Eosinophilic esophagitis (EoE) is a chronic immune-mediated inflammatory disease that affects the esophagus. Its epidemiology in Chile and Latin America is unknown due to the absence of population studies. Our aim was to describe the clinical, endoscopic, and histologic characteristics of adult patients diagnosed with EoE, as well as their treatment response.
Material and methodsA descriptive prospective study was conducted on a cohort of patients ≥ 18 years of age with an eosinophil count greater than 15 eosinophils/high power field.
ResultsA total of 62 patients were included, 75.8% of whom were men. Mean patient age was 38 years, mean age at diagnosis was 34 years, and diagnosis was made later in men. Sixty-five percent had a concomitant immunoallergic disease, and allergic rhinitis was the most frequent. Dysphagia was the most frequent referral, with a predominance of men. Women presented more often with food allergies and peripheral eosinophilia. The most frequent endoscopic finding was edema, followed by rings, with a mean eosinophilic esophagitis endoscopic reference score (EREFS) of 3.5 and a mean eosinophil count in biopsies of 37.5 eosinophils/high power field. Men presented with a higher EREFS and eosinophil count at diagnosis. All patients received treatment and the most frequent was with proton pump inhibitors, followed by combination treatment with corticosteroids. Endoscopic (partial/total) and histologic response rates were 93.5 and 77%, respectively.
ConclusionWe found characteristics in our cohort similar to those described in international groups. Women presented with greater autoimmune comorbidity, peripheral eosinophilia, and food allergies, but had a lower eosinophil count and endoscopic score. We found no differences between the different therapeutic regimens.
La esofagitis eosinofílica (EEo) es una enfermedad inflamatoria crónica inmunomediada que afecta al esófago. En Chile y Latinoamérica se desconoce su epidemiología debido a la ausencia de estudios poblacionales. El objetivo de es describir características clínicas, endoscópicas e histológicas de pacientes adultos con el diagnóstico de EEo, así como la respuesta al tratamiento.
Material y metodosEstudio descriptivo prospectivo de cohorte pacientes ≥ 18 años con recuento mayor a 15 eosinófilos/campo.
Resultados62 pacientes, 75,8% hombres edad promedio 38 años, edad promedio al diagnóstico 34 años, siendo más tardío en hombres; 65% tenía otra enfermedad inmunoalérgica concomitante, siendo más frecuente rinitis alérgica. El motivo de consulta más frecuente fue disfagia, predominando en hombres. Mujeres presentaron mayor frecuencia de alergias alimentarias y eosinofilia periférica. El hallazgo endoscópico más frecuente fue edema seguido de anillos con un puntaje EREFS (Eosinophilic Esophagitis Endoscopic Reference Score) promedio de 3,5 y un recuento promedio de eosinófilos en biopsias de 37,5 eosinófilos/campo. Hombres presentaron EREFS y recuento de eosinófilos más altos al diagnóstico. Todos los pacientes recibieron tratamiento, siendo lo más frecuente el uso de inhibidores de la bomba protones seguido por tratamiento combinado con corticoides. La tasa de respuesta endoscópica (parcial/total) e histológica fue 93,5% y 77% respectivamente.
ConclusiónEn esta cohorte, observamos características similares a lo descrito en grupos internacionales. Las mujeres presentaron mayor comorbilidad autoinmune, eosinofilia periférica y alergias alimentarias pero un menor recuento de eosinófilos y score endoscópico. No observamos diferencias entre los distintos esquemas terapéuticos.
Eosinophils can be physiologically present in the entire gastrointestinal tract, but under normal conditions, they are not found in the esophageal mucosa. The presence of eosinophils in the esophageal squamous epithelium can be secondary to several systemic or local diseases, of which eosinophilic esophagitis (EoE) and gastroesophageal reflux disease stand out.1 EoE is an immune-mediated chronic inflammatory disease that exclusively affects the esophagus and is defined by symptoms secondary to esophageal dysfunction in the presence of more than 15 eosinophils/high power field in the esophageal mucosa and in the absence of other diseases associated with an increase in eosinophils in the esophageal epithelium.1,2 Initially considered a rare entity, studies have shown that its incidence and prevalence have increased in recent decades, especially in the West.3,4 A meta-analysis published in 2023, using diagnostic criteria from 2018, that included 40 studies primarily from North America and Europe, showed an incidence of 5.31/100,000 per year and a prevalence of 40.04/100,000 in adults.4 Said incidence is likely due to a greater knowledge about the disease, with higher esophageal biopsy rates. Nevertheless, evidence suggests an increased incidence beyond having greater awareness of the disease, a phenomenon that has also been described in other atopic diseases.5 In Chile and Latin America, its epidemiology is unknown due to the absence of population studies and the fact that there are very few reported cases.6–9 Between approximately 2 and 7% of adults undergoing upper gastrointestinal endoscopy due to suspected EoE, in whom biopsies are taken, meet the histologic criteria for EoE.10,11
Thus, considering the current evidence on the increase of persons affected by EoE, the unfavorable clinical evolution that can develop without adequate treatment, and the lack of local clinical studies, our aim was to describe the clinical aspects and treatment of patients with EoE that were evaluated in the celiac disease and immune-mediated gastrointestinal diseases program of our university center.
Material and methodsA descriptive, prospective study was designed that included patients diagnosed with EoE, seen in the celiac disease and immune-mediated gastrointestinal diseases program of the Clínica Universidad de los Andes, within the time frame of September 2021 and July 2023. The STROBE verification checklist for observational cohort studies was utilized. Patients ≥ 18 years of age diagnosed with EoE, according to clinical, endoscopic, and histologic criteria,1,2 were included in the study. The diagnosis was made from a minimum of 6 esophageal biopsies, including the distal and proximal esophagus, that showed an increase in the number of eosinophils ≥ 15 eosinophils/high power field. Patients were to suspend the use of proton pump inhibitors at least 7 days before the index biopsy was taken. The findings of the upper gastrointestinal endoscopy were systematized, utilizing the Eosinophilic Esophagitis Endoscopic Reference score (EREFS), which describes the presence of exudates, rings, edema, furrows, and strictures.12,13 Treatment was evaluated by the symptomatic, endoscopic, and histologic response; the histologic response was considered successful if the eosinophil count was below 15 eosinophils per high power field.14 Partial endoscopic response was considered when there was a reduction of at least one point on the EREFS and total endoscopic response was considered when the endoscopic aspect of the esophagus was normal. Follow-up was multidisciplinary, involving gastroenterologists, nutritionists, and immunologists. Patients that presented with another systemic or local disease that could explain the symptoms, with histologic alterations in the esophageal mucosa, or with the presence of systemic eosinophilia were excluded from the study.
Statistical analysisDescriptive statistics were carried out to report the results of the study variables. The continuous variables were described through median and range and the categorical variables were determined through absolute and relative percentage frequency. Comparative chi-square statistics were utilized, and statistical significance was set at a p ≤ 0.05. The Stata® v.12 statistics program was employed.
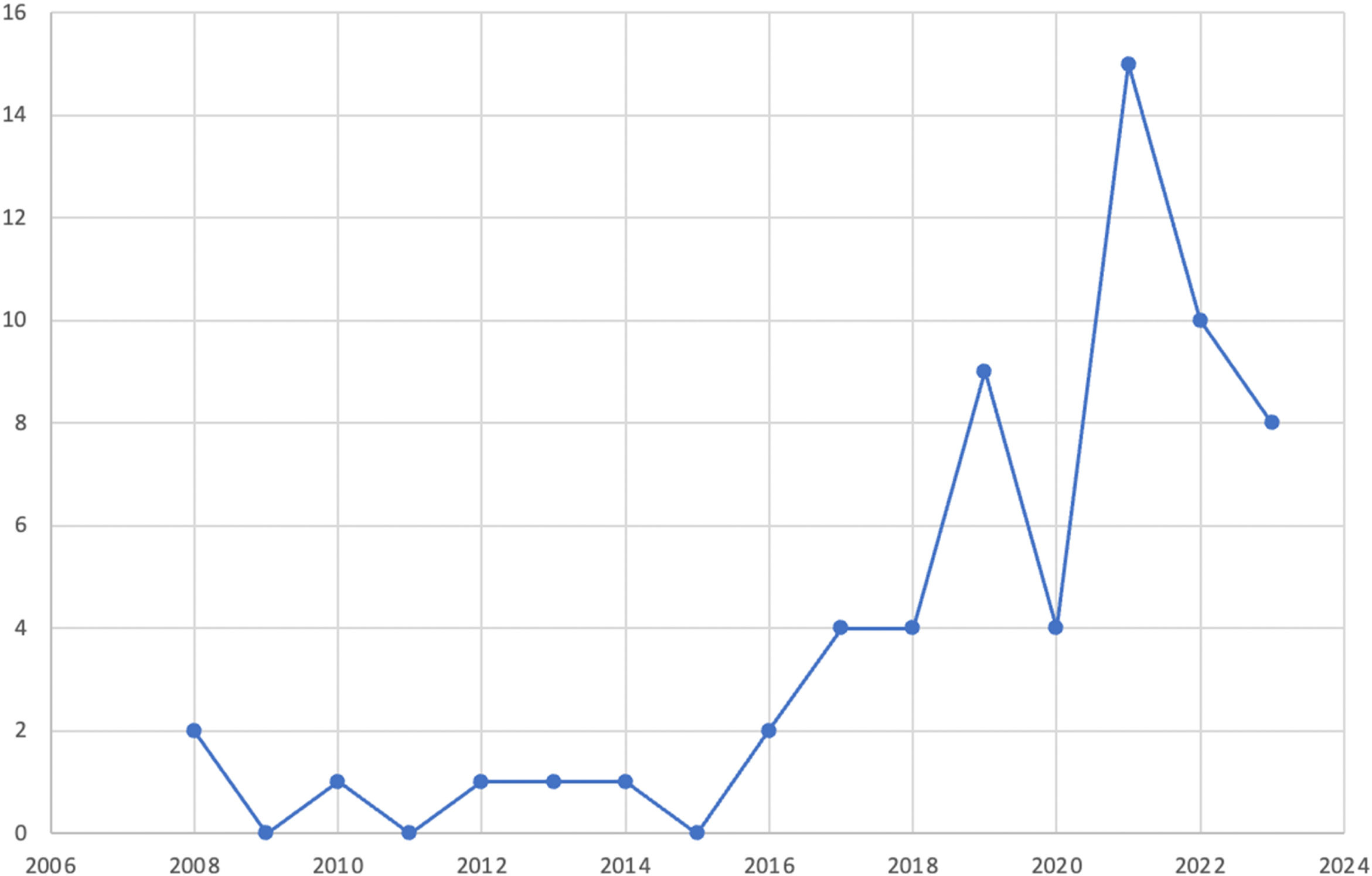
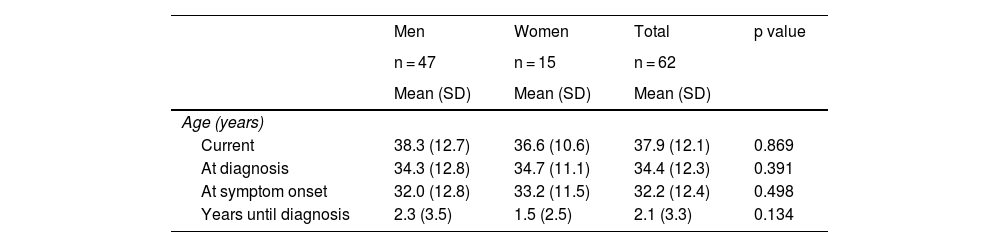
ResultsThe study included 62 patients, 75.8% of whom were men. Mean patient age was 38 years (range 18-77) and an increase in cases diagnosed over the past 4 years was observed. Mean patient age at diagnosis was 34 years, and diagnosis was made later in men. The most frequent concomitant immunoallergic disease was allergic rhinitis. The most common reason for consultation was dysphagia, followed by heartburn. Peripheral eosinophilia was significantly higher in women (> 0.5 × 109/l), at 33 vs 6.4% in men, p = 0.007. Twenty-two percent of the patients presented with a food allergy, more frequently in women (67 vs 26%, p = 0.004), associated with fish-seafood, dairy products, and foods with a high lipid content (defined at evaluation by the nutritional team). Regarding procedures, the patients presented with a median of 4 points on the EREFS and 25 eosinophils/high power field in the eosinophil count in the biopsies. Table 1 shows the demographic and clinical characteristics of the patients.
Demographic and clinical characteristics of the patients with eosinophilic esophagitis.
| Men | Women | Total | p value | |
|---|---|---|---|---|
| n = 47 | n = 15 | n = 62 | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | ||||
| Current | 38.3 (12.7) | 36.6 (10.6) | 37.9 (12.1) | 0.869 |
| At diagnosis | 34.3 (12.8) | 34.7 (11.1) | 34.4 (12.3) | 0.391 |
| At symptom onset | 32.0 (12.8) | 33.2 (11.5) | 32.2 (12.4) | 0.498 |
| Years until diagnosis | 2.3 (3.5) | 1.5 (2.5) | 2.1 (3.3) | 0.134 |
| N (%) | N (%) | N (%) | ||
|---|---|---|---|---|
| Reason for consultation | 0.019* | |||
| Dysphagia | 30 (63.8) | 7 (46.7) | 37 (59.7) | |
| Heartburn | 10 (21.3) | 3 (20) | 13 (21) | |
| Pain | 0 | 3 (20) | 3 (4.8) | |
| Other | 7 (14.9) | 2 (13.3) | 9 (14.5) | |
| Emergency endoscopy | 10 (21.3) | 4 (26.7) | 14 (22.6) | 0.664 |
| Immunologic evaluation | 13 (27.7) | 9 (60) | 22 (35.5) | 0.023* |
| Food allergy | 12 (25.5) | 10 (66.7) | 22 (35.5) | 0.004* |
| Cereals and gluten | 5 (10.6) | 4 (26.7) | 9 (14.5) | 0.125 |
| Legumes | 3 (6.4) | 3 (20) | 6 (9.7) | 0.120 |
| Fruits and vegetables | 4 (8.5) | 1 (6.7) | 5 (8.1) | 0.819 |
| Foods rich in lipids | 5 (10.6) | 6 (40) | 11 (17.7) | 0.010* |
| Fish and seafood | 2 (4.3) | 5 (33.3) | 7 (11.3) | 0.002* |
| Dairy products | 1 (2.1) | 3 (20) | 4 (6.5) | 0.014* |
| Eggs | 2 (4.3) | 1 (6.7) | 3 (4.8) | 0.705 |
| Other allergies (ATB, pollen, latex) | 9 (19.1) | 5 (33.3) | 14 (22.6) | 0.253 |
| Concomitant immunoallergic disease | ||||
| Allergic rhinitis | 27 (57.4) | 5 (33.3) | 32 (51.6) | 0.104 |
| Asthma | 10 (21.3) | 2 (13.3) | 12 (19.4) | 0.498 |
| Dermatitis | 6 (12.8) | 3 (20.0) | 9 (14.5) | 0.489 |
| Eosinophilic colitis | 1 (2.1) | 1 (6.7) | 2 (3.2) | 0.386 |
| Celiac disease | 1 (2.1) | 0 | 1 (1.6) | 0.569 |
| Peripheral eosinophilia | 3 (6.4) | 5 (33.3) | 8 (12.9) | 0.007* |
| Initial treatment | ||||
| Elimination diet | 1 (2.1) | 1 (6.7) | 2 (3.2) | 0.686 |
| Corticosteroids | 1 (2.1) | 0 | 1 (1.6) | |
| Proton pump inhibitors | 27 (57.4) | 7 (46.7) | 34 (54.8) | |
| Mixed (PPI + corticosteroids) | 18 (38.3) | 7 (46.7) | 25 (40.3) |
| Me (Min-Max) | Me (Min-Max) | Me (Min-Max) | ||
|---|---|---|---|---|
| Procedures | ||||
| Initial endoscopic score (EREFS) | 4 (1–7) | 4 (0-5) | 4 (0-7) | 0.602 |
| Initial biopsy eosinophils | 25 (15-100) | 30 (20-100) | 25 (15-100) | 0.565 |
Data are expressed through frequencies (absolute [N] and relative percentage [%]) and measures of central tendency.
Mean (x¯); ATB: antibiotic; EREFS: Endoscopic Reference Eosinophilic Esophagitis Score; Me: median; range: minimum and maximum; SD: standard deviation.
Kolmogorov-Smirnov normality test, non-normal distribution. Pearson chi-square tests.
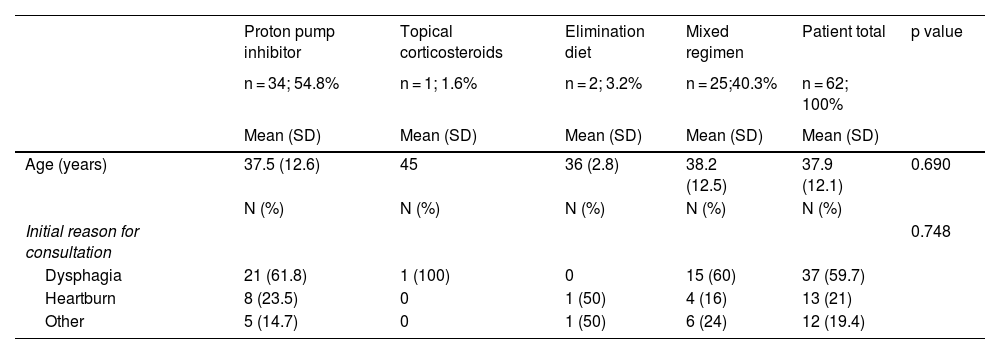
All patients received permanent treatment, with control endoscopy at a mean of 6 months (range 2-12 months); 2 patients had an elimination diet (dairy products, gluten, peanuts, soy), 34 had exclusive PPI treatment (40 mg of esomeprazole), one patient had exclusive topical corticosteroid treatment (1 mg of budesonide in artificial saliva after breakfast and 1 mg in artificial saliva after dinner), and 25 patients received combination therapy (PPIs, diet, and topical corticosteroids; 2 mg of budesonide in artificial saliva daily was the most frequently used corticosteroid). Combination therapy of PPIs, diet, and corticosteroids was opted for in patients with a higher EREFS (7 points). The endoscopic response rate (partial/total) defined by improvement in the EREFS was 93.5% and the histologic response rate (eosinophil count under 15 eosinophils/high power field) was 77%. No patient needed endoscopic dilatation. Table 2 shows the details of the types of treatment.
Response to the different treatments, with respect to endoscopic and histologic response in Chilean adults with eosinophilic esophagitis.
| Proton pump inhibitor | Topical corticosteroids | Elimination diet | Mixed regimen | Patient total | p value | |
|---|---|---|---|---|---|---|
| n = 34; 54.8% | n = 1; 1.6% | n = 2; 3.2% | n = 25;40.3% | n = 62; 100% | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 37.5 (12.6) | 45 | 36 (2.8) | 38.2 (12.5) | 37.9 (12.1) | 0.690 |
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Initial reason for consultation | 0.748 | |||||
| Dysphagia | 21 (61.8) | 1 (100) | 0 | 15 (60) | 37 (59.7) | |
| Heartburn | 8 (23.5) | 0 | 1 (50) | 4 (16) | 13 (21) | |
| Other | 5 (14.7) | 0 | 1 (50) | 6 (24) | 12 (19.4) |
| Me (min-max) | Me (min-max) | Me (min-max) | Me (min-max) | Me (min-max) | ||
|---|---|---|---|---|---|---|
| Endoscopic findings | ||||||
| Initial EREFS | 0.964 | |||||
| Edema (0-1) | 1 (0-1) | 1 | 1 (0-1) | 1 (0-1) | 1 (0-1) | |
| Rings (0-3) | 1 (0-2) | 1 | 2 (1-2) | 1 (0-2) | 1 (0-2) | |
| Exudate (0-2) | 1 (0-2) | 0 | 0 | 1 (0-2) | 1 (0-2) | |
| Furrows (0-2) | 1 (0-2) | 1 | 2 (1-2) | 1 (0-2) | 1 (0-2) | |
| Stricture (0-1) | 0 (0-1) | 0 | 0 | 0 (0-1) | 0 (0-1) | |
| Total score (0-9) | 4 (2-7) | 3 | 4 (3-4) | 4 (0-5) | 4 (0-7) | |
| Control EREFS | 0.802 | |||||
| Edema (0-1) | 0 (0-1) | 0 | 1 (0-1) | 0 (0-1) | 0 (0-1) | |
| Rings (0-3) | 0 (0-2) | 0 | 0 | 0 (0-2) | 0 (0-2) | |
| Exudate (0-2) | 0 (0-2) | 0 | 0 | 0 (0-2) | 0 (0-2) | |
| Furrows (0-2) | 0 (0-1) | 0 | 0 | 0 (0-1) | 0 (0-1) | |
| Stricture (0-1) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | |
| Total score (0-9) | 0 (0-5) | 0 | 1 (0-1) | 0 (0-4) | 5 (0-5) | |
| Histologic findings | ||||||
| Initial eosinophil count | 25 (15-100) | 34 | 20 (20-25) | 30 (15-100) | 25 (15-100) | <0.001* |
| Control eosinophil count | 0 (0-40) | 0 | 2 (0-4) | 0 (0-100) | 0 (0-100) | 0.293 |
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
|---|---|---|---|---|---|---|
| Histologic response (<15 eos/field) | 26 (76.5) | 1 (100) | 2 (100) | 20 (80) | 49 (79) | 0.815 |
| Endoscopic improvement | 0.903 | |||||
| Total | 19 (55.9) | 1 (100) | 1 (50) | 13 (52) | 34 (54.8) | |
| Partial | 13 (38.2) | 0 | 1 (50) | 9 (36) | 23 (37.1) | |
| No changes | 2 (5.9) | 0 | 0 | 1 (4) | 3 (4.8) | |
| Worsened | 0 | 0 | 0 | 2 (8) | 2 (3.2) |
Data are expressed through frequencies (absolute [N] and relative percentage [%]) and measures of central tendency.
Mean (x¯); ATB: antibiotic; EREFS: Endoscopic Reference Eosinophilic Esophagitis Score; Me: median; range: minimum and maximum; SD: standard deviation.
Kolmogorov-Smirnov normality test, non-normal distribution. Pearson chi-square tests.
Ours is the first cohort of adult patients with EoE currently described in Latin America. Previous studies were limited to case reports and the majority were pediatric patients.15,16 The clinical picture of EoE is associated with esophageal dysfunction or fibrosis and its presentation and progression vary, according to patient age.17 The most frequent symptoms in children are vomiting, abdominal pain, dysphagia, and food impaction, whereas in adults, they are dysphagia and other less characteristic symptoms. Said symptoms can be confused with other diagnoses, such as gastroesophageal reflux disease. This was observed in our cohort, in which more than 20% of patients were initially seen for heartburn.
In persons with EoE without treatment, inflammation sustained at the level of the esophagus is associated with a greater prevalence of chest pain, fibrosis, dysphagia, and food impaction.18 A study that included 721 subjects with EoE, showed that in patients with progression for more than 21 years at the time of diagnosis, the frequency of strictures and food impactions at the level of the esophagus was 52 and 57%, respectively. In patients with symptom duration of fewer than 2 years at the time of diagnosis, said frequency was 19 and 24%, respectively. The risk of stricture for each year of symptoms of untreated EoE increases 9%,19,20 a situation not seen in our cohort. The mean time to diagnosis in our case series was 2.1 years, which could explain the low complication rate observed. Between 28 and 86% of adult patients with EoE have a concurrent allergic disease, including food allergies, allergic rhinitis, atopic dermatitis, and asthma,21–23 which was also observed in our cohort, affecting almost two-thirds of our population.
Genetic, environmental, and allergenic factors are most likely involved in the pathogenesis of EoE. In susceptible individuals, exposure to food allergens, mainly dairy products and wheat, are associated with eosinophil and mastocyte infiltration into the esophageal mucosa, causing damage to the integrity of the epithelial barrier.24,25 In our group, more than one-third of the patients presented with a history of food allergy studied by an immunology team, highlighting allergy to foods rich in lipids, flours, and avocado, differing from that described in international case series, in which egg, milk, and peanut allergies predominate.23
Our case series showed endoscopic findings similar to those described by other groups, in which linear furrows, rings, and edema stand out, but we had a low rate of strictures. For the diagnosis and management of EoE, taking a minimum of 6 biopsies, including the distal and proximal esophagus,26 is recommended, which was carried out in all our patients. In up to 25% of patients with EoE, the esophageal mucosa can appear normal at endoscopy,27 which occurred in 5% of our cohort, probably indicating a low rate of clinical suspicion.
Treatment of EoE requires a multidisciplinary evaluation and follow-up by a gastroenterologist, nutritionist, and immunologist,14 which occurred in the majority of our patients. Treatment goals are to achieve symptomatic and endoscopic remission and reduce the eosinophil count to under 15 eosinophils/high power field, consequently reducing the risk for maintained inflammatory activity and the development of fibrosis and stricture.14,28 The therapeutic strategy should be selected, based on efficacy, ease of administration, cost, and patient preference.1,2 Our group based treatment on the initial endoscopic findings, with a tendency to prefer PPI therapy, followed by mixed therapy, elimination diet, and topical corticosteroids. None of the patients at present have required the start of biologic therapy with dupilumab (the monoclonal antibody targeting IL-4 and IL-13 that is available in our environment) or endoscopic dilatation, probably as a result of early diagnosis and the low number of cases, compared with that reported in the literature.18
One of the main strengths of our study is the characterization of a cohort of adult patients with EoE in Latin America, contributing significantly to the scant data available in our region. In addition, it is important to point out that the patients in our cohort were thoroughly studied, underwent a close follow-up, and were evaluated by a multidisciplinary team in a university environment. A limitation of our study was its descriptive design, in addition to the fact that some of the patients were diagnosed at other medical centers, resulting in a lack of uniformity in the therapeutic regimens and follow-up protocols between participants. This situation has been recognized and addressed by our immune-mediated disease group since 2022, producing an internal protocol for diagnosis, management, and follow-up, which will provide us with the opportunity to improve and broaden our results (Fig. 1). Conducting future multicenter studies in Chile and Latin America in the adult population with EoE is essential.
ConclusionIn the present case series, we found that patients with EoE had characteristics similar to those described in other studies, highlighting the differences seen by sex. The therapeutic strategies employed in our cohort showed rates of endoscopic and histologic effectiveness similar to those described in the literature. Incidence and prevalence studies at the local level and in other Latin American countries will determine the true impact of this disease.
Ethical considerationsThe data obtained during the controls were collected in Excel®. Patient names were not included, to protect their confidentiality, and they previously signed statements of informed consent. The study was approved by the Bioethics Committee of the Universidad de los Andes, CEC2023013. The authors declare that this article contains no personal information that could identify patients.
AuthorshipChristian von Muhlenbrock: management, formal analysis, conceptualization, data curation, writing, methodology, and patient acquisition.
Paulina Núñez and Rodrigo Quera: research, methodology, writing, review, and editing.
Javier Venegas, Victoria Gatica, and Nicole Pacheco: data collection.
Karin Herrera: data analysis.
Fabiola Castro: review and editing.
FundingThe authors declare that they have not received any funding for the conduct of this work.
See related content at DOI: https://doi.org/10.1016/j.rgmxen.2025.01.001, Remes-Troche J.M. Tradition and advancement: 90 years of excellence of the Asociación Mexicana de Gastroenterología. Rev Gastroenterol Mex. 2025;90:1-3.