Fontan surgery, originally described in 1971, is a surgical procedure to correct univentricular congenital heart defects. It consists of creating an anastomosis between the systemic venous return from the inferior vena cava and superior vena cava and the branches of the pulmonary artery, thus achieving passive ventricular filling. This disposition eliminates the mixing of intracardiac blood (oxygenated and deoxygenated) and increases arterial oxygen saturations in the blood, while at the same time reducing volume overload in the single ventricle. The aim is to alleviate hypoxemia and prolong patient survival, but the procedure elevates central venous pressure.1,2
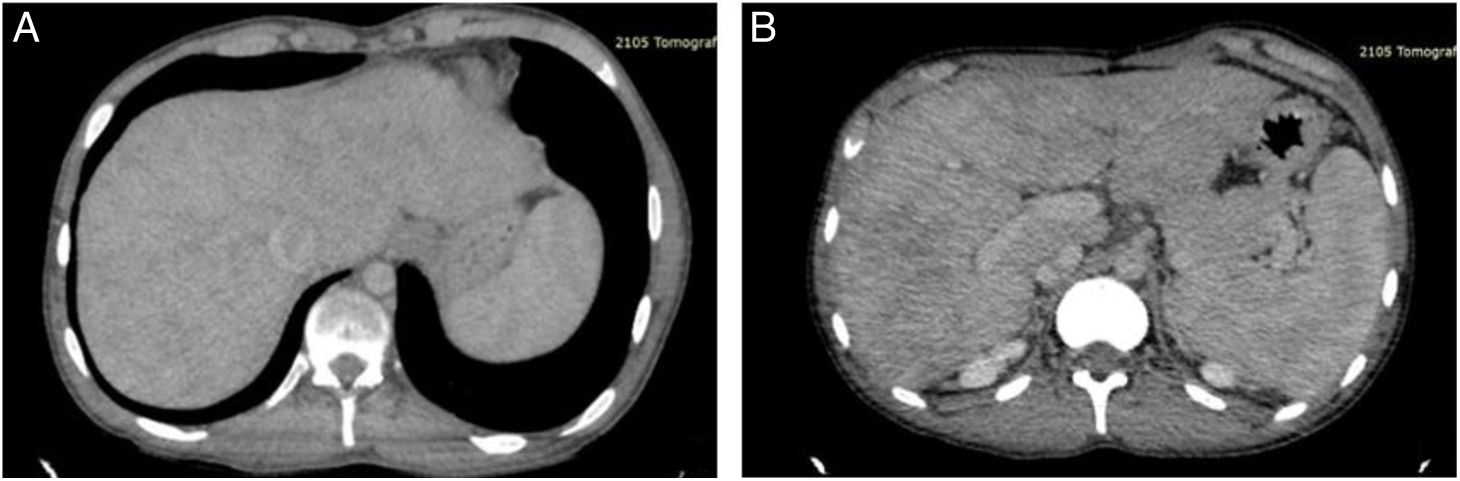
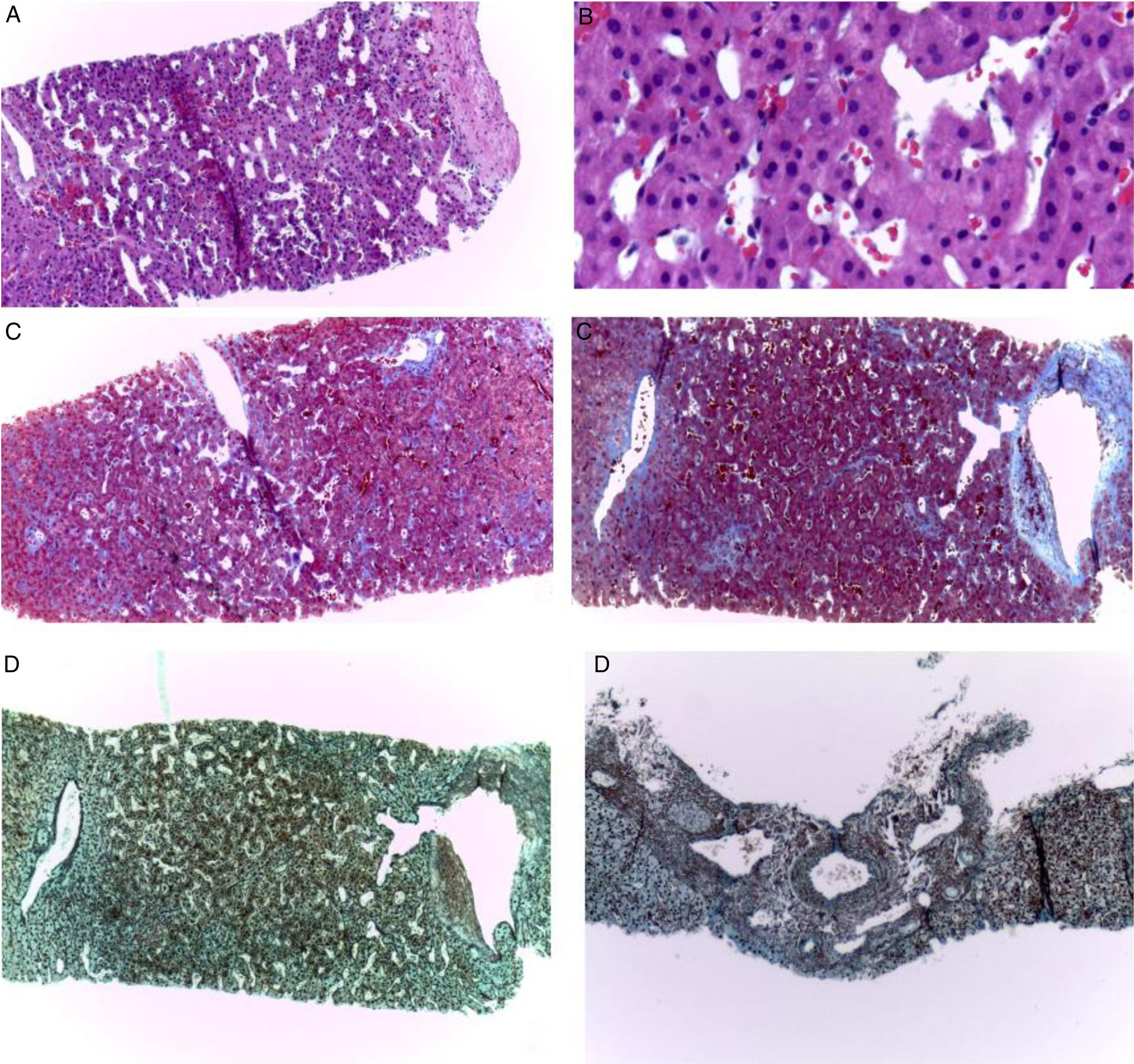
A 19-year-old man had a past medical history of hypoplastic left heart syndrome that was corrected through the Norwood procedure in 2005, the bidirectional Glenn procedure in 2009, and the extracardiac fenestrated Fontan procedure in 2017. He was referred from the Instituto Nacional de Cardiología for consultation at the hepatology service, as the approach to portal hypertension due to the presence of postprandial hematemesis. Upper gastrointestinal endoscopy was performed that reported small esophageal varices, with no signs of poor prognosis (Baveno IV), and mild portal hypertensive gastropathy (McCormack). Vibration-controlled transient elastography (VCTE®) was ordered to evaluate liver fibrosis grade and reported 5.9 kPa and a controlled attenuation parameter of 185 dB/m, corresponding to steatosis grade S0 (0-5% steatosis). The lower esophageal varices, ascites, splenomegaly, and thrombocytopenia (VAST) score for this patient was 3 points, indicating probable advanced Fontan-associated liver disease (FALD). Triphasic abdominal tomography was ordered (Fig. 1) to rule out portal vein and suprahepatic vein thromboses. A percutaneous liver biopsy was carried out to confirm the diagnosis (Fig. 2), in which liver fibrosis was reported as stage 2b, Ishak 2b, and modified Metavir F2, according to the congestive hepatic fibrosis score (CHFS), and as stage 2, according to the 3-scale scoring system for changes related to chronic congestion.2
A) Computed tomography with contrast in the portal venous phase, showing an enlarged liver with dilatation of the inferior vena cava and an enhanced reticular pattern that affects the entire liver, with severe congestive changes in the periphery. B) Computed tomography with contrast in the portal venous phase, showing splenomegaly, accompanied by congestive hepatopathy. Portal vein and suprahepatic vein thromboses were ruled out.
Liver biopsy. Fragments of the liver parenchyma with preserved architecture, with their respective well-defined portal spaces. The higher magnification of the liver parenchyma shows sinusoidal dilatation and congestion, with no inflammation or steatosis (A, H&E ×100 and B, higher magnification, ×400). The same changes of fibrosis (Metavir grade 2, CHFS 2b, Ishak 2b) are shown in Masson’s trichrome staining (C, ×100) and reticular staining (D, ×100). An irregular fibrosis pattern is apparent through acinar atrophy and the alteration of the arrangement and architecture of the liver.
FALD, arising from different hemodynamic changes secondary to the Fontan procedure, was first described in 1983. Due to the effect of the connection of the pulmonary circulation with the systemic circulation, patients undergoing this palliative procedure can present with an increase in pulmonary pressure. This causes an increase in pulmonary resistance with a consequently chronic elevation of the central venous pressure, which is associated with reduced cardiac output with ventricular systolic and diastolic dysfunction that produces liver congestion with sinusoidal dilatation.2–4 Said sinusoidal dilatation with perisinusoidal edema causes oxidative stress activation in the sinusoidal endothelial cells, which decreases nitric oxide production, and also causes stellate cell activation, resulting in liver fibrosis. Sinusoidal dilatation also causes lymphatic congestion that contributes to the depositing of collagen. There is also a decrease in hepatic blood flow due to reduced cardiac output, which causes hypoxic/ischemic damage, altering oxygen diffusion and causing atrophy and the potential apoptosis of centrilobular hepatocytes. All this is associated with the presence of an increase in intestinal permeability due to intestinal lymphatic and venous congestion, contributing to the hepatic and systemic inflammatory process. Likewise, patients with FALD show a decrease in anticoagulant proteins, presenting with a thrombophilic state, with the risk of systemic and sinusoidal thromboses.2 The above-described situation favors the development of portal hypertension, arterialization of the blood supply to the liver, and as a consequence, chronic liver injury.3 Liver congestion severity is heterogeneous due to the technique-dependent flow characteristics of the Fontan connection in each patient, which partially explains the interindividual variability regarding liver injury.2,4 The complication rates at the level of the liver after Fontan surgery are liver fibrosis (0.85-58.3%), cirrhosis of the liver (20.5-23.0%), hepatocellular carcinoma (1.2-9.8%), gastroesophageal varices (19.2-33.3%), and protein-losing gastroenteropathy (3.7-24.0%).4 A retrospective study showed that 1, 6, and 43% of patients presented with cirrhosis at 10, 20, and 30 years, respectively.2
The severity of FALD has been shown to mainly be related to time from the performance of the Fontan procedure, increasing at 15 years after the surgery. Other risk factors considered to lead to advanced FALD are older age at the time of the procedure, the atriopulmonary surgery variant, female sex, an extracardiac connection, hypoplastic left ventricular syndrome, the presence of intrapulmonary shunts, and chronic hypoxemia.2 The detection of FALD is limited due to the lack of significant findings in the physical examination, laboratory test results that tend to be within normal ranges, and the lack of conclusive characteristics in imaging studies. As a result, liver biopsy continues to be the most widely accepted diagnostic method. Different surveillance studies have shown that almost 100% of young adults that have undergone the Fontan procedure present with histologic signs of liver fibrosis. In addition, a significant percentage of those patients (between 35 and 68%) show bridging fibrosis.5 The case presented herein underlines the importance of strict surveillance in patients that have undergone Fontan surgery, given that the majority of them will develop some degree of liver alteration that can range from mild fibrosis to the severe complications of cirrhosis or hepatocellular carcinoma. The progression to liver injury tends to be insidious and silent, reinforcing the all-important need for multidisciplinary follow-up and treatment. This focus is essential for preventing the progression to severe liver disease and for improving the quality of life of these patients.
Ethical considerationsThe authors declare that this article contains no personal information that could identify the patient, meeting the current regulations, and was authorized by the institutional research and ethics committee.
Financial disclosureNo financial support was received in relation to this article.
Our thanks to the Hepatology Department and Pathologic Anatomy Division of the Hospital General Dr. Manuel Gea González, Mexico City, Mexico.