transport time for the grafts transported by commercial airline was 240 min (4 h), a minimum of 220 min (3 h and 10 min) and a maximum of 305 min (5 h and 5 min). The transport duration in the commercial flight to Hermosillo was 220 min, to Monterrey was 226 to 275 min, to Guadalajara was 240 to 287 min, and to Mexico City was 240 to 305 min.
One of the potential recipients died during the total hepatectomy and before the implant, and so the liver graft was used in another patient at the same hospital. Follow-up at 30 days revealed initial normal functioning in 10 grafts and one case of early graft dysfunction (case #17). There were no cases of primary nonfunction. All the liver graft recipients survived more than 30 days, with no need for retransplantation.
Discussion and conclusionsThe utilization of kidney grafts from brain-dead donors in Mexico in 2017 was similar to that observed in the United States of America (USA) (USA 7,549/8,591, 90.5% vs. Mexico 499/558, 89.9%), whereas the utilization rate in liver grafts was dramatically different (USA 7,647/8,403, 91% vs. Mexico 168/558, 30.1%).1,14 Given that nationally, only ~20% of the liver grafts from cadaveric donors are discarded for medical contraindications in the donor before procurement,15 the difference between kidney grafts and liver grafts cannot be explained by that problem alone.
In 2017, there were 558 brain-dead donor donations in Mexico, of which 168 liver grafts and 499 kidney (one or both kidneys) grafts (utilization rate 30.1% vs. 89.9%) were retrieved. In 340 cases (61.2%), kidney procurement, but not liver procurement, was performed, whereas in only 9 cases (1.6%), liver procurement, but not kidney procurement, was carried out.1 The main causes of discarding liver grafts from cadaveric donors in Mexico are unrelated to the donor and quality of the graft. The 3 principal general causes, according to the CENATRA, are: lack of report of multiorgan donations (51.6% of cadaveric donations are not reported before procurement), the failure of local committees to make the liver grafts from their donors available (11.2%), and the inability to find a team to perform graft extraction within the time stipulated by the local committee (10.1%).15
In Mexico, it is not obligatory by law to offer a liver graft from a cadaveric donor in a donation process. Instead, it is left to the discretion of the internal donor coordination committee of each hospital to make the offer or not. The committee can even not notify about the donation before procurement, given that according to the current legislature, it can make that notification up to 48 h after the retrieval. Neither the internal committees nor the CENATRA systematically has a liver transplantation expert available, in place or remotely, to evaluate all the potential cadaveric donors. The present study demonstrates that the presence of a local liver evaluation and procurement team increases the number of potential liver donors reported to the CENATRA (12/14, 85.7% of the donations offered), as well as the graft utilization rate (11/14, 78.5%). Evaluation by an expert and adequate medical care enable more aggressive offers (ECDs) to be carried out and a greater number of grafts with good results to be utilized in Mexico.
Consent by the donor’s family to donate kidneys, but not the liver, is frequently cited as a cause for liver graft discard. However, it is difficult to believe that a family that has accepted to donate life to a stranger would selectively authorize the procurement of only some abdominal organs, given that the approach is the same for all of them. In reality, the main obstacle to the offer and distribution of liver grafts for donation teams is the time required for the distribution of the liver and the need to wait for the arrival of a team that has no planned transport logistics before the offer of the organ. The strategy described in the present report shows that if family consent is not withdrawn and there are no events of circulatory arrest during the wait, liver grafts can be offered within a median time of ~9 h (555 min) from the start of the first offer to procurement.
Mexico does not have an air transport system for organs. The cost of private executive flights limits the capacity to utilize liver graft offers, at the national level. The time involved when using commercial flights is often a detractor, given that a round trip is required so the team from the transplantation center can perform the procurement and return to the hospital to carry out the transplant. Few cities in the country have the number of commercial flights needed for that to happen. Our study showed that the use of commercial flights made it possible for the local team to transport the retrieved liver grafts, with cold ischemia times < 6 h, to all the active liver transplantation centers in Mexico, within the study period.
The evaluation protocol was modified during the study period, after the detection of macrovesicular steatosis >30% in donor #6. It became clear that the grade of alcohol use was underestimated in the donor, who had no other risk factors for fatty liver. After that case, we performed ultrasound on all donors, even if they had an imaging study carried out with another method.
Ours is the first report on the specific causes for rejecting liver graft offers from cadaveric donors in Mexico and is limited by the small number of cases. All the ideal donors were accepted by the first hospital center to which they were offered, whereas more than 8 offers were made per case to place the grafts coming from ECDs (up to 28 offers in one case).
The utilization rate of private hospitals was almost 4 times higher than that of the public hospitals combined. During the study period, twice as many liver transplantations were performed at public hospitals than at private hospitals (160 vs. 80),1 making it very unlikely that the trend observed in our study would be reproduced at the national level. The main difficulty for interpreting that finding is that we do not know the percentage of ECDs in Mexico, their acceptance rate, or the causes of declined liver graft offers by hospital centers, nationally. The declined offers from ECDs by public hospitals could be a reflection of the attempt to administer scant resources, given that even though grafts from ECDs are associated with the same long-term survival, they are also associated with greater morbidity and resource use.16,17 Another factor that could explain said phenomenon is the expense of organ transport. Although the cost of commercial flights is dramatically lower than private flights, most public hospitals cannot afford them.
In conclusion, the implementation of a local evaluation and procurement team increased the total number of liver grafts for liver transplantation, as well as their relative use. The lack of an air transport system for transplantation purposes in Mexico was partially resolved through effective coordination and the use of commercial flights.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Zamora-Valdés D, et al. Utilización de injertos hepáticos de donantes cadavéricos: impacto de la implementación de un equipo local de valoración y procuración en México. Rev Gastroenterol México. 2021;86:220–228.