Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare neoplasms originating in neuroendocrine cells from the gastric mucosa and submucosa, small intestine, large intestine, rectum, and pancreas. Our aim was to describe their histopathologic, endoscopic, and clinical characteristics and the experience with these tumors at a tertiary care hospital center in the Colombian Southwest.
Materials and methodsA retrospective, analytic, observational, and descriptive study included 93 patients diagnosed with GEP-NETs, within the time frame of 2018 and 2022. Their clinical histories were reviewed to collect the sociodemographic, clinical, endoscopic, pathologic, treatment, follow-up, and survival data.
ResultsMedian patient age was 55.8 years, and 60.2% were women. A total of 78.5% of the patients presented with symptoms, the most common of which was abdominal pain (78.1%). The tumors were mainly located in the stomach (32.3%) and small intestine (23.7%). Histopathologically, 53.8% of the tumors were grade1, 30.1% were grade 2, 9.68% were grade 3, and 7.52% were carcinomas. Tumor location was significantly related to stage; the majority of tumors in stage I were in the stomach, whereas the stage IV tumors were in the small intestine. At the last evaluation, 40.9% of the patients were disease-free, disease was stable in 24.7% and progressive in 11.8%, and 18.3% of the patients died.
ConclusionsGEP-NETs are clinically heterogeneous, and their early diagnosis is dependent on the recognition of lesions in endoscopic and imaging studies. Early tumors are mainly located in the stomach and advanced tumors in the small intestine, with metastases in the liver and regional lymph nodes. The present study suggests the importance of disease awareness in the early detection of GEP-NETs; said factor, combined with timely interdisciplinary management, could significantly impact patient outcomes.
Los tumores neuroendocrinos gastroenteropancreáticos (TNE-GEP) son neoplasias infrecuentes originadas en células neuroendocrinas de la mucosa y submucosa gástrica, intestino delgado, grueso, recto y páncreas. Este estudio describe las características clínicas, endoscópicas, histopatológicas y la experiencia de un centro de alta complejidad en el suroccidente colombiano.
Materiales y métodosSe realizó un estudio observacional descriptivo con alcance analítico retrospectivo con 93 pacientes diagnosticados con TNE-GEP entre 2018 y 2022. Se revisaron historias clínicas para recolectar datos sociodemográficos, clínicos, endoscópicos, patológicos, tratamientos, seguimiento y supervivencia.
ResultadosLa mediana de edad fue de 55.8 años, y el 60.2% eran mujeres. El 78.5% presentó síntomas, siendo el dolor abdominal el más común (78.1%). Los tumores se localizaron principalmente en el estómago (32.3%) e intestino delgado (23.7%). Histopatológicamente, el 53.8% de los tumores eran de grado 1, el 30.1% de grado 2, el 9.68% grado 3 y 7.52% carcinomas. La localización del tumor mostró una relación significativa con su estadio; la mayor proporción de tumores en estadio I se encontraron en estómago, mientras que los de estadio IV en intestino delgado. En la última valoración, 40.9% de los pacientes estaban libres de enfermedad, 24.7% tenían enfermedad estable, 11.8% progresiva y 18.3% falleció.
ConclusionesLos TNE-GEP presentan heterogeneidad clínica, su diagnóstico temprano depende del reconocimiento endoscópico e imagenológico de las lesiones. Los tumores tempranos se localizan principalmente en el estómago, y los avanzados en el intestino delgado, con metástasis en hígado y ganglios linfáticos regionales. Este trabajo sugiere la importancia de la sensibilización en la detección temprana de los TNE-GEP; este factor combinado con un manejo interdisciplinario oportuno, podrían impactar significativamente en los desenlaces de los pacientes.
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare neoplasms that originate from pluripotential neuroendocrine cells distributed in the gastric mucosa and submucosa, the small and large intestines, the rectum, and the pancreas.1 These cells have the capacity to secrete diverse monoamines, peptides, and physiologically active hormones, resulting in heterogeneity and a complex clinical presentation, encompassing both well-differentiated tumors known as neuroendocrine tumors (NETs) and poorly-differentiated and aggressive tumors, such as neuroendocrine carcinomas (NECs).2 The majority are asymptomatic and are incidentally detected in endoscopic studies, presenting as polyps or ulcers.2,3
The 2022 WHO classification of NETs defines GEP-NETs as well-differentiated tumors that are categorized into three grades (G1, G2, G3), according to the mitotic rate and Ki-67 proliferation index. High-grade and poorly differentiated NECs are divided into small cell and large cell subtypes.4 The therapeutic option for localized tumors is surgical or endoscopic resection, with some exceptions. Somatostatin analogues, chemotherapy, or tyrosine kinase inhibitors are used for locally advanced or metastatic disease.1,3 According to the NANETS consensus, stable progression indicates that the disease presents no significant changes in lesion size or number, signifying that the tumor maintains controlled growth. In contrast, persistent progression may mean a change in the biologic behavior of the tumor, manifesting as a continuous increase in lesion size or number.5,6
GEP-NETs can clinically manifest in a very nonspecific manner, with symptoms, such as abdominal pain that can be secondary to mass effects, gastrointestinal bleeding, and symptoms related to excess hormone secretion, diarrhea due to serotonin release, or flushing from prostaglandin secretion.7 In the United States, a retrospective study utilizing the Surveillance, Epidemiology, and End Results (SEER) program data, evaluated 64,971 patients with NETs. The age-adjusted incidence rate increased 6.4 times, augmenting from 1.09 per 100,000 in 1973 to 6.98 per 100,000 in 2012, with increases seen at all sites, stages, and grades. In the SEER 18 register (2000–2012), the highest rate was 3.56 per 100,000 at gastroenteropancreatic sites.8
In Colombia, few studies describe NETs. In a 2020 study conducted in Medellín that included 111 cases, 59.4% corresponded to primary NETs, 34.8% of which were located in the pancreas, 25.7% in the small intestine, and 13.6% in the stomach.9
The Fundación Valle del Lili is a referral center in the Colombian Southwest that has a multidisciplinary group specializing in the management of these types of tumors. For the present study, a four-year follow-up was carried out on patients with GEP-NETs, to describe their clinical, histopathologic, endoscopic, and treatment characteristics.
Materials and methodsA retrospective, descriptive, analytic, observational study was conducted that included patients above 18 years of age of both sexes, with gastrointestinal neuroendocrine neoplasms that were diagnosed previously or during the study period, and seen at the Fundación Valle del Lili between January 1, 2018, and December 31, 2022. From the institution’s hospital cancer registry, patients with a histopathologic diagnosis of a NET of the stomach, small intestine, appendix, pancreas, colon, or rectum were included. Twenty-five patients met the following exclusion criteria: 18 had incomplete information in the pathology report of the specimen obtained, 3 patients had a histopathologic diagnosis of a hepatic neuroendocrine neoplasm, and 4 patients were minors. After applying the exclusion criteria to the initial sample of 118 patients, the final sample was made up of 93 patients.
All the data were collected from the clinical histories, including the outcomes defined by the treating physician. Data collection was carried out using the REDCap application, and the STROBE checklist was employed.
Statistical analysisAn exploratory analysis was carried out to verify and correct outliers and missing values in the data, ensuring data quality and completeness. A univariate analysis was then carried out to study the behavior of the numerical variables. The normality of the variables was evaluated using the Shapiro-Wilk test; those whose value was a p > 0.05 were considered to have normal distribution and were expressed as mean and standard deviation. The variables with non-normal distribution were expressed as median and interquartile range. The categorical variables were expressed as proportions. The Fisher’s exact test was utilized to verify the significant differences between variables. All the analyses were performed utilizing the RStudio version 2023.03.1 + 446 statistics package. The Kaplan-Meier life table was calculated and the importance of the difference in survival between the subgroups was evaluated using the logarithmic range test. For the survival and outcome analysis, patient status during the study period was taken into account; the cause of death could not be established in 4 patients because they were treated outside of our institution.
Ethical considerationsThe Biomedical Research Ethics Committee approved the present study, with approval number 2023.208, in accordance with the Declaration of Helsinki and the 1993 Resolution 8430 of the Colombian Department of Health and Social Protection. To safeguard the identity of the participants, the medical records were codified, and access was restricted to the researchers. The authors declare that this article contains no personal information that could identify the patients.
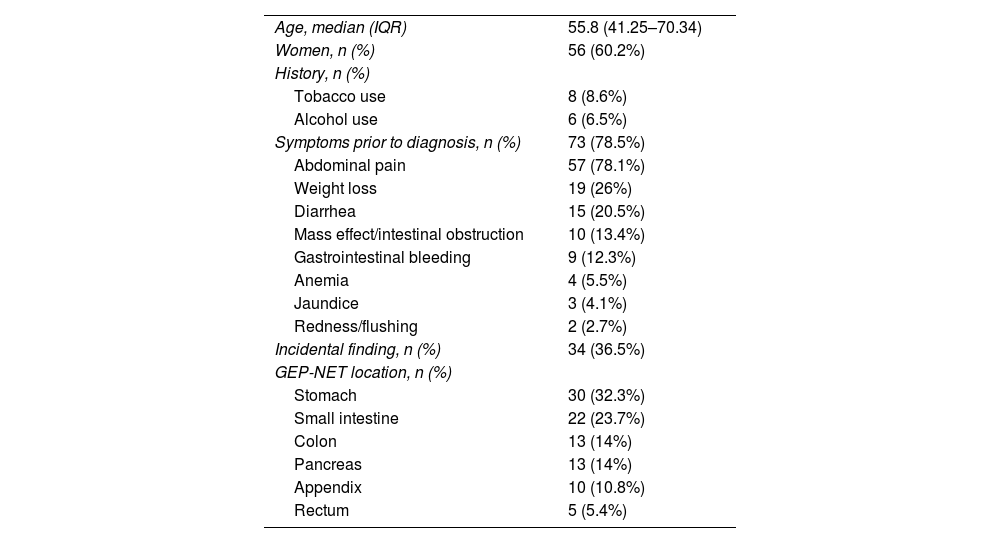
ResultsA total of 93 patients with a median age of 55.8 years (41.25–70.34 IQR), 60.2% of whom were women, were analyzed. Four patients were identified with a personal history of autoimmune disease: two with autoimmune thyroiditis and two with autoimmune atrophic gastritis. No patient presented with a history of GEP-NET-related tumor or neoplasm. Two patients had a family history of medullary thyroid carcinoma in a first-degree relative. A total of 91.4% (n = 85) patients had no history of smoking and 87 (93.5%) did not use alcohol (Table 1).
Sociodemographic and clinical characteristics of the patients with GEP-NETs treated at the Fundación Valle del Lili from 2018 to 2022.
| Age, median (IQR) | 55.8 (41.25–70.34) |
| Women, n (%) | 56 (60.2%) |
| History, n (%) | |
| Tobacco use | 8 (8.6%) |
| Alcohol use | 6 (6.5%) |
| Symptoms prior to diagnosis, n (%) | 73 (78.5%) |
| Abdominal pain | 57 (78.1%) |
| Weight loss | 19 (26%) |
| Diarrhea | 15 (20.5%) |
| Mass effect/intestinal obstruction | 10 (13.4%) |
| Gastrointestinal bleeding | 9 (12.3%) |
| Anemia | 4 (5.5%) |
| Jaundice | 3 (4.1%) |
| Redness/flushing | 2 (2.7%) |
| Incidental finding, n (%) | 34 (36.5%) |
| GEP-NET location, n (%) | |
| Stomach | 30 (32.3%) |
| Small intestine | 22 (23.7%) |
| Colon | 13 (14%) |
| Pancreas | 13 (14%) |
| Appendix | 10 (10.8%) |
| Rectum | 5 (5.4%) |
GEP-NET: gastroenteropancreatic neuroendocrine tumor; IQR: interquartile range.
Regarding the clinical characteristics, 40.9% of the patients had a normal BMI (18.5–24.9), 21.5% were overweight, and 18.3% were obese. Of the 73 patients (78.5%) that had symptoms before diagnosis, the most common were abdominal pain (78.1%), followed by weight loss (26%), diarrhea (20.5%), a mass effect or obstruction (13.4%), and gastrointestinal bleeding (12.3%). In 36.5% of the patients, the GEP-NET was an incidental finding, and the majority were discovered in the appendix (26.4%), stomach (23.5%), and small intestine (23.5%).
A total of 32.3% of the tumors were found in the stomach (gastric neuroendocrine tumors [GNETs], 23.7% in the small intestine, 14% in the colon, 14% in the pancreas, 10.8% in the appendix, and 5.4% in the rectum, as described in Table 1.
With respect to symptoms related to tumor location, abdominal pain was the most prevalent, standing out in the gastric tumors (28.07%) and in those of the small intestine (26.31%). Diarrhea was reported with greater frequency in the tumors of the stomach (33.33%) and small intestine (20%), whereas gastrointestinal bleeding was more common in the patients with tumors of the small intestine (44.44%) and rectum (22.22%). Weight loss was notably high in the gastric tumor cases (52.63%). The mass effect or obstruction was reported in several locations, especially in the small intestine (40%) and pancreas (20%).
Endoscopic findings were documented in 32 patients (34.4%), and the majority corresponded to polypoid lesions (46.8%), followed by ulcers (15.6%) and tumor mass (15.6%).
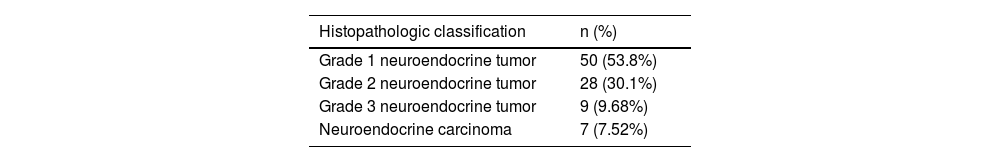
According to their histopathologic classification, 53.8% of cases presented with a grade 1 neuroendocrine neoplasm, 30.1% with grade 2, 9.68% with grade 3, and 7.52% with NECs (6.45% were poorly differentiated small cell NECs and 1.07% were poorly differentiated large cell NECs), with proliferation indexes (Ki67) below 3% for grade 1, 3–20% for grade 2, and above 20% for grade 3 and the carcinomas (Table 2). Immunohistochemistry performed on the samples of 72 patients revealed that chromogranin A, synaptophysin, and CD56 were more frequently stained in the diseased tissues.
Histopathologic classification of GEP-NETs treated at the Fundación Valle del Lili from 2018 to 2022.
| Histopathologic classification | n (%) |
|---|---|
| Grade 1 neuroendocrine tumor | 50 (53.8%) |
| Grade 2 neuroendocrine tumor | 28 (30.1%) |
| Grade 3 neuroendocrine tumor | 9 (9.68%) |
| Neuroendocrine carcinoma | 7 (7.52%) |
GEP-NETs: gastroenteropancreatic neuroendocrine tumors.
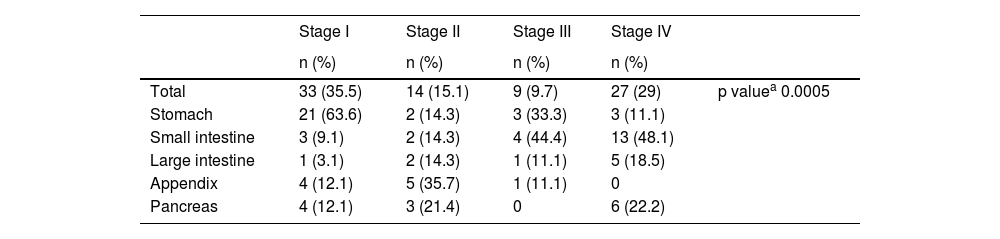
The result of the bivariate analysis between stage and tumor location showed a statistically significant difference (p = 0.0005). The majority of stage I tumors were found in the stomach (n = 21), whereas the stage IV tumors were located in the small intestine (n = 13) (Table 3). Stage in 10 patients could not be established due to a lack of information on their TNM classification.
Relation between stage and location of GEP-NETs treated at the Fundación Valle del Lili from 2018 to 2022.
| Stage I | Stage II | Stage III | Stage IV | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Total | 33 (35.5) | 14 (15.1) | 9 (9.7) | 27 (29) | p valuea 0.0005 |
| Stomach | 21 (63.6) | 2 (14.3) | 3 (33.3) | 3 (11.1) | |
| Small intestine | 3 (9.1) | 2 (14.3) | 4 (44.4) | 13 (48.1) | |
| Large intestine | 1 (3.1) | 2 (14.3) | 1 (11.1) | 5 (18.5) | |
| Appendix | 4 (12.1) | 5 (35.7) | 1 (11.1) | 0 | |
| Pancreas | 4 (12.1) | 3 (21.4) | 0 | 6 (22.2) |
GEP-NETs: gastroenteropancreatic neuroendocrine tumors.
Imaging studies were carried out on 37 patients: ultrasounds in 6.5% of cases, computed tomography (CT) in 20.4%, and magnetic resonance imaging (MRI) in 12.9%. Of the patients that had an ultrasound study, a tumor was identified in 6 of them: three in the stomach, two in the pancreas, and one in the rectum, and the majority were under 3 cm, round, and hypoechoic. The CT studies identified 20 tumors, 8 of which were located in the small intestine; 17.2% of the lesions were larger than 1 cm, 10.8% had an irregular shape, and 16.1% were solid. In the MRI studies, the majority of lesions were in the pancreas (7.5%); they were solid, larger than 1 cm, and showed hypointensity in T1 and hyperintensity in T2, a homogeneous enhancement pattern, and early enhancement time.
A total of 46.2% of the study population presented with metastasis. Metastases were most frequently located in the liver, at 65.1%, followed by regional lymph nodes, at 30.2%, and in other sites of the gastrointestinal tract, at 4.7%, of which the most frequent primary tumor locations were the small intestine (38.4%) and large intestine (23%).
The complementary treatments for the neuroendocrine neoplasms were endoscopic resection in 22 patients (23.7%), surgical resection in 63 patients (67.7%), and systemic treatment in 30 patients (32.3%).
Regarding outcomes at the final evaluation, 38 patients (40.9%) were disease-free, 23 (24.7%) had stable persistent disease, 11 (11.8%) had persistent disease in progression, and 17 (18.3%) patients died; 13 of the deaths were related to a neuroendocrine neoplasm, 10 of which were high-grade tumors. Eleven patients died during the study period and 6 died after the study period. There was no follow-up information for 4 patients. The median number of months from diagnosis to death was 13.26 (5.42–25.01 IQR) and the median follow-up time from diagnosis to the final evaluation was 27.01 months (17.82–56.43 IQR). For that analysis, patient status was determined according to the status registered in the clinical history by the treating physician, applying no additional criteria by the researchers for its definition (Table 4).
Treatment and outcomes of patients with GEP-NETs treated at the Fundación Valle del Lili from 2018 to 2022.
| Treatment, n (%) | |
| Surgical resection | 63 (67.7%) |
| Systemic treatment | 30 (32.3%) |
| Endoscopic resection | 22 (23.7%) |
| Outcomes, n (%) | |
| Disease-free | 38 40.9%) |
| Stable persistent disease | 23 (24.7%) |
| Persistent disease in progression | 11 (11.8%) |
| Deceased patients | 17 (18.3%) |
| Patients lost to follow-up | 4 (4.3%) |
| NET-related deaths | 13 (14%) |
| Months elapsed from diagnosis to death, median (IQR) | 13.26 (5.42–25.01) |
| Months elapsed from diagnosis to last evaluation in living patients, median (IQR) | 27.01 (17.82–56.43) |
IQR: interquartile range; GEP-NETs; gastroenteropancreatic neuroendocrine tumors; NET: neuroendocrine tumor.
Having in mind that the behavior of pancreatic tumors can be different, 13 patients with pancreatic neuroendocrine tumors (pNETs) were analyzed. Median patient age was 68 years (IQR: 49.5–72), with male predominance (61.5%). The most frequent symptoms were abdominal pain (77%), weight loss (15.3%), and diarrhea (15.3%). Three cases were incidental findings. Metastasis was present in 61.5% of patients, mainly in the liver (75%) and lymph nodes (15%). The histopathologic classification was 23% cases of grade 1 tumors, 46.1% were grade 2, 7.7% were grade 3, and 23% were carcinomas. A total of 46.1% cases were in stage IV, 30.8% in stage I, and 23.1% in stage II. Treatment included surgical resection (84.6%), systemic therapy (38.5%), and endoscopic resection (7.7%), and they were not mutually exclusive. Median follow-up time was 22.3 months (12.20–29.39 IQR); 38.5% of patients presented with stable disease, 30.8% had progression, 15.4% had complete remission, and 23.1% died due to pNET. Median survival was 14.36 months (10.99–34.15 IQR).
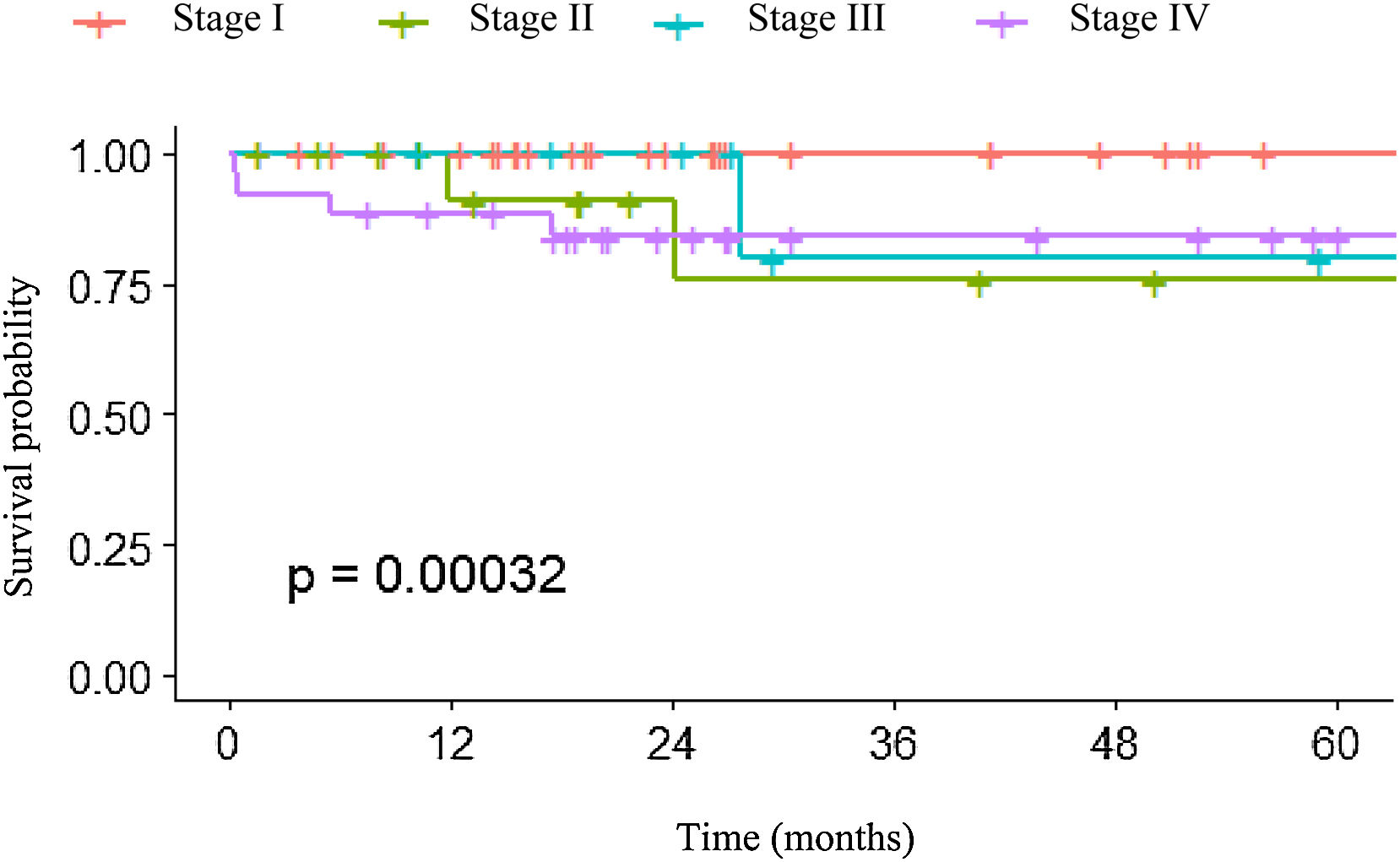
In relation to overall survival of the patients with GEP-NETs, survival was higher in the first months after diagnosis in patients with more advanced-stage tumors. However, over time, patients with stage II and III disease had a lower survival rate than the patients with stage IV disease. In particular, patients in stage I had a higher survival probability, which remained constant over time (Fig. 1).
The survival analysis by stage included only 7 of the 11 patients that died during the study period; 4 were excluded from the graph due to a lack of sufficient information for TNM classification. A survival percentage analysis by stage was carried out, taking into consideration time up to death (Fig. 1). Survival was 100% in stage I. It decreased to 90.9% at 11.9 months and to 75.8% at 24.1 months in stage II patients. Survival decreased to 80% at 27.6 months in stage III. Lastly, in stage IV, survival had an early descent at 0.3 months to 96% and a rapid decrease to 84% at 17.4 months, stabilizing at that value. The differences between survival percentages were not statistically significant.
The evaluation of survival related to histologic grade revealed that patients with grade 1 GNET had a median survival of 26.99 months (16.34–56.08 IQR); survival was 25.66 months (17.64–52.44 IQR) in patients with grade 2, 14.84 months (6.07–29.82 IQR) in patients with grade 3, and 16.01 months (12.52–27.32 IQR) in patients with carcinomas. Nevertheless, those differences were not statistically significant (p = 0.38).
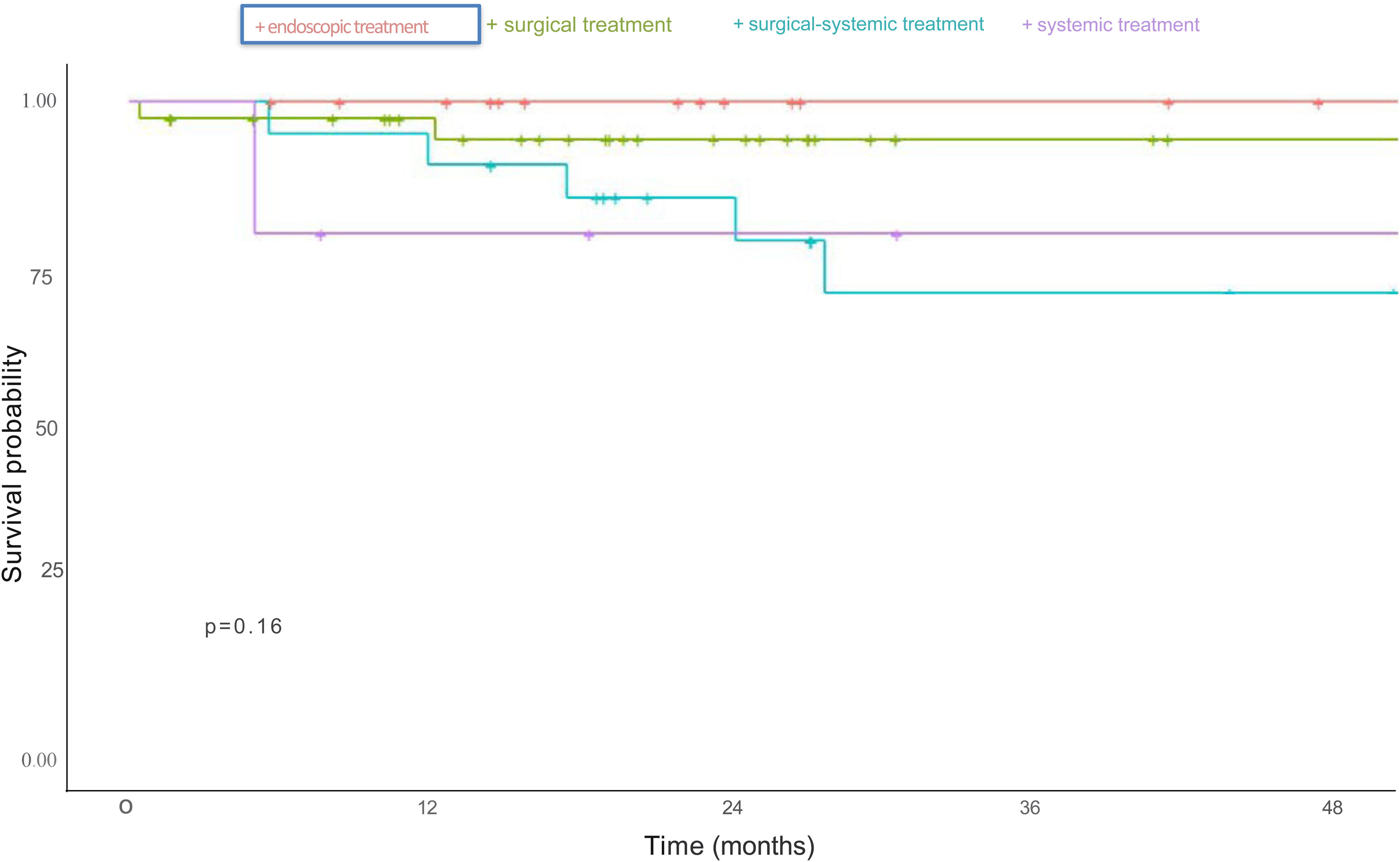
For the survival evaluation according to treatment type, patients that received at least one treatment were included. Surgical and endoscopic treatments had a better survival rate, compared with systemic treatment and the combination of surgical and systemic treatment, but no treatment type reached statistical significance (p = 0.16) (Fig. 2).
DiscussionGEP-NETs can present in patients at any age, with a greater incidence in the fifth decade of life.1 In the present study, the median patient age was 55.8 years (41.25–70.34), with a greater prevalence in women (60.2%). This coincides with the descriptions of demographic characteristics in the literature, which indicate that GEP-NETs are more prevalent in middle-aged women, possibly due to biologic differences or behaviors in seeking medical attention.10
Smoking and alcohol use are factors associated with various gastrointestinal neoplasms but their specific relation to GEP-NETs continues to be an area of investigation. A recent study found no significant association between smoking and the incidence of GEP-NETs, suggesting a greater relevance of other etiologic factors.10 Similarly, moderate alcohol use does not appear to significantly increase the risk for developing GEP-NETs,10–11 which was reflected in the relatively low prevalence of a history of smoking and alcohol use (8.6% and 6.5%, respectively) in our study population.
Regarding symptoms prior to the diagnosis of GEP-NETs, we found a diverse and nonspecific presentation in our study. The most common symptom was abdominal pain, followed by weight loss, diarrhea, mass effect or obstruction, and gastrointestinal bleeding, concurring with the literature, in which abdominal pain is reported as a common presentation symptom.10,12 The diagnosis of these tumors was incidental in 36.5% of our cases, with the appendix as the most common anatomic site; reports in the literature show that the clinical presentation in many patients is similar to acute appendicitis.7
The stomach and small intestine were the most frequent tumor locations in our study, unlike the literature that reports the rectum as the most common GEP-NET location.12 This could be related to the increased incidence of GNETs due to the higher number of upper gastrointestinal endoscopies performed for different gastrointestinal diseases, resulting in a greater detection of GNETS, previously considered rare.2,10,13–15 In our study population, polypoid lesions were the most common endoscopic gastric lesions, concurring with reports in the literature that identify polyps as the most frequent manifestation of GEP-NETs in the stomach.16–18 Distinguishing GNETs from non-neoplastic gastric polyps is a challenge, which could be analyzed in additional studies that evaluate the characteristics that facilitate their identification through endoscopy.
The majority of tumors were classified as grade 1 (53.8%), followed by grade 2 (30.1%), grade 3 (9.68%), and NECs (7.52%). Said distribution underscores a predominance of low-grade tumors that generally indicates more favorable prognosis and is supported in the literature, with reports showing that most GEP-NETs (approximately 90%) are low-grade NETs (grade 1 or 2), whereas under 10% are high-grade GEP-NETs.1 With respect to prognostic staging, stage I was more prevalent in the stomach, reflecting effective early detection or intrinsic differences in tumor biology that predispose to less aggressive diseases in that area.1,9 In contrast, visualization of lesions in the small intestine is more difficult, which could explain why stage IV disease was more frequently found in that location in our study, also suggesting a different tumor progression route or possible delays in the diagnoses in the small intestine.1,9
Our study results concur with those reported in the international literature, regarding the positivity of immunohistochemical markers in NET cases.9,19 The majority of samples were positive for chromogranin A, synaptophysin, and CD56. As in the study by Flórez et al.,9 a high number of samples were positive for CD56. Most of the metastatic lesions presented in tumors originating in the small intestine, corresponding to that described in the literature, in which the probability of metastatic involvement is reported to be associated with the primary tumor site, more commonly in patients with tumors in the small intestine than in those with tumors at other sites.10,20
Imaging studies are considered essential tools for the diagnosis, staging, and follow-up of GEP-NETs. In CT studies, primary tumor detection is related to its size and location, and the pancreas is the most commonly identified site.21 In the present study, most of the lesions larger than 1 cm were identified through CT studies and the most frequent locations were the small intestine and pancreas. On the other hand, MRI is useful for evaluating GEP-NETs in the liver and pancreas; tumors can present with hyperintensity in T2 weighted sequences.22 In our study, MRI identified tumors in the pancreas; they were predominantly solid tumors, with hyperintensity in T2.
Concerning management, surgical and endoscopic resections were the most common modalities, followed by systemic treatment, which could be explained by the fact that the majority of patients presented with localized disease. Treatment data reinforce the current consensus that surgical resection is the cornerstone of GEP-NET management, especially for localized tumors. Endoscopic resection is a less invasive option in early-stage tumors, whereas systemic therapy is reserved for advanced or metastatic diseases.10 Said findings support the role of multimodal treatment strategies adapted to tumor stage and location.1,23
In the present study, we found that the patients with GEP-NETs had a median follow-up period of 27 months. In 2018, the Colombian Instituto Nacional de Cancerología carried out a study that evaluated the outcomes of patients with a histologically confirmed diagnosis of a NET of intestinal origin and reported that a majority of patients had stable persistent disease (37.5%), 25% were disease-free, and 11.8% presented with persistent disease in progression. There was a higher mortality rate and lower survival rate related to high-grade neuroendocrine neoplasms. The medical literature also describes the strong influence of tumor grade and characteristics, as well as the effectiveness of treatment regimens, on the clinical outcome of patients with NETs.24–26
Our study results showed a difference in the overall survival of patients with GEP-NETs, according to stage. Initially, survival was greater in the patients with low-grade tumors, reflecting the importance of early diagnosis. In the literature, early detection and aggressive treatment are shown to improve survival rates, whereas advanced disease is associated with less favorable results, despite the therapeutic interventions carried out.24,27 Upon analyzing survival over time, we found that patients with stage II and stage III disease, had a lower survival rate than those with stage IV disease. Previous studies have reported that, in cases of GEP-NETs in the small intestine, patients in stage III can have a better survival rate than those with stages I or II; this is associated with surgical tumor resection, given that tumors of the small intestine, even in advanced stages, are often susceptible to definitive surgical treatment, which can improve survival in those patients, even though they have advanced disease.25 In our study, 30% of the patients presented with advanced disease (stage IV) and approximately half of those patients had tumors in the small intestine. Said distribution could explain our survival results, in which the more advanced stage of disease had a better survival rate than the earlier stages.
On the other hand, patients in stage I had a probability of constant and significantly higher survival, underlining the importance of early diagnosis and intervention in that patient population. These results suggest the need for continuous follow-up and personalized treatment, according to tumor stage. Nevertheless, additional studies are needed to evaluate intervention effectiveness and their impact on patient survival.
Study strengths and limitationsOur study is the first descriptive analysis to profile a Southwestern Colombian population with GEP-NETs treated at a referral center for cancer patients. It provides a valuable understanding of the clinical, histopathologic, endoscopic, and treatment characteristics of those patients, filling an information gap about GEP-NETs in the region and country. Given that the study population was made up of patients treated at a center with an integrated care team for the management of their disease, there was a high number of patients with tumors in advanced stages. This could result in an optimistic perception of survival, due to the adequate interventions performed at the center. A limitation of our study was the size of the population, which did not enable more robust analyses to be carried out or conclusive relations between variables to be established. This limitation could be addressed through multicenter studies that would enable the performance of more specific analyses.
ConclusionsEven though the clinical manifestations of GEP-NETs tend to be nonspecific, early diagnosis is largely dependent on accurately recognizing lesions through endoscopic and imaging studies. This factor, together with timely management, can influence the clinical results. In our study, the majority of the GEP-NETs were detected in early stages, whereas the pNETs were more frequently found in more advanced stages and grades at diagnosis. The main metastatic sites identified were the liver and lymph nodes. Survival was better in the patients that received surgical treatment. These findings emphasize the importance of disease awareness for the early detection of GEP-NETs and underline the need for further studies to be conducted to broaden the knowledge related to their diagnosis, treatment, and prognosis.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
The authors declare that there is no conflict of interest.