Introduction
Inflammatory bowel diseases (IBD) are chronic inflammatory conditions of the gastrointestinal tract which clinically present as one of two disorders: Crohn's disease (CD) or ulcerative colitis (UC). In a short proportion of patients differentiation between CD and UC is not possible; these cases constitute what is called indeterminate colitis.1,2 There is increasing evidence about the association of serological markers with specific forms of IBD, disease behavior and phenotype of IBD. Anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are serological markers associated with CD and UC, respectively. Combined measurement of pANCA and ASCA has been suggested as a valuable diagnostic approach for discriminating CD and UC. Several antibodies are produced against various antimicrobial products and autoantigens. This antibody production may be explained by loss of the immune tolerance, which triggers or maintain the inflammatory process of IBD.
Here we describe and analyze the most recent evidence of the utility of traditional and new sero-logic markers in patients with IBD, making special emphasis on their clinical utility. Furthermore, when it is allowed by actual evidence, we comment about the behavior of antibodies between populations, as there is thought that a wide range of variations of the immunological, genetic and environmental patterns can exist.3,4
The objective of this review is to provide a summarized and practical view of the clinical utility of serological markers in IBD.
Material and methods
We performed a computer-assisted search in Pub-Med with the key words "inflammatory bowel disease", "serological markers", "antibodies", "Latin-American", and "Hispanic" since January 1979 to December 2008. We selected studies that include large cohort of patients and that have shown some practical utility. Review articles, letters, and memories of medical meetings were also included. We excluded abstracts, personal communications and articles published in any other language different to English and Spanish.
Results
Antibodies in inflammatory bowel disease
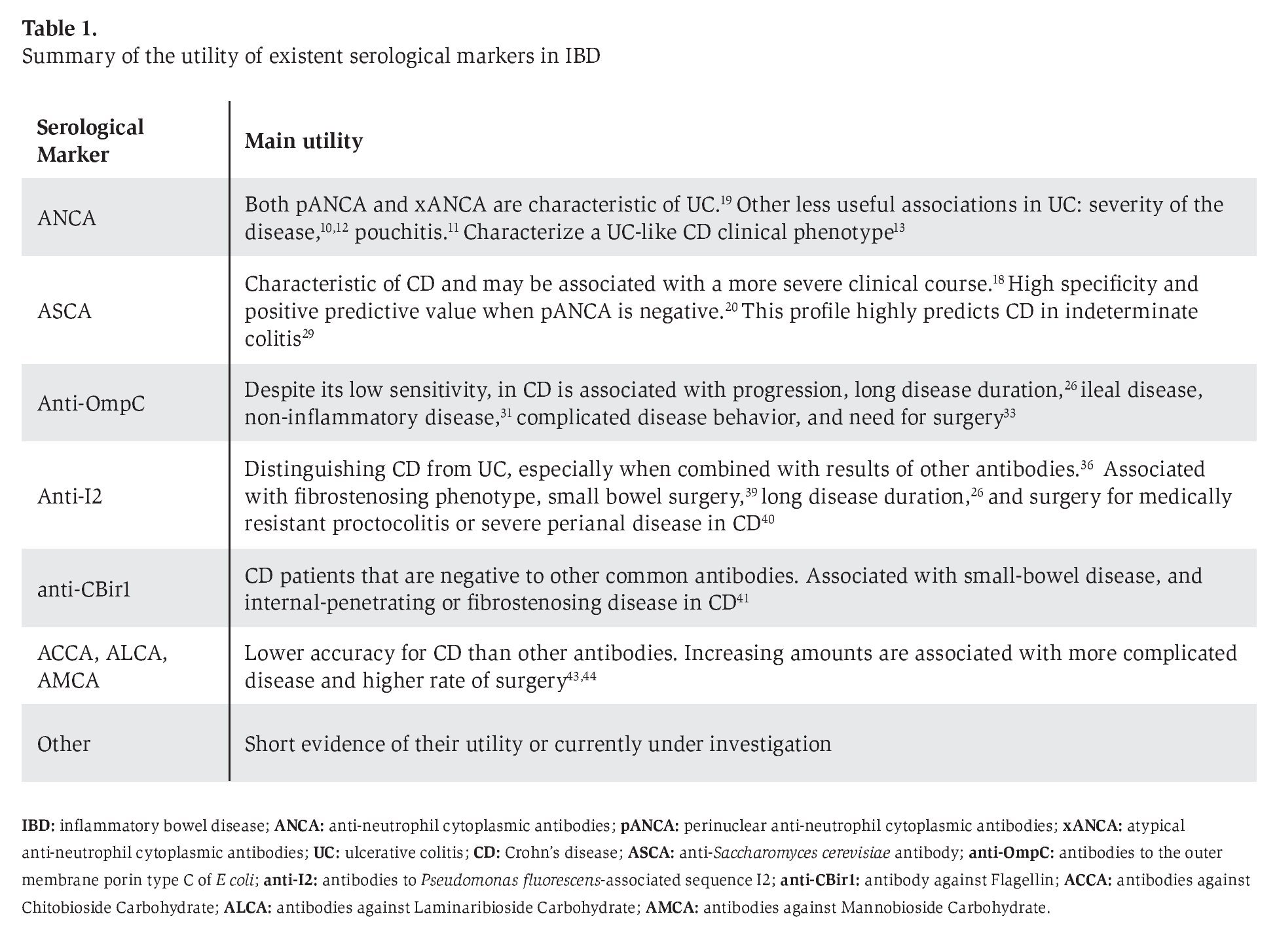
At present, serologic antibodies lead the pack for distinguishing between IBD and non-IBD, CD and UC, and for stratifying CD phenotypes (Table 1). The pANCA, atypical ANCA (xANCA), and ASCA are the best studied. They have the highest accuracy on distinguishing between CD and UC and they also are used to identify certain phenotypes.
Other serologic tests of special and crescent interest include: antibodies against the outer membrane porin type C of E. coli (anti-OmpC), antibodies against a protein expressed by Pseudomonas fl uorescens(anti-I2), antibody against flagellin (anti-CBir1), antibodies to chitobioside carbohydrate (ACCA), laminaribioside carbohydrate (ALCA), mannobioside carbohydrate (AMCA), antibodies against exocrine pancreas (PAB), anti-calreticulin antibodies (anti-CRT), anti-alpha-enolase antibodies, antibodies against porcine pancreatic amylase (anti-PPA), and antinuclear antibodies (ANAs). These antibodies are less used in the distinction between CD and UC, but they have a promising role on identifying phenotypes, especially when they are combined, or when pANCA, x-ANCA, and ASCA bring insufficient information regarding the diagnosis of a subtype of IBD.
It is important that pANCA, xANCA, and ASCA support the diagnosis of a specific subtype of IBD, but they may not be sufficiently sensitive or specific to be practical as screening tools or for routine clinical use.5,6 Because pANCA and ASCA may be present years before IBD is clinically diagnosed, or may be present in other diseases, clinicians must use them and interpret their results with caution and diligence. The indiscriminate use of serological assays will predictably lead to inappropriate treatment of individuals who do not have UC or CD, as well as delay the diagnosis and treatment of individuals who have negative serology.7,8
Anti-Neutrophil Antibodies
The anti-neutrophil antibodies (ANCA) and their patterns of reactivity are present in a wide range of autoimmune diseases. Prevalence of pANCA in IBD varies widely suggesting differences between populations in the autoimmune patterns of the disease. A prospective study between two different populations showed a pANCA prevalence in UC of 44% for Chinese and 64% for Caucasian patients (p=0.046), and for CD of 14% for Chinese and 10% for Caucasian patients (p=NS). Given these data, sensitivity, specificity, positive predictive value and negative predictive value to distinguish UC patients from controls are different (44%, 94%, 88% and 63%, respectively for Chinese, and 64%, 94%, 91% and 72% for Caucasian UC patients).9 In Latin-American patients with UC the prevalence of pANCA is around 50%.10 Based on the higher prevalence of this antibody in UC than CD patients, its main utility remains on distinguishing between forms of IBD and probably on identifying subgroups of patients. Some other associations have been described among UC patients, but their clinical usefulness remains limited because pANCA may not offer additional information than the obtained with clinical methods, for example, its association with severity of the disease,10 development of chronic pouchitis after ileo-anal pouch,11 and treatment-resistant left-sided UC.12 In CD patients, pANCA has less utility, its expression only had shown to characterize a UC-like clinical phenotype.13
Atypical ANCA (xANCA) is present in the sera of 59 to 84% of patients with UC and in 10 to 20% in patients with CD,14,15 and a higher prevalence have been reported in other autoimmune diseases.16,17 Recently, Desplat-Jégo et al. confirmed that in French patients there is higher frequency of this antibody in UC (71.8%) compared with CD patients (11%) and healthy blood donors (0%). Furthermore, in an attempt to increase its utility, combination with ASCA results were analyzed, showing that negativity of xANCA and positivity of ASCA have a sensitivity of 46.2%, specificity of 97.6%, positive predictive value of 94.2%, and negative predictive value of 68.7% for CD.18 It seems that the prevalence of this antibody is lower in Latin-American patients. In a recent study that included 184 IBD Mexican patients (160 patients with UC and 24 patients with CD) found that the sensitivity, specificity, positive predictive value and negative predictive of xANCA for the diagnosis of UC is 50, 96, 99 and 22%, respectively; while for the diagnosis of CD is 4, 50, 1 and 78%, respectively.19 The utility of xANCA, alone or in combination with ASCA, remains on distinguishing between CD and UC, but its clinical utility has not been widely studied; further studies are necessary to determine other potential clinical uses.
Anti-Saccharomyces cerevisiae Antibodies
A positive test for anti-saccharomyces cerevisiae antibodies (ASCA) IgA or IgG is significantly more frequent in CD (49.5%) compared with UC (5.1%) or healthy blood donors (4%).18 Combination of a positive ASCA test with a negative pANCA test has a positive predictive value of 96% and a specificity of 97% for CD.20
Several studies have shown that ASCA is present in 20% to 25% of first-degree relatives of patients with CD, suggesting that generation of ASCA may be related to genetic influences although environmental factors may also play a certain role.21,22 The stability of ASCA, and its non-induction after oral yeast exposure in murine models suggests that environment may play only a minor role in inducing ASCA.23 As occurs with pANCA, it seems that there is a variation between ethnic groups on ASCA sensitivity and predictive vales. Lawrence et al found this variation particularly on IgA between Chinese and Caucasian populations, therefore careful interpretation of this test is necessary between populations.9
Israeli et al detected ASCA in 31% of patients before the clinical diagnosis of CD. Furthermore, an increase in ASCA frequency was observed over time, with the highest frequency during the 36 months before diagnosis.7 Titers of ASCA were though not to correlate with disease activity and seemed to be stable over long periods, even after achieving remission.23,24 But in contrast, a recent study showed that positivity of ASCA is associated with active CD (37.9 vs. 0%), a more severe clinical profile suggested by anal complications (perianal abscess, anal fissure or fistula), and age of onset under 20 years. ASCA levels are not different between treated and non-treated patients.18 Other clinical parameters that correlate with ASCA positivity are ileal involvement of disease, penetrating as well as stricturing disease behavior, disease progression, and development of postoperative fistulas following ileal pouch-anal anastomosis. 9, 25-27 In children and young adults, ASCA is also highly specific for CD, and can identify a subset of children with disease of the ileum and ascending colon who may be at increased risk for surgery.28
This antibody determination is also of interest in patients with indeterminate colitis (IC). Despite 48.5% of these patients do not develop ASCA or pANCA, the ASCA+/pANCA- profile predicts CD in 80% of patients with IC colitis and ASCA-/pANCA+ predicts UC in 63.6%.29
Antibodies to the Outer Membrane Porin type C of E coli
It seems that antibodies to the outer membrane porin type C of E coli (anti-OmpC) is a heritable immunophenotype as the expression in unaffected relatives of CD patients is high.30 The prevalence of anti-OmpC has been reported from 31% to 55% in CD patients,26,31,32 24% of UC patients, and 20% of healthy controls, and its main utility remains on the differentiation between CD and UC patients.31
In children and young adults, a positive test for anti-OmpC is present in 24% of CD and 11% of UC patients, and displayed a 5% false-positive rate. However, anti-OmpC identified a small number of IBD patients not detected by other assays (7%). This low sensitivity rises to 65% for CD, with a specificity of 94% if anti-OmpC test is combined with determination of other antibodies (ASCA, DNase-sensitive and pANCA).28
In CD patients, anti-OmpC demonstrated correlation with progression, long disease duration,26 ileal disease, and non-inflammatory disease, but not with risk for surgery, or response to steroids or infliximab.31 In counterpart, Papp et al recently found in a large cohort of eastern European CD patients that is associated with a more complicated disease behavior and need for surgery.33
In a prospective study of pediatric CD patients, a positive test for anti-OmpC was associated with development of internal penetrating and/ or stricturing disease after a median follow-up of 18 months, suggesting that immune responses to microbial antigens are associated with more aggressive disease phenotypes.34
In patients with indeterminate colitis the prevalence of anti-OmpC is 17.2%, while for healthy controls was 2.2%. Sensitivity, specificity, positive predictive value, and negative predictive value were 17.2%, 88.5%, 47.1% and 64.4%, respectively.35 Therefore, this antibody could be useful, but probably not more other tools. We think that future studies are required in order to establish correlation or association with genetic phenotype.
Antibodies to Pseudomonas fluorescens-associated sequence I2
These antibodies are present in 50% of CD patients,32 and its main role remains in differentiating between CD and UC.36 Its utility rises if results are combined with tests for antibodies to Bacteroides caccae TonB-linked outer membrane protein (OmpW) and ASCA (sensitivity 94% for CD), or anti-OmpW and pANCA (sensitivity 83% for UC).36
A study performed to determine if defects in innate immunity are similarly associated with increased adaptive immune responses to microbial antigens found that anti-I2, ASCA, anti-OmpC, and antibody against flagellin (CBir1) increase in frequency as NOD2 gene variant carriage increase in CD patients, suggesting that patients carrying this gene variant have increased adaptive immune responses to microbial antigens.37 Reactivity against microbial components lead to think in a theoretical response to antibiotics in CD patients; but no statistically significant response was noted in a group of patients treated with steroids plus antibiotics when compared with the group that received only steroids.38
Anti-I2 has shown association with fibrosteno-sing phenotype, small bowel surgery,39 long disease duration,26 and clinical response to fecal diversion indicated for medically resistant proctocolitis or severe perianal disease in CD patients.40
In children with CD, anti-I2, as well as anti-OmpC, has shown association with internal penetrating and/or stricturing disease. This immune reactivity patterns predicted a faster development of disease complications suggesting that the presence and magnitude of immune responses to microbial antigens are significantly associated with more aggressive disease phenotypes.34
In patients with indeterminate colitis the prevalence of anti-I2 has been reported in 41.9% while in healthy controls in 17.2%. The sensitivity, specificity, positive predictive value, and negative predictive value of anti-I2 in indeterminate colitis were 41.9%, 76.4%, 48.1% and 71.6%.35
Antibody against Flagellin
This is present in 50% of CD patients, 6% of UC patients, and 8% of healthy controls. It is also present in 46% of ASCA-, 64% of ASCA+, and in 38% of seronegative CD patients (ASCA, anti-OmpC and ANCA),41 so its utility may remain on the subset of patients with CD that are negative to other more common antibodies.
The presence and levels of anti-CBir1 are associated with CD and with small-bowel disease, and internal-penetrating or fibrostenosing disease features.41 Quantitative, but not qualitative, response to CBir1 is also significantly associated with the CD-associated NOD2 variants.42
Antibodies against Chitobioside Carbohydrate, Laminaribioside Carbohydrate, and Mannobioside Carbohydrate
Initial findings of the utility of antibodies against chitobioside carbohydrate (ACCA), Laminaribio-side Carbohydrate (ALCA) and Mannobioside Carbohydrate (AMCA) determined that these antibodies have lower accuracy for CD than ASCA and/or pANCA. Interestingly increasing amounts were associated with more complicated disease behavior, and a higher frequency of CD-related abdominal surgery, suggesting that the number and magnitude of immune responses to different microbial antigens are associated with severity of disease.43,44 These results were confirmed recently by Papp et al who also found association with NOD2/CARD15 genotype,33 supporting the finding that variants in innate immune receptor genes influence these antibodies formation.45
It seems that there is a complex interplay between antibodies and innate immunity that is not completely understood, but there may remain the trigger for IBD or for the development of certain disease phenotype.
Antibodies to Exocrine Pancreas
The antibodies to exocrine pancreas (PAB) are present in 26 to 39% of CD patients, 4% to 23% of UC patients, 22% of their unaffected first-degree relatives, 13% of celiac disease patients, and 0% to 4% of healthy controls.18,46-49 PAB gets higher accuracy when combined with other antibodies: positivity of PAB and/or ASCA combined with negativity for tissue transglutaminase antibody yields a sensitivity, specificity, positive and negative predictive value of 60%, 100%; 100% and 90% respectively, regarding the diagnosis of CD.50
There was found that PAB is highly specific for CD and is associated with long disease duration and early onset of disease, but differences in their prevalence in CD subtypes suggest that is not useful in the discrimination of CD phenotypes.18,51 PAB titers have shown to be dependent on the inflammatory activity but independent on the therapy.46
Anti-Calreticulin Antibodies
The anti-calreticulin antibodies (anti-CRT) are present in a variety of autoimmune diseases.52,53 The prevalence of this antibody is about 30% in IBD, and is significantly higher in the initial phase of UC.54 There are needed more studies to determine its clinical utility.
Anti-Alpha-Enolase Antibodies
Antibodies against alpha-enolase have been detected in a large variety of infectious and autoimmune diseases and seems to arise as a consequence of a microbial infection or uncontrolled cell growth or proliferation in specific organs.55 Anti-alpha-enolase antibodies are present in 49% of UC patients, 50% of CD patients, 30.5% of primary sclerosing cholangitis patients, 37.8% of autoimmune hepatitis patients, 34% of ANCA-positive vasculitides, 31% of non-IBD gastrointestinal controls, and 8.5% of healthy controls. Therefore, anti-alpha-enolase antibodies are of limited diagnostic value for the diagnosis of IBD.56
Antibodies to Porcine Pancreatic Amylase
These antibodies are present in 38% of CD patients (especially in patients with small bowel disease), in 9% of UC patients, and in 5% of healthy controls, suggesting that dietary proteins could play a role in the inflammatory response of CD patients with small bowel disease. The antibodies to porcine pancreatic amylase (Anti-PPA) combined with ASCA and anti-I2 may be useful for the diagnosis of CD as 72% of these patients were found positive for at least one antibody.57 Clinical utility or usefulness on distinguishing CD phenotypes remains unknown.
Antinuclear antibodies
The antinuclear antibodies (ANAs) are present in a wide range of autoimmune disorders. Folwaczny et al reported that the prevalence of ANAs in UC and CD patients is 43% and 18%, respectively.58 Recently, there were found in Mexican UC patients that ANAs were positive in 53.6%, and were associated with steroid dependence. Further studies are necessary to confirm its utility.59
Conclusions
Serological markers are useful for distinguishing between IBD and non-IBD, and between forms and phenotypes of IBD, but they are far to be perfect. They have several limitations, especially because of the variability of results and lack of technique standardization. Furthermore, as we mentioned above, differences between populations make difficult their application and interpretation. Finally, more studies are warranted to establish the complete utility of these antibodies.
Correspondence author: Dr. Josue Barahona-Garrido.
Instituto de Enfermedades Digestivas y Nutricionales. Avenida Reforma 7-62 zona 9, Edificio
Aristos Reforma, oficina 109. CP 01009. Guatemala City, Guatemala. Telephone/Fax: +502-23859606.
E-mail: gastromedic@gmail.com
Received: January 13th, 2009 Accepted on: May 26th, 2009