Crohn’s disease (CD) is a chronic inflammatory disease, characterized by periods of activity and remission, many cases of which require personalized treatment.1
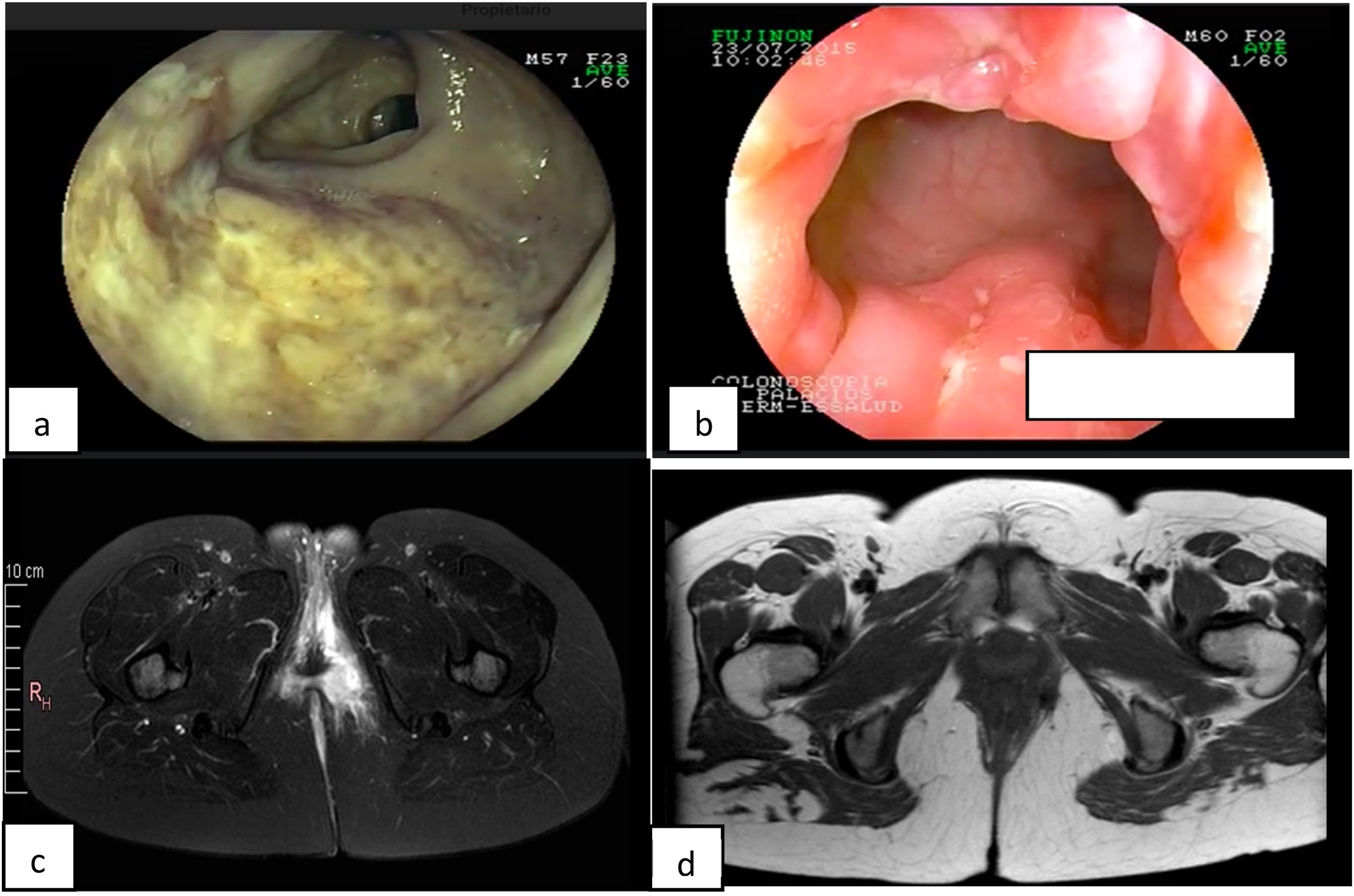
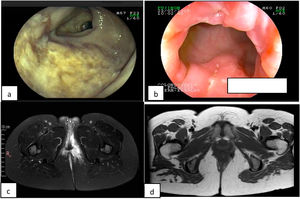
A 34-year-old woman had an 8-year history of CD. She initially presented with a moderate bout of the disease, with ileocolonic involvement and anal fissures (Fig. 1a and b). Induction therapy was infliximab 5 mg/kg IV at weeks 0, 2, and 6, followed by 5 mg/kg IV infusion every 8 weeks as maintenance therapy. The patient was in clinical and endoscopic remission for one year, after which she presented with numerous bouts of mild disease, characterized by deep anal fissures that remitted with prednisone 1 mg/kg/d VO, but became corticoid dependent. She then presented with a left-sided perianal abscess and 2 complex perianal fistulas that were treated with ciprofloxacin 500 mg every 12 h and metronidazole 500 mg every 8 h for 3 months, along with setons for 8 months, curing said complications (Fig. 1c and d). The patient continued to present with several mild bouts of anal fissures that improved with prednisone 1 mg/kg/d, but they were corticoid dependent. Infliximab dose was increased to 10 mg/kg IV every 4 weeks, combined with azathioprine 2 mg/kg/d. At one year there was no clinical improvement, and so anti-infliximab antibodies and serum infliximab levels were measured (0.1 AU/mL and 17.74 µg/mL, respectively) and the patient was considered to have secondary failure. Medication was switched to ustekinumab 390 mg IV and then 90 mg SC every 8 weeks, achieving clinical and endoscopic remission at 6 months. The patient remains in remission at the follow-up visit at 2 years.
Anti-tumor necrosis factor agents provide significant and lasting remission in CD. However, 30% of patients do not respond to induction therapy and another 30% lose response during the first year of treatment, leading to the recommendation of switching biologics to agents, such as ustekinumab,2 which inhibits interleukin 12 and interleukin 23 in the inflammatory pathway.3 Perianal CD is a therapeutic challenge with high failure rates. Recent studies support the superior effectiveness of ustekinumab, compared with other biologic treatments.4–6 Importantly, all the studies reported that ustekinumab safely maintained clinical response and remission in patients with CD. Likewise, short-term and long-term treatments were beneficial in patients with refractory disease, avoiding invasive procedures (surgical therapy).7,8
In conclusion, ustekinumab has been shown to be a good option for achieving remission in both bio-naïve and bio-failure patients, as occurred in our patient presenting with a complex case of perianal CD.
Ethical considerationsThe authors declare that no experiments were conducted on humans or animals for the present study, that they have followed the protocols of their work center on the publication of patient data, and that they have preserved patient anonymity at all times (thus informed consent was not requested). This study meets the current bioethical research norms.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Alcántara-Figueroa CE, Coronado-Rivera EF, Estela-Vásquez EF, Calderón-Cabrera DC, Alcántara-Ascón RA. Ustekinumab y remisión en enfermedad de Crohn con compromiso perianal: reporte de un caso. Rev Gastroenterol Mex. 2023;88:431–432.